Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pectin Isolation
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Isobolographic Analysis
2.6. Flow Cytometry Analysis
2.7. Caspase-3 Activation Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. E. coli Strains
2.10. Adherence Assay
2.11. E. coli Proliferation
2.12. β-Glucuronidase (GUS) Activity Assay
2.13. Statistical Analysis
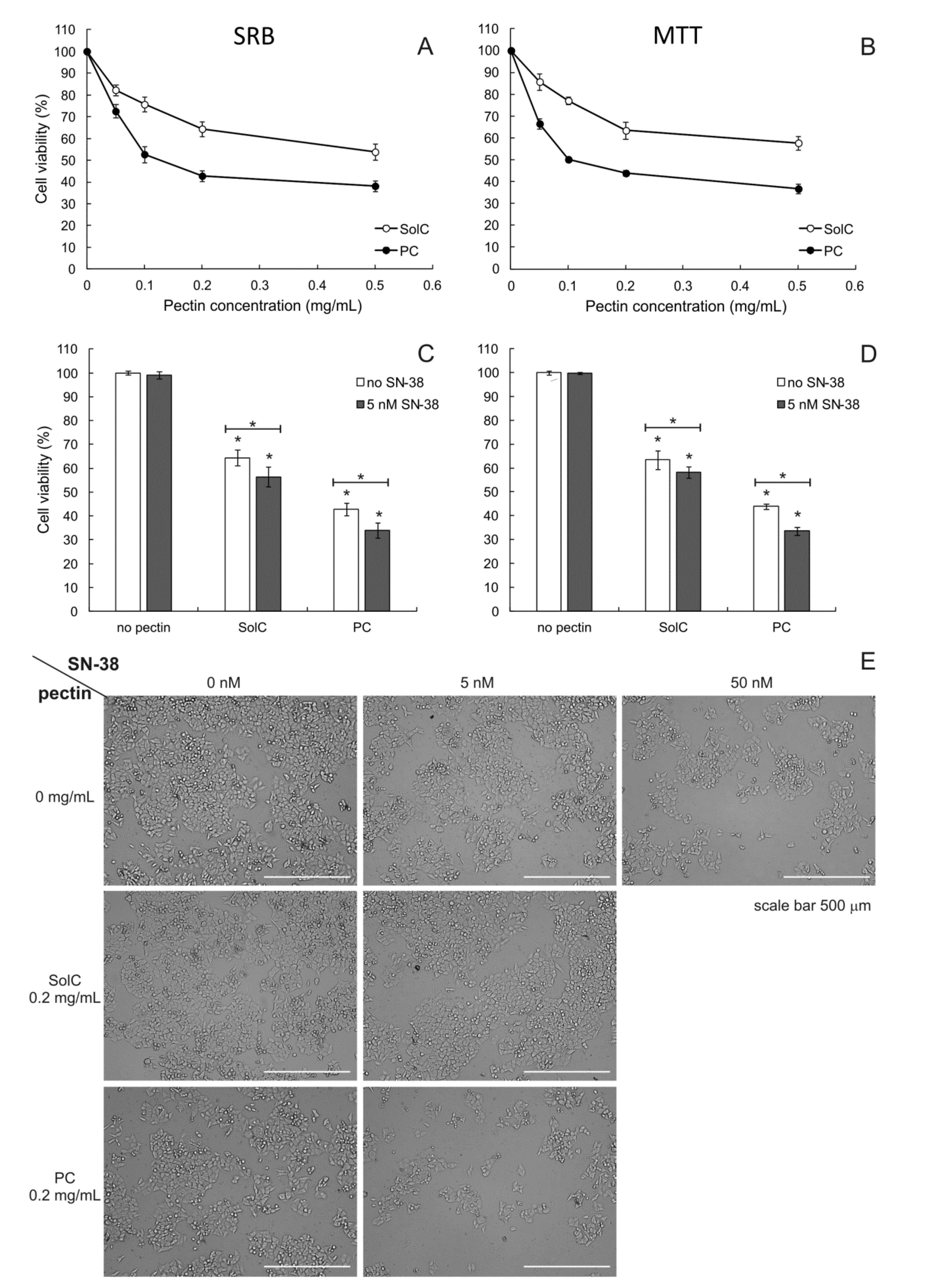
3. Results
3.1. Anticancer Activity of Pectins
3.1.1. Cytotoxicity of Pectins
3.1.2. Cytotoxicity of Pectins Combined with SN-38
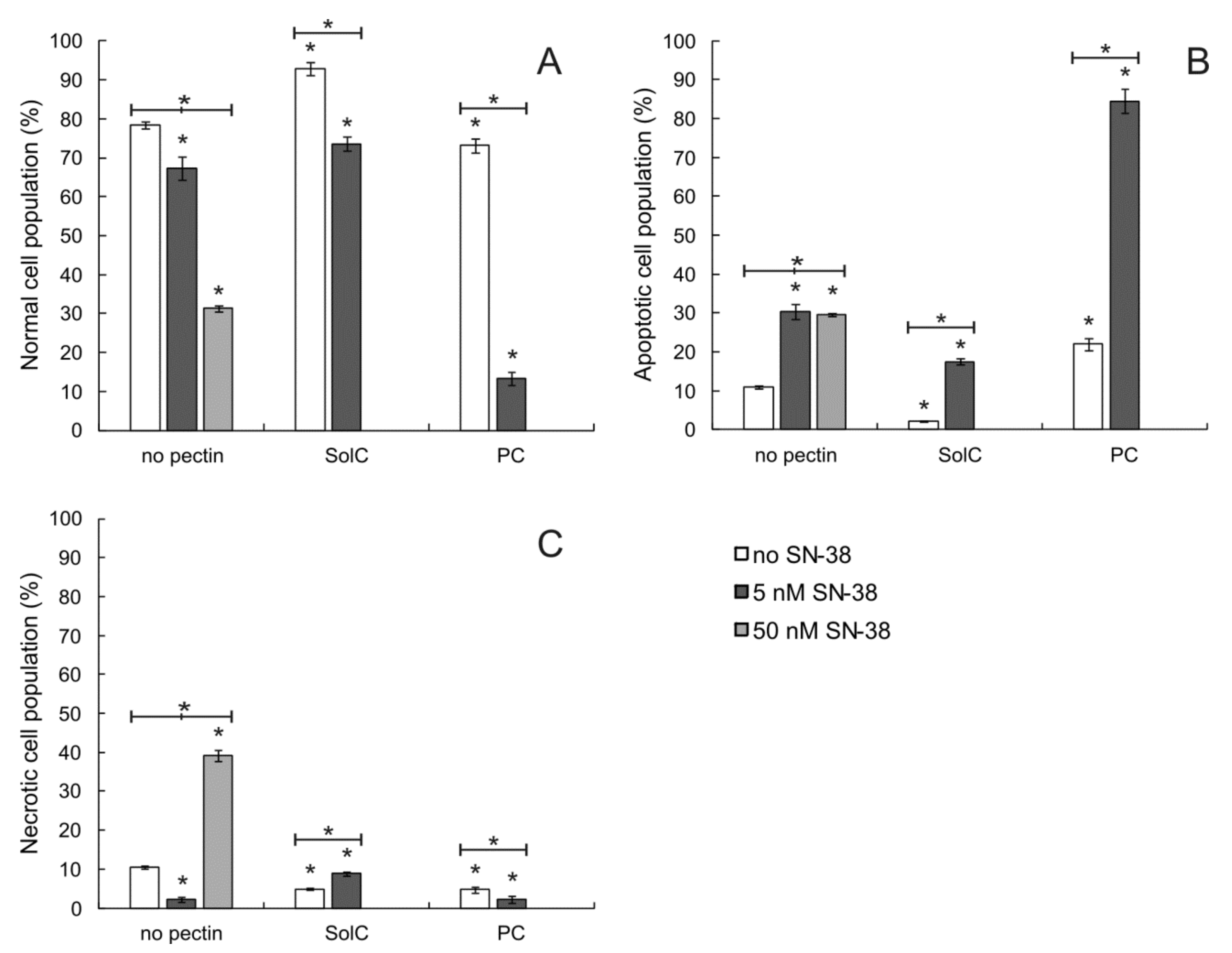
3.2. Proapoptotic Activity of Pectins
3.2.1. Annexin V/PI Double Staining Assay
3.2.2. Caspase-3 Activation
3.3. The Influence of Pectins on Cell Cycle
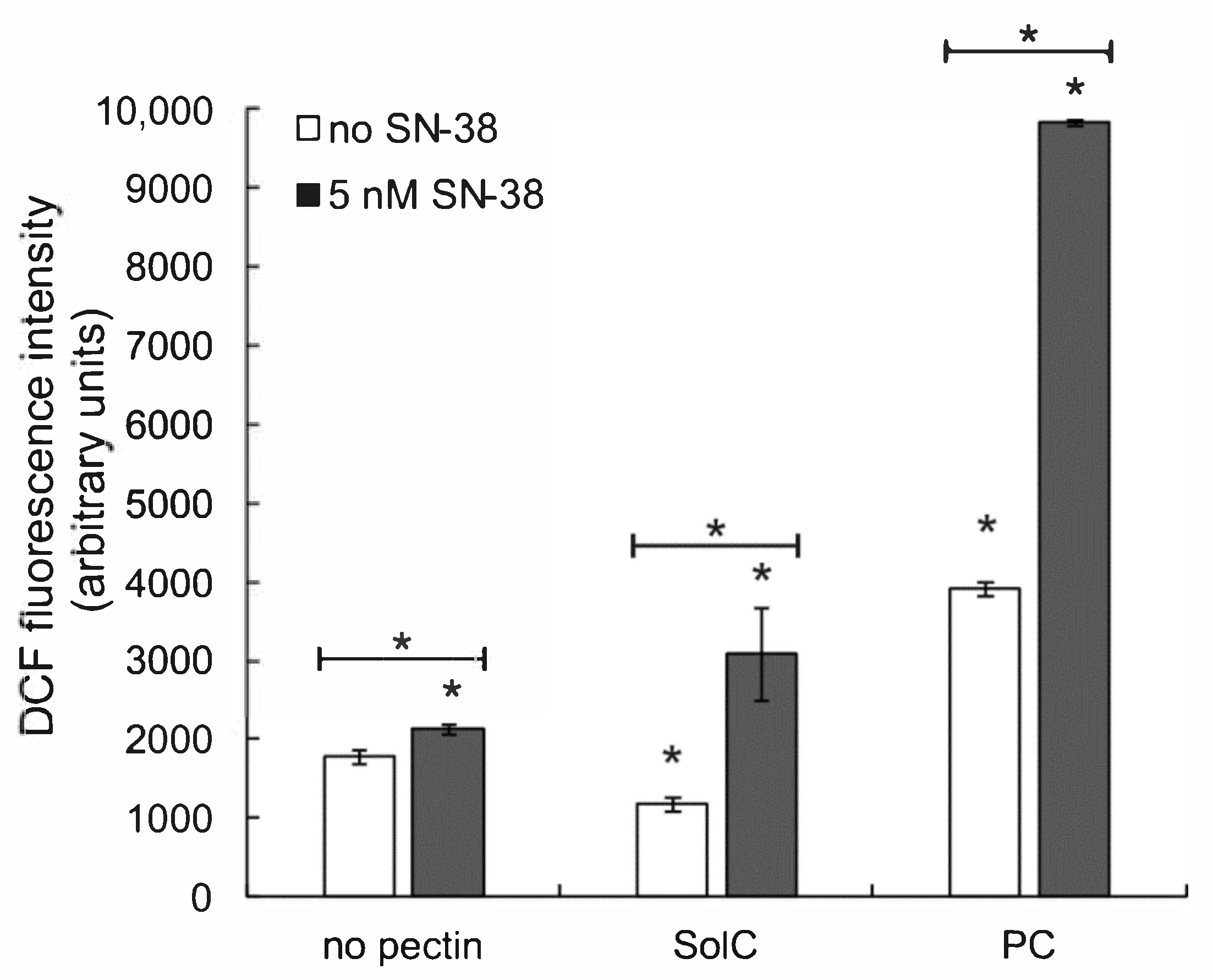
3.4. The Effect of Pectins on Reactive Oxygen Species (ROS) Production
3.5. Anti-Inflammatory Activity of Pectins
3.6. Adherence of E. coli to Colon Cancer Cells
3.7. The Effect of Pectins on β-Glucuronidase (GUS) Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 14 March 2021).
- Recio-Boiles, A.; Cagir, B. Colon Cancer. In Stat Pearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fantini, M.C.; Guadagni, I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021, 53, 558–565. [Google Scholar] [CrossRef]
- Peddareddigari, V.G.; Wang, D.; Dubois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of gut microbiota in the development and treatment of colorectal cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.; Lee, Y.; Im, E. Interplay between the gut microbiota and inflammatory mediators in the development of colorectal cancer. Cancers 2021, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgard, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [Green Version]
- Karwowska, Z.; Szemraj, J.; Karwowski, B. Microbiota alterations in gastrointestinal cancers. Appl. Sci. 2020, 10, 585. [Google Scholar] [CrossRef] [Green Version]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchro-nous unresectable metastases (JCOG1007; iPACS): A randomized clinical trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef]
- Rivory, L.P. Irinotecan (CPT-11): A brief overview. Clin. Exp. Pharmacol. Physiol. 1996, 23, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO Initiative Endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Gibson, R.J.; Stringer, A.M. Chemotherapy-induced diarrhoea. Curr. Opin. Support. Palliat. 2009, 3, 31–35. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, L.; Gupta, S.; Daily, J.; Kelly, L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.T.; Chen, K.C.; Cheng, C.M.; Cheng, T.L.; Tao, M.H.; Roffler, S.R. impediments to enhancement of CPT-11 anticancer activity by E. coli directed beta-glucuronidase therapy. PLoS ONE 2015, 10, e0118028. [Google Scholar] [CrossRef] [Green Version]
- Willats, W.G.T.; Mccartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant. Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Binhamad, H.A.S. Isolation and characterisation of pectin. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer Nature: Basingstoke, UK, 2020; pp. 61–82. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic effects of modified citrus pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef] [Green Version]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-Xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Wikiera, A.; Grabacka, M.; Byczyński, Ł.; Stodolak, B.; Mika, M. Enzymatically extracted apple pectin possesses antioxidant and antitumor activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C modified citrus pectin targets galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism In Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Aich, S.; Delbaere, L.T.J.; Chen, R. Continuous spectrophotometric assay for β-glucuronidase. Biotechniques 2001, 30, 846–850. [Google Scholar] [CrossRef] [Green Version]
- Leclere, L.; van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Leng, J.; Liu, D.; Hao, M.; Gao, X.; Tai, G.; Zhou, Y. The inhibitory effects and mechanisms of rhamnogalacturonan I pectin from potato on HT-29 colon cancer cell proliferation and cell cycle progression. Int. J. Food Sci. Nutr. 2013, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Motwani, M.; Jung, C.; Shah, M.A.; Schwartz, G.K.; Sirotnak, F.M.; She, Y.; Gonen, M. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in HCT116 colon cancer monolayers and xenografts. Clin. Cancer Res. 2001, 7, 4209–4219. [Google Scholar] [PubMed]
- Hossein, G.; Keshavarz, M.; Ahmadi, S.; Naderi, N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 7561–7568. [Google Scholar] [CrossRef] [Green Version]
- Tehranian, N.; Sepehri, H.; Mehdipour, P.; Biramijamal, F.; Hossein-Nezhad, A.; Sarrafnejad, A.; Hajizadeh, E. Combination effect of PectaSol and doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell. Biol. Int. 2012, 36, 601–610. [Google Scholar] [CrossRef]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V.; et al. Pectin induces apoptosis in human prostate cancer cells: Correlation of apoptotic function with pectin structure. Glycobiology 2007, 17, 805–819. [Google Scholar] [CrossRef]
- Yan, J.; Katz, A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer Ther. 2010, 9, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Olano-Martin, E.; Rimbach, G.H.; Gibson, G.R.; Rastall, R.A. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2003, 23, 341–346. [Google Scholar]
- Ogutu, F.O.; Mu, T.H.; Sun, H.; Zhang, M. Ultrasonic modified sweet potato pectin induces apoptosis like cell death in colon cancer (HT-29) cell line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef]
- Fang, T.; Liu, D.; Ning, H.M.; Liu, D.; Sun, J.Y.; Huang, X.J.; Dong, Y.; Geng, M.Y.; Yun, S.F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- Avivi-Green, C.; Madar, Z.; Schwartz, B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int. J. Mol. Med. 2000, 6, 689–698. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.; Mironov, V.; Arkhipova, D.; Khabibullina, A.; Mironova, L.; Zakirova, Y.; Milyukov, V. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Conti, S.; Vexler, A.; Hagoel, L.; Kalich-Philosoph, L.; Corn, B.W.; Honig, N.; Shtraus, N.; Meir, Y.; Ron, I.; Eliaz, I.; et al. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr. Cancer Ther. 2018, 17, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. Increased NFκ-B activity in HCT116 colorectal cancer cell line harboring TLR4 Asp299Gly variant. Iran. J. Allergy Asthma Immunol. 2012, 11, 121–132. [Google Scholar]
- Chung, Y.H.; Kim, D. Enhanced TLR4 expression on colon cancer cells after chemotherapy promotes cell survival and epithelial-mesenchymal transition through phosphorylation of GSK3β. Anticancer Res. 2016, 36, 3383–3394. [Google Scholar] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, H. The Role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Sheu, M.T.; Chen, T.F.; Wang, Y.C.; Hou, W.C.; Liu, D.Z.; Chung, T.C.; Liang, Y.C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Sun, Y.; Zhang, D.; Yue, Z.; Niu, Y.; Meng, J.; Yang, T.; Liu, W.; Mei, Q. An apple oligogalactan suppresses endotoxin-induced cyclooxygenase-2 expression by inhibition of LPS pathways. Int. J. Biol. Macromol. 2013, 61, 75–81. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.H.; Mi, M.; Jiang, F.L.; Yue, Z.G.; Sun, Y.; Fan, L.; Meng, J.; Zhang, X.; Liu, L.; et al. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr. Res. 2013, 33, 839–848. [Google Scholar] [CrossRef]
- Ishisono, K.; Yabe, T.; Kitaguchi, K. Citrus pectin attenuates endotoxin shock via suppression of Toll-Like Receptor signaling in Peyer’s patch myeloid cells. J. Nutr. Biochem. 2017, 50, 38–45. [Google Scholar] [CrossRef]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin Oligosaccharides ameliorate colon cancer by regulating oxidative stress- and inflammation-activated signaling pathways. Front. Immunol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochet, F.; Peri, F. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011, 12, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Lodge, J.B.; Viappiani, S.; et al. How to feed the mammalian gut microbiota: Bacterial and metabolic modulation by dietary fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Mou, H.; Luo, B.; Jiang, X. Inhibition of adhesion of intestinal pathogens (Escherichia coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella typhimurium) by common oligosaccharides. Foodborne Pathog. Dis. 2015, 12, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Manderson, K.; Wells, A.; Hotchkiss, A.T., Jr.; Gibson, G.R.; Formentin, K.; Beer, M.; Rastall, R.A. Oligosaccharide-mediated inhibition of the adhesion of pathogenic Escherichia coli strains to human gut epithelial cells in vitro. J. Food Prot. 2008, 71, 2272–2277. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Ganan, M.; Collins, M.; Rastall, R.; Hotchkiss, A.T.; Chau, H.K.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Inhibition by pectic oligosaccharides of the invasion of undifferentiated and differentiated Caco-2 cells by Campylobacter jejuni. Int. J. Food Microbiol. 2010, 137, 181–185. [Google Scholar] [CrossRef]
- Wilkowska, A.; Nowak, A.; Antczak-Chrobot, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally different pectic oligosaccharides produced from apple pomace and their biological activity in vitro. Foods 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, M.K.; Chung, M.S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Ganzle, M.G. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.J.; Lin, T.C.; Lin, H.Y.; Chen, P.Y.; Hsu, C.Y.; Lin, C.H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, A.P.; Pellock, S.J.; Biernat, K.A.; Walton, W.G.; Wallace, B.D.; Creekmore, B.C.; Letertre, M.M.; Swann, J.R.; Wilson, I.D.; Roques, J.R.; et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl. Acad. Sci. USA 2020, 117, 7374–7381. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.K.; Leklem, J.E. The effect of dietary citrus pectin on the excretion of human fecal neutral and acid steroids and the activity of 7alpha-dehydroxylase and beta-glucuronidase. Am. J. Clin. Nutr. 1981, 34, 2068–2077. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Bakutova, L.A.; Latkin, D.S.; Golovchenko, V.V.; Vityazev, F.V. Interaction of microbial β-glucuronidase with vegetable pectins. J. Agric. Food Chem. 2011, 59, 9922–9926. [Google Scholar] [CrossRef]
- Bauer, H.G.; Asp, N.G.; Dahlqvist, A.; Fredlund, P.E.; Nyman, M.; Oste, R. Effect of two kinds of pectin and guar gum on 1,2-dimethylhydrazine initiation of colon tumors and on fecal beta-glucuronidase activity in the rat. Cancer Res. 1981, 41, 2518–2523. [Google Scholar] [PubMed]
- Ohno, K.; Narushima, S.; Takeuchi, S.; Itoh, K.; Itoh, T.; Hioki, K.; Nomura, T. Effect of bacterial metabolism in the intestine on colorectal tumors induced by 1,2-dimethylhydrazine in transgenic mice harboring human prototype c-Ha-ras genes. J. Exp. Clin. Cancer Res. 2001, 20, 51–56. [Google Scholar] [PubMed]
- Chen, H.L.; Lin, Y.M.; Wang, Y.C. Comparative effects of cellulose and soluble fibers (pectin, konjac glucomannan, inulin) on fecal water toxicity toward Caco-2 cells, fecal bacteria enzymes, bile acid, and short-chain fatty acids. J. Agric. Food Chem. 2010, 58, 10277–10281. [Google Scholar] [CrossRef] [PubMed]
- Lindop, R.; Tasman-Jones, C.; Thomsen, L.L.; Lee, S.P. Cellulose and pectin alter intestinal beta-glucuronidase (EC 3.2.1.31) in the rat. Br. J. Nutr. 1985, 54, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkami, H.; Tazawa, K.; Yamashita, I.; Shimizu, T.; Murai, K.; Kobashi, K.; Fujimaki, M. Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn. J. Cancer Res. 1995, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Okami, H.; Yamashita, I.; Ohnishi, Y.; Kobashi, K.; Fujimaki, M. Effects of apple pectin on fecal enzyme activities and prostaglandin E2 levels in azoxymethane-induced rat colon carcinogenesis. In Food Factors for Cancer Prevention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 178–181. [Google Scholar] [CrossRef]
- Rao, C.V.; Chou, D.; Simi, B.; Ku, H.; Reddy, B.S. Prevention of colonic aberrant crypt foci and modulation of large bowel microbial activity by dietary coffee fiber, inulin and pectin. Carcinogenesis 1998, 19, 1815–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Concentration (mg/mL) | Ratio | Combination Index (CI) | |
|---|---|---|---|
| SN-38 | SolC | ||
| 1.96 × 10−6 | 0.2 | 102,000:1 | 1000 |
| SN-38 | PC | ||
| 0.96 x 10−6 | 0.1 | 104,400:1 | 0.822 |
| 1.96 x 10−6 | 0.2 | 102,000:1 | 0.779 |
| 3.92 x 10−6 | 0.5 | 127,550:1 | 0.792 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers 2021, 13, 2952. https://doi.org/10.3390/cancers13122952
Palko-Łabuz A, Maksymowicz J, Sobieszczańska B, Wikiera A, Skonieczna M, Wesołowska O, Środa-Pomianek K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers. 2021; 13(12):2952. https://doi.org/10.3390/cancers13122952
Chicago/Turabian StylePalko-Łabuz, Anna, Jerzy Maksymowicz, Beata Sobieszczańska, Agnieszka Wikiera, Magdalena Skonieczna, Olga Wesołowska, and Kamila Środa-Pomianek. 2021. "Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity" Cancers 13, no. 12: 2952. https://doi.org/10.3390/cancers13122952







