Three-Dimensional Cell Metabolomics Deciphers the Anti-Angiogenic Properties of the Radioprotectant Amifostine
Abstract
:Simple Summary
Abstract
1. Introduction
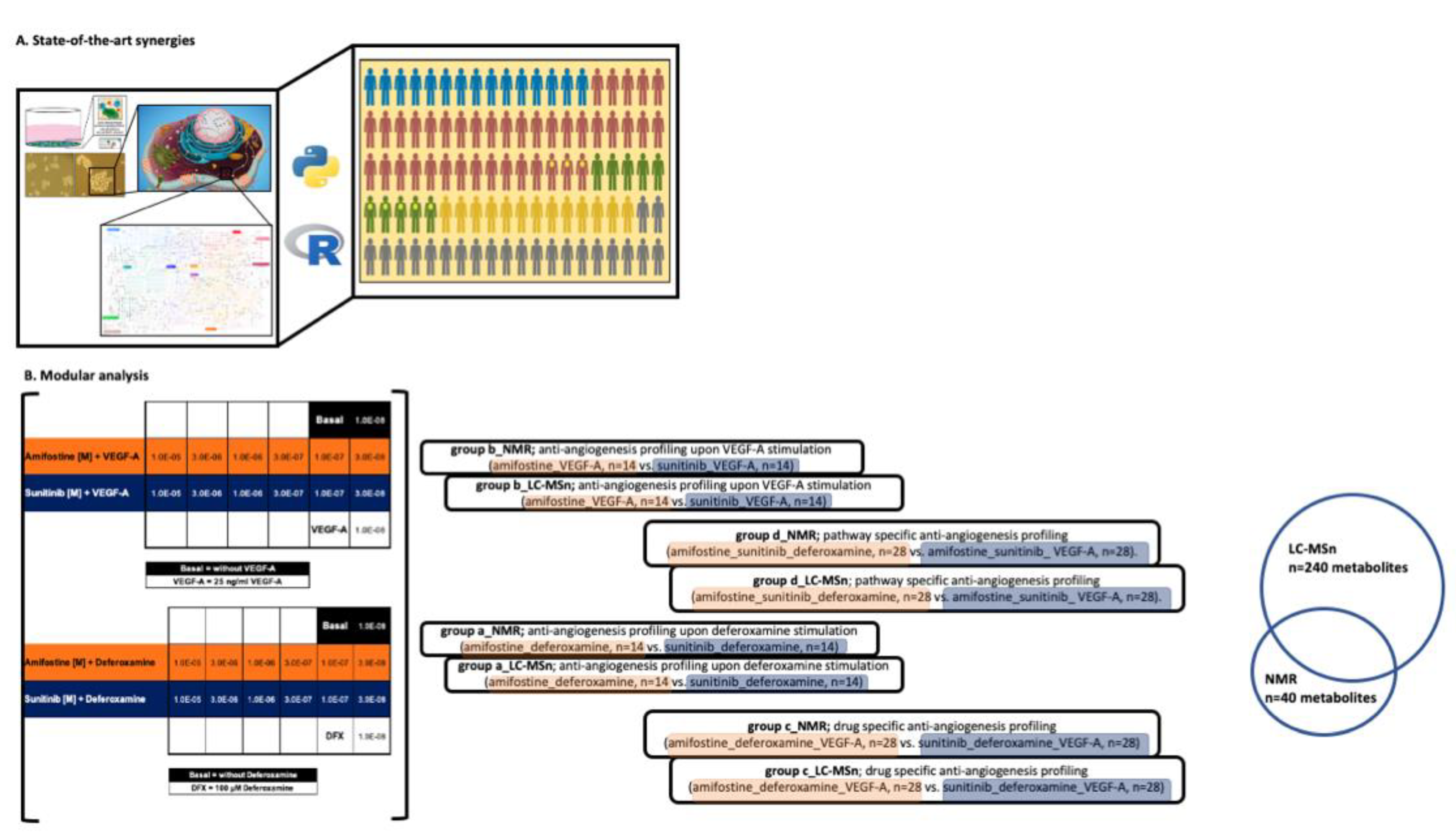
2. Materials and Methods
3. Results
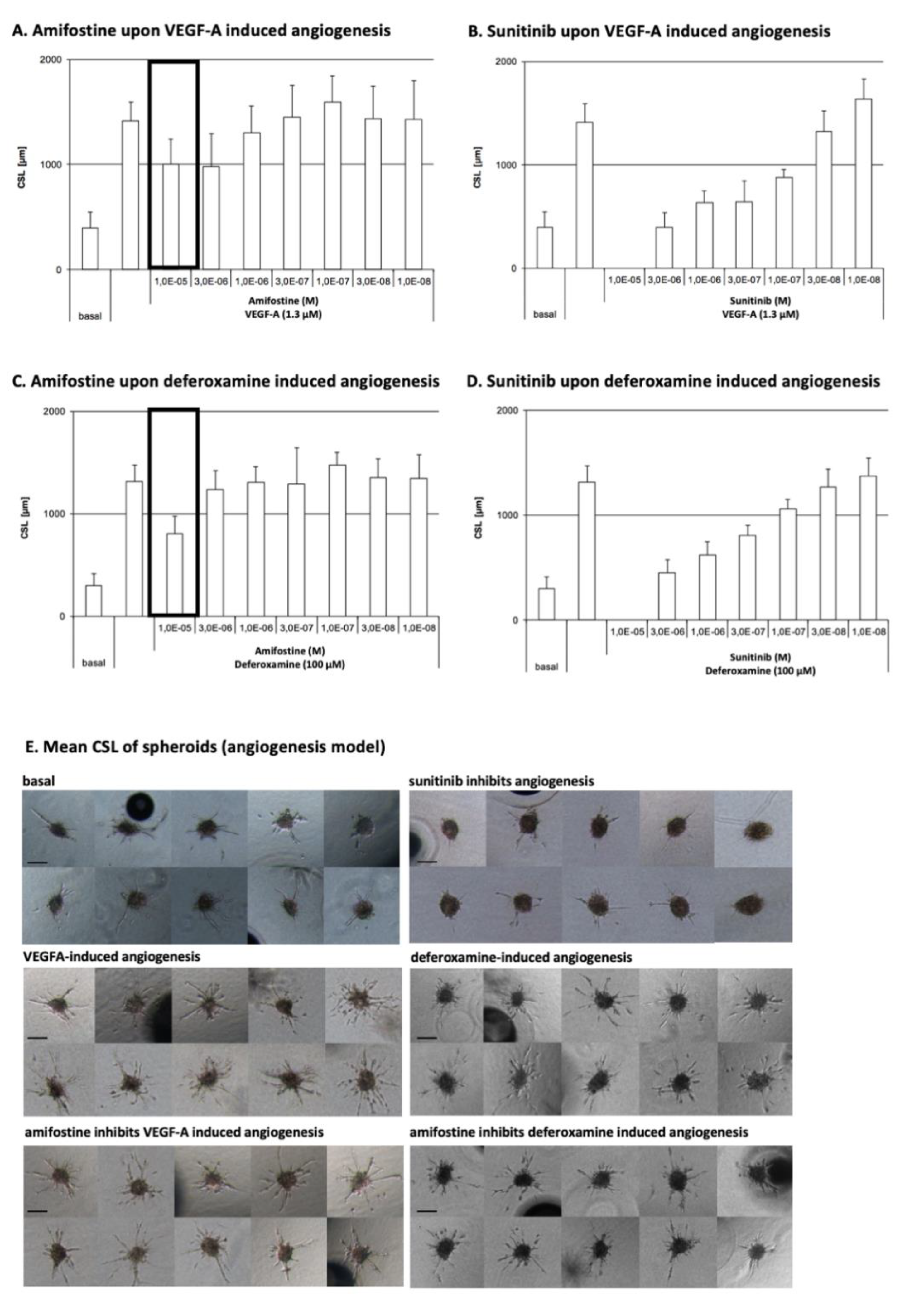
3.1. Amifostine Inhibits VEGF-A- and Deferoxamine-Induced Cell Sprouting
3.2. LC-MS- and NMR-Based 3D Cell Metabolomes Reveal the Anti-Angiogenic Profile of Amifostine
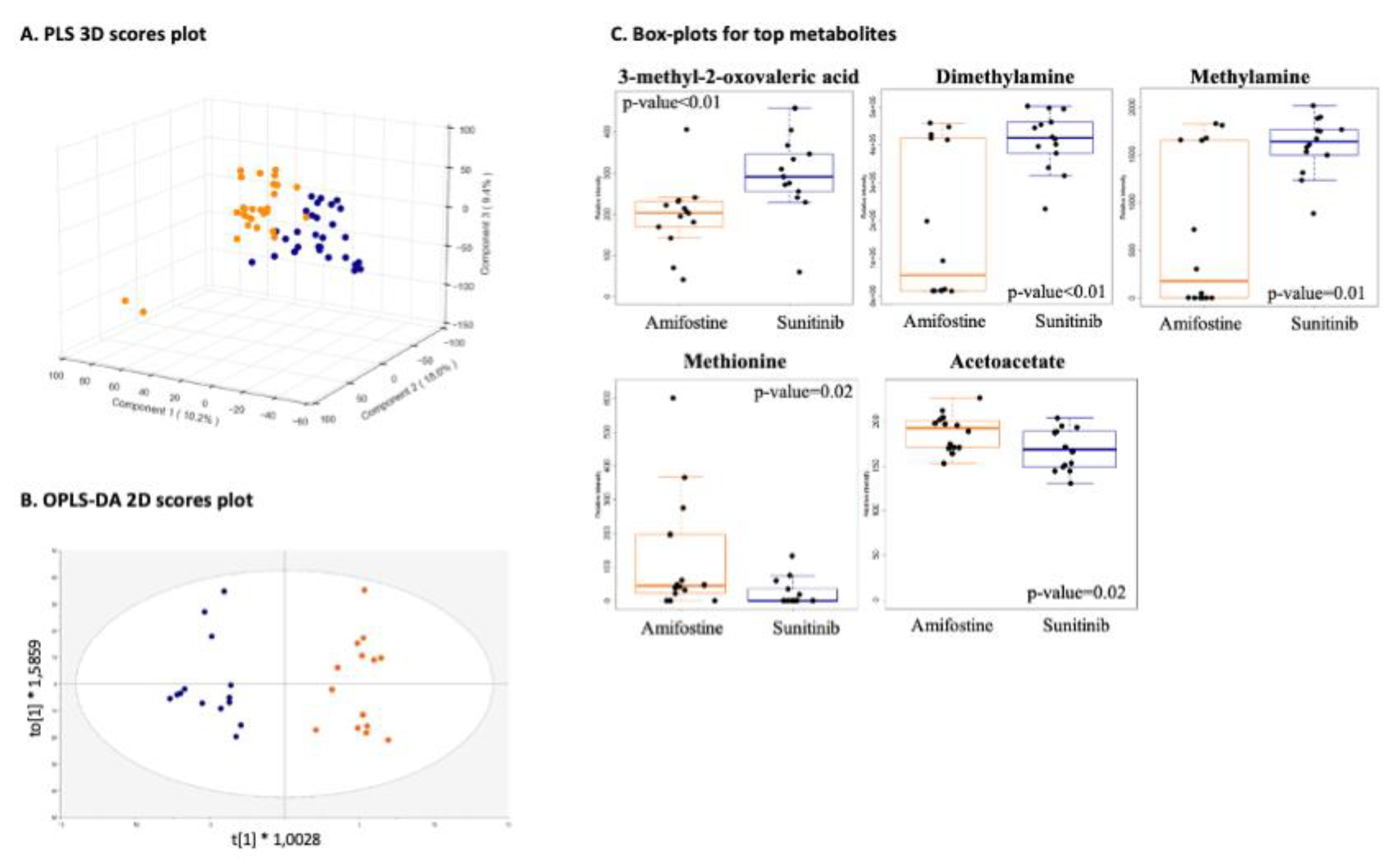
3.2.1. Drug-Specific Anti-Angiogenesis Profiling
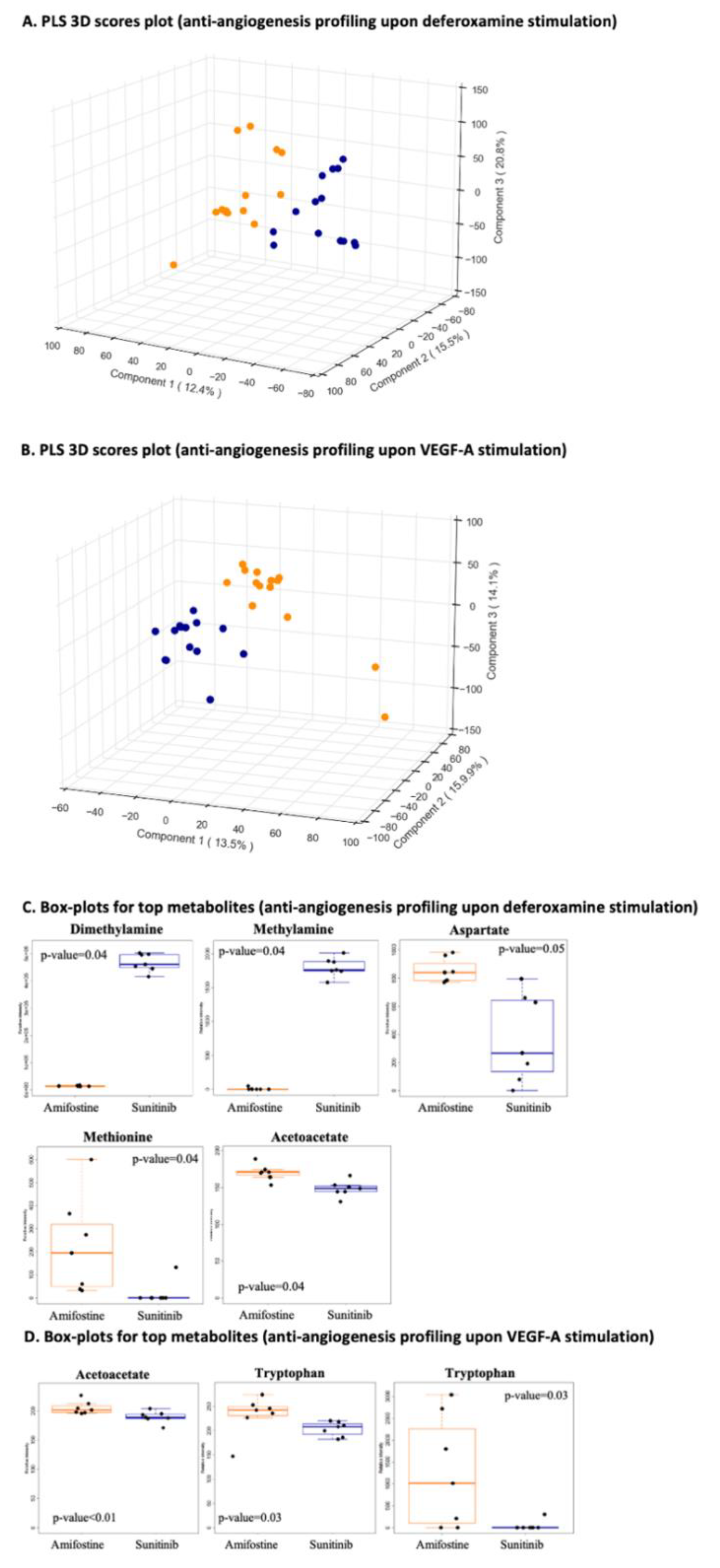
3.2.2. Pathway-Specific Anti-Angiogenesis Profiling
3.2.3. Anti-Angiogenesis Profiling Upon Deferoxamine Stimulation and/or Anti-Angiogenesis Profiling Upon VEGF-A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tartaglia, L.A. Complementary new approaches enable repositioning of failed drug candidates. Expert Opin. Investig. Drugs 2006, 15, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Xuan, P.; Yangkun, C.; Tiangang, Z.; Xiao, W.; Shuxiang, P.; Tonghui, S. Drug repositioning through integration of prior knowledge and projections of drugs and diseases. Bioinformatics 2019, 35, 4108–4119. [Google Scholar] [CrossRef] [PubMed]
- Grdina, D.J.; Kataoka, Y.; Murley, J.S. Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol. Drug Interact. 2000, 16, 237–279. [Google Scholar] [CrossRef]
- McCumber, L.M. The potential influence of cell protectors for dose escalation in cancer therapy: An analysis of amifostine. Med. Dosim. 2004, 29, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, R.L. The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine (ethyol®). Eur. J. Cancer 1996, 32, S5–S16. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Zois, C.E.; Kalamida, D.; Pouliliou, S.; Karagounis, I.V.; Yeh, T.L.; Abboud, M.I.; Claridge, T.D.W.; Schofield, C.J.; et al. Normal tissue radioprotection by amifostine via Warburg-Type effects. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Matsumoto, S.; Matsumoto, A.; Manda, S.; Nakanishi, I.; Matsumoto, K.-I.; Mitchell, J.B.; Krishna, M.C.; Anzai, K. Effect of amifostine, a radiation-protecting drug, on oxygen concentration in tissue measured by EPR oximetry and imaging. J. Clin. Biochem. Nutr. 2017, 60, 151–155. [Google Scholar] [CrossRef]
- Savoye, C.; Swenberg, C.; Hugot, S.; Sy, D.; Sabattier, R.; Charlier, M.; Spotheim-Maurizot, M. Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug Ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int. J. Radiat. Biol. 1997, 71, 193–202. [Google Scholar] [CrossRef]
- Hofer, M.; Falk, M.; Komůrková, D.; Falková, I.; Bačíková, A.; Klejdus, B.; Pagáčová, E.; Štefančíková, L.; Weiterová, L.; Angelis, K.J.; et al. Two New Faces of Amifostine: Protector from DNA Damage in Normal Cells and Inhibitor of DNA Repair in Cancer Cells. J. Med. Chem. 2016, 59, 3003–3017. [Google Scholar] [CrossRef]
- Grdina, D.J.; Nagy, B.; Hill, C.K.; Wells, R.L.; Peraino, C. The radioprotector wr1065 reduces radiation-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in v79 cells. Carcinogenesis 1985, 6, 929–931. [Google Scholar] [CrossRef]
- Giannopoulou, E.; Katsoris, P.; Parthymou, A.; Kardamakis, D.; Papadimitriou, E. Amifostine protects blood vessels from the effects of ionizing radiation. Anticancer Res. 2002, 22, 2821–2826. [Google Scholar] [PubMed]
- Polytarchou, C.; Kardamakis, D.; Katsoris, P.; Papadimitriou, E. Antioxidants modify the effect of X rays on blood vessels. Anticancer Res. 2006, 26, 3043–3047. [Google Scholar]
- Giannopoulou, E.; Katsoris, P.; Kardamakis, D.; Papadimitriou, E. Amifostine inhibits angiogenesis in vivo. J. Pharmacol. Exp. Ther. 2003, 304, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Polytarchou, C.; Gligoris, T.; Kardamakis, D.; Kotsaki, E.; Papadimitriou, E. X-rays affect the expression of genes involved in angiogenesis. Anticancer Res. 2004, 24, 2941–2945. [Google Scholar] [PubMed]
- Giannopoulou, E.; Papadimitriou, E. Amifostine has antiangiogenic properties in vitro by changing the redox status of human endothelial cells. Free Radic. Res. 2003, 37, 1191–1199. [Google Scholar] [CrossRef]
- Auerbach, R.; Lewis, R.; Shinners, B.; Kubai, L.; Akhtar, N. Angiogenesis assays: A critical overview. Clin. Chem. 2003, 49, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef]
- Donovan, D.; Brown, N.J.; Bishop, E.T.; Lewis, C.E. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis 2001, 4, 113–121. [Google Scholar] [CrossRef]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane as an in vivo assay to study antiangiogenesis. Pharmaceuticals 2010, 3, 482–513. [Google Scholar] [CrossRef] [PubMed]
- Katsila, T.; Juliachs, M.; Gregori, J.; Macarulla, T.; Villarreal, L.; Bardelli, A.; Torrance, C.; Elez, E.; Tabernero, J.; Villanueva, J. Circulating pEGFR is a candidate response biomarker of cetuximab therapy in colorectal cancer. Clin. Cancer Res. 2014, 20, 6346–6356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischel, L.L.; Young, E.W.K.; Mader, B.R.; Beebe, D.J. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 2013, 34, 1471–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Katsila, T.; Spyroulias, G.A.; Patrinos, G.P.; Matsoukas, M.T. Computational approaches in target identification and drug discovery. Comput. Struct. Biotechnol. J. 2016, 14, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsila, T.; Liontos, M.; Patrinos, G.P.; Bamias, A.; Kardamakis, D. The New Age of -omics in Urothelial Cancer—Re-wording Its Diagnosis and Treatment. EBioMedicine 2018, 28, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differenatiation. J. Cell Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef]
- Korff, T.; Augustin, H.G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 1999, 112(Pt 19), 3249–3258. [Google Scholar] [CrossRef]
- Silva, L.P.; Lorenzi, P.L.; Purwaha, P.; Yong, V.; Hawke, D.H.; Weinstein, J.N. Measurement of DNA concentration as a normalization strategy for metabolomic data from adherent cell lines. Anal. Chem. 2013, 85, 9536–9542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulaki, A.; Katsila, T.; Stergiou, I.E.; Giannouli, S.; Gόmez-Tamayo, J.C.; Piperaki, E.-T.; Kambas, K.; Dimitrakopoulou, A.; Patrinos, G.P.; Tzioufas, A.; et al. Bioenergetic Profiling of the Differentiating Human MDS Myeloid Lineage with Low and High Bone Marrow Blast Counts. Cancers 2020, 12, 3520. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, I.; Chasapi, S.A.; Bariamis, S.E.; Varvarigou, A.; Spraul, M.; Spyroulias, G.A. Metabolic changes in early neonatal life: NMR analysis of the neonatal metabolic profile to monitor postnatal metabolic adaptations. Metabolomics 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chasapi, S.A.; Karagkouni, E.; Matzarapi, K.; Marousis, K.D.; Varvarigou, A.; Spyroulias, G.A. NMR and Metabolomics. eLS 2019, 1–9. [Google Scholar] [CrossRef]
- Narayanaswamy, P.; Teo, G.; Ow, J.R.; Lau, A.; Kaldis, P.; Tate, S.; Choi, H. MetaboKit: A comprehensive data extraction tool for untargeted metabolomics. Mol. Omics 2020, 16, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucl. Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Eisner, R.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic. Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Kostidis, S.; Addie, R.D.; Morreau, H.; Mayboroda, O.A.; Giera, M. Quantitative NMR analysis of intra-and extracellular metabolism of mammalian cells: A tutorial. Anal. Chim. Acta 2017, 980, 1–24. [Google Scholar] [CrossRef]
- Marks, V.; Munoz, A.; Rai, P.; Walls, J.D. (1)H NMR studies distinguish the water soluble metabolomic profiles of untransformed and RAS-transformed cells. PeerJ 2016, 4, e2104. [Google Scholar] [CrossRef] [Green Version]
- Chenomx, N. Suite. In Edmonton; Chenomx Inc.: Edmonton, AB, Canada, 2015. [Google Scholar]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Aihara, K.-I.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef]
- Bletsa, E.; Filippas-Dekouan, S.; Kostara, C.; Dafopoulos, P.; Dimou, A.; Pappa, E.; Chasapi, S.; Spyroulias, G.; Koutsovasilis, A.; Bairaktari, E.; et al. Effect of dapagliflozin on urine metabolome in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Diez, M.; Adam, J.; Adamski, J.; Chasapi, S.A.; Luchinat, C.; Peters, A.; Prehn, C.; Santucci, C.; Spyridonidis, A.; Spyroulias, G.A.; et al. Plasma and serum metabolite association networks: Comparability within and between studies using NMR and MS profiling. J. Proteome. Res. 2017, 16, 2547–2559. [Google Scholar] [CrossRef]
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N.L. 3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro. Biotechnol. Bioeng. 2020, 117, 748–762. [Google Scholar] [CrossRef]
- Kozyra, M.; Johansson, I.; Nordling, Å.; Ullah, S.; Lauschke, V.M.; Ingelman-Sundberg, M. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco Song, H.-H.; Park, K.M.; Gerecht, S. Hydrogels to Model 3D in vitro Microenvironment of Tumor Vascularization. Adv. Drug Deliv. Rev. 2014, 15, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Donneys, A.; Nelson, N.S.; Perosky, J.E.; Polyatskaya, Y.; Rodriguez, J.J.; Figueredo, C.; Vasseli, C.A.; Ratliff, H.C.; Deshpande, S.S.; Kozloff, K.M.; et al. Prevention of radiation-induced bone pathology through combined pharmacologic cytoprotection and angiogenic stimulation. Bone 2016, 84, 245–252. [Google Scholar] [CrossRef] [Green Version]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of Amifostine for Cytoprotection during Radiation Therapy: A Review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Dedieu, S.; Canron, X.; Rezvani, H.R.; Bouchecareilh, M.; Mazurier, F.; Sinisi, R.; Zanda, M.; Moenner, M.; Bikfalvi, A.; North, S. The cytoprotective drug amifostine modifies both expression and activity of the pro-angiogenic factor VEGF-A. BMC Med. 2010, 8, 19. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsila, T.; Chasapi, S.A.; Gomez Tamayo, J.C.; Chalikiopoulou, C.; Siapi, E.; Moros, G.; Zoumpoulakis, P.; Spyroulias, G.A.; Kardamakis, D. Three-Dimensional Cell Metabolomics Deciphers the Anti-Angiogenic Properties of the Radioprotectant Amifostine. Cancers 2021, 13, 2877. https://doi.org/10.3390/cancers13122877
Katsila T, Chasapi SA, Gomez Tamayo JC, Chalikiopoulou C, Siapi E, Moros G, Zoumpoulakis P, Spyroulias GA, Kardamakis D. Three-Dimensional Cell Metabolomics Deciphers the Anti-Angiogenic Properties of the Radioprotectant Amifostine. Cancers. 2021; 13(12):2877. https://doi.org/10.3390/cancers13122877
Chicago/Turabian StyleKatsila, Theodora, Styliani A. Chasapi, Jose Carlos Gomez Tamayo, Constantina Chalikiopoulou, Eleni Siapi, Giorgos Moros, Panagiotis Zoumpoulakis, Georgios A. Spyroulias, and Dimitrios Kardamakis. 2021. "Three-Dimensional Cell Metabolomics Deciphers the Anti-Angiogenic Properties of the Radioprotectant Amifostine" Cancers 13, no. 12: 2877. https://doi.org/10.3390/cancers13122877






