Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Aim of the Study
1.2. Source of the Data
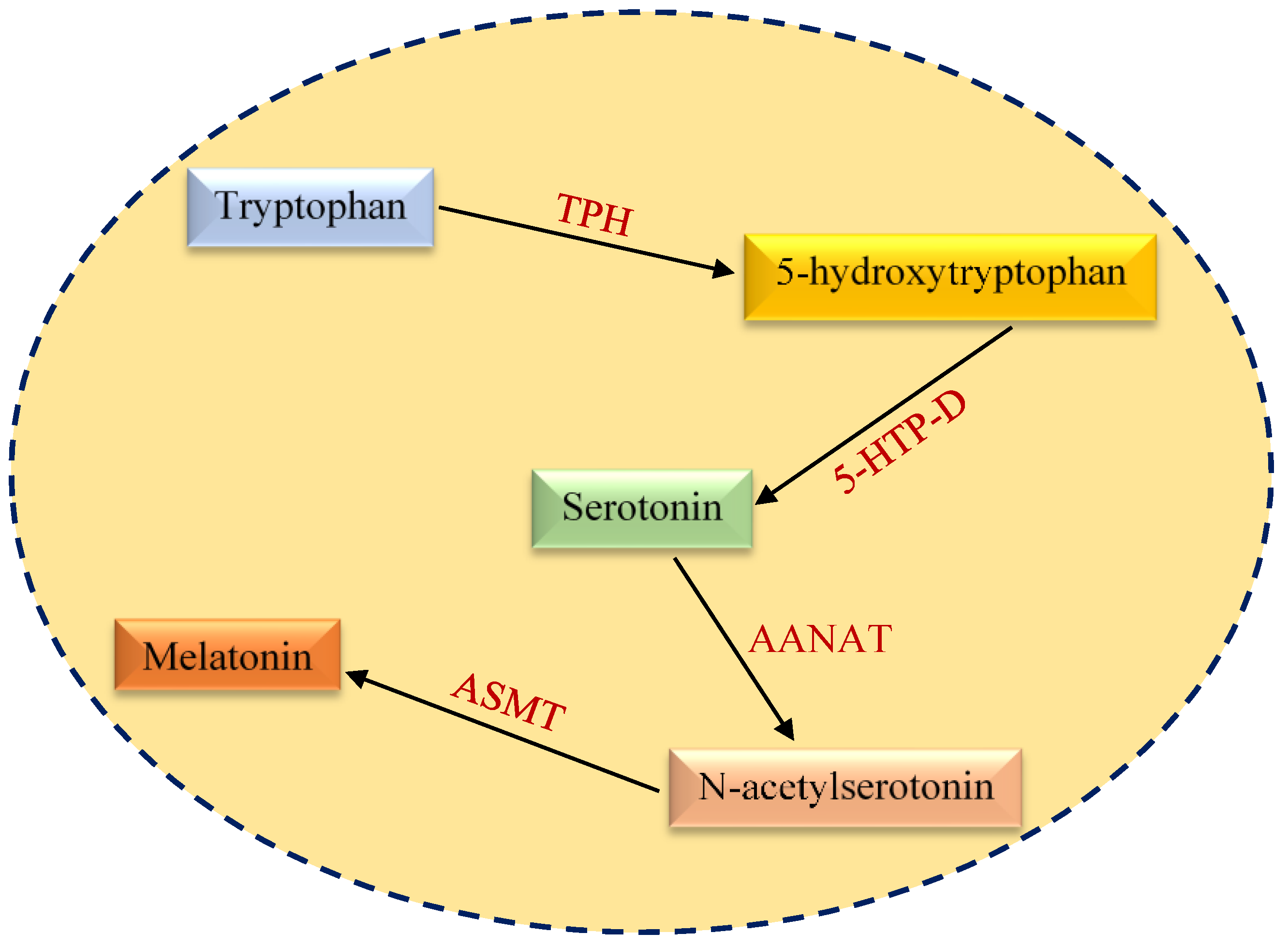
2. Structural and Functional Aspects of Melatonin
2.1. Aberrations in Cancer Metabolism
2.2. Links between Mitochondrial Dysfunction, Melatonin and Cancer
2.3. Melatonin Regulating Cancer Metabolism In Vitro
2.4. Impact of Melatonin on the Metabolic Reprogramming In Vivo
2.5. Melatonin’s Impact on the “Critical Players” of Metabolic Reprogramming
3. Expert Recommendations in the Framework of Predictive, Preventive and Personalized (3P) Medicine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and t lymphocytes. Immunology 2017, 152, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic reprogramming in triple-negative breast cancer. Front. Oncol. 2020, 10, 428. [Google Scholar] [CrossRef]
- Giannattasio, S.; Mirisola, M.G.; Mazzoni, C. Editorial: Cell stress, metabolic reprogramming, and cancer. Front. Oncol. 2018, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Cernuda, R.; Quiros, I.; Rodriguez, P.; Garcia, J.I.; Hevia, D.; Sainz, R.M. Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2019, 2, 76–104. [Google Scholar] [CrossRef]
- Hansen, M.V.; Andersen, L.T.; Madsen, M.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: A randomized, double-blind, placebo-controlled trial. Breast Cancer Res. Treat. 2014, 145, 683–695. [Google Scholar] [CrossRef]
- Kostoglou-Athanassiou, I. Therapeutic applications of melatonin. Ther. Adv. Endocrinol. Metab. 2013, 4, 13–24. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mociková-Kalická, K.; Bojková, B.; Adámeková, E.; Mníchová-Chamilová, M.; Kubatka, P.; Ahlersová, E.; Ahlers, I. Preventive effect of indomethacin and melatonin on 7,12-dimethybenz/a/anthracene-induced mammary carcinogenesis in female sprague-dawley rats. A preliminary report. Folia Biol. 2001, 47, 75–79. [Google Scholar]
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin potentiates the anti-tumour effect of pravastatin in rat mammary gland carcinoma model. Int. J. Exp. Pathol. 2014, 95, 401–410. [Google Scholar] [CrossRef]
- Bojková, B.; Kubatka, P.; Qaradakhi, T.; Zulli, A.; Kajo, K. Melatonin may increase anticancer potential of pleiotropic drugs. Int. J. Mol. Sci. 2018, 19, 3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S. Anti-warburg effect of melatonin: A proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 2021, 22, 764. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, J.; Hillier, J.; Jones, M.; Lu, W.; Jones, E.Y. Structural characterization of melatonin as an inhibitor of the wnt deacylase notum. J. Pineal. Res. 2020, 68, e12630. [Google Scholar] [CrossRef] [Green Version]
- Rani, P.; Pal, D.; Hegde, R.R.; Hashim, S.R. Acetamides: Chemotherapeutic agents for inflammation-associated cancers. J. Chemother. 2016, 28, 255–265. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Saha, S.; Singh, K.M.; Gupta, B.B.P. Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: Similarities and differences. Gen. Comp. Endocrinol. 2019, 279, 27–34. [Google Scholar] [CrossRef]
- Benyassi, A.; Schwartz, C.; Coon, S.L.; Klein, D.C.; Falcón, J. Melatonin synthesis: Arylalkylamine N-acetyltransferases in trout retina and pineal organ are different. Neuroreport 2000, 11, 255–258. [Google Scholar] [CrossRef]
- Low, M.J. Neuroendocrinology. In Williams Textbook of Endocrinology, 13th ed.; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 109–175. ISBN 978-0-323-29738-7. [Google Scholar]
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin differentially affects vascular blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H670–H674. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Circadian and non-circadian melatonin: Influences on glucose metabolism in cancer cells. J. Curr. Sci. Technol. 2020, 10, 85–98. [Google Scholar] [CrossRef]
- Hardeland, R. Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus—Consequences to melatonin dysfunction. Int. J. Mol. Sci. 2013, 14, 5817–5841. [Google Scholar] [CrossRef] [Green Version]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. S3), 34–42. [Google Scholar] [CrossRef]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in sleep disorders. Neurología 2020. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-inflammatory activity of melatonin: A focus on the role of NLRP3 inflammasome. Inflammation 2021. [Google Scholar] [CrossRef]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The effect of melatonin on superoxide dismutase and glutathione peroxidase activity, and malondialdehyde levels in the targeted and the non-targeted lung and heart tissues after irradiation in xenograft mice colon cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Impact of melatonin on immunity: A review. Cent. Eur. J. Med. 2013, 8, 369–376. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. Melatonin and the immune system therapeutic potential in cancer, viral diseases, and immunodeficiency states. In The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy; Bartsch, C., Bartsch, H., Blask, D.E., Cardinali, D.P., Hrushesky, W.J.M., Mecke, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 384–394. ISBN 978-3-642-59512-7. [Google Scholar]
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Campos, L.A.; Bueno, C.; Barcelos, I.P.; Halpern, B.; Brito, L.C.; Amaral, F.G.; Baltatu, O.C.; Cipolla-Neto, J. Melatonin therapy improves cardiac autonomic modulation in pinealectomized patients. Front. Endocrinol. 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 7895–7908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin anticancer effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Liu, B.; Guan, Y.; Gong, M.; Zhang, W.; Pan, J.; Liu, Y.; Liang, R.; Yuan, Y.; Ye, L. Melatonin inhibits the proliferation of breast cancer cells induced by bisphenol a via targeting estrogen receptor-related pathways. Thorac. Cancer 2018, 9, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.M.; Frasch, T.; Xiang, S.; Yuan, L.; Duplessis, T.; Mao, L. Molecular mechanisms of melatonin anticancer effects. Integr. Cancer Ther. 2009, 8, 337–346. [Google Scholar] [CrossRef]
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, A.M.; Suárez, S.; Puente-Moncada, N.; Anítua, M.J.; et al. Mechanisms involved in the pro-apoptotic effect of melatonin in cancer cells. Int. J. Mol. Sci. 2013, 14, 6597–6613. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.M.; Marelli, M.M.; Maggi, R.; Dondi, D.; Motta, M.; Limonta, P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol. Rep. 2000, 7, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, Z.; Zhu, X.; Zhang, J. New insights into molecules and pathways of cancer metabolism and therapeutic implications. Cancer Commun. 2021, 41, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef]
- Koklesova, L.; Samec, M.; Liskova, A.; Zhai, K.; Büsselberg, D.; Giordano, F.A.; Kubatka, P.; Golunitschaja, O. Mitochondrial impairments in aetiopathology of multifactorial diseases: Common origin but individual outcomes in context of 3P medicine. EPMA J. 2021, 1–14. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Wang, L.; Chen, S. The sweet trap in tumors: Aerobic glycolysis and potential targets for therapy. Oncotarget 2016, 7, 38908–38926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, M.-H.; Chang, W.-W.; Yeh, B.-W.; Chu, Y.-S.; Lee, Y.-C.; Lee, H.-T. Glucose transporter 3 is essential for the survival of breast cancer cells in the brain. Cells 2019, 8, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, Y.R.; Bandyopadhyay, S.; Semaan, A.; Ahmed, Q.; Albashiti, B.; Jazaerly, T.; Nahleh, Z.; Ali-Fehmi, R. Glut-1 expression correlates with basal-like breast cancer. Transl. Oncol. 2011, 4, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Menendez, P.; Hevia, D.; Alonso-Arias, R.; Alvarez-Artime, A.; Rodriguez-Garcia, A.; Kinet, S.; Gonzalez-Pola, I.; Taylor, N.; Mayo, J.C.; Sainz, R.M. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018, 17, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.R.R.; Rocha, R.M.; Carvalho, K.C.; Carvalho, A.L.; da Cunha, I.W.; Lourenço, S.V.; Soares, F.A. Glut1 and Glut3 as potential prognostic markers for oral squamous cell carcinoma. Molecules 2010, 15, 2374–2387. [Google Scholar] [CrossRef] [PubMed]
- Chiba, I.; Ogawa, K.; Morioka, T.; Shimoji, H.; Sunagawa, N.; Iraha, S.; Nishimaki, T.; Yoshimi, N.; Murayama, S. Clinical significance of GLUT-1 expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Oncol. Lett. 2011, 2, 21–28. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, M.-C.; Hung, W.-C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From Cellular metabolism, transcriptional regulation to extracellular signaling. Mol. Cancer 2018, 17, 35. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Clark, P.M.; Mason, D.E.; Keenan, M.C.; Hill, C.; Goddard, W.A.; Peters, E.C.; Driggers, E.M.; Hsieh-Wilson, L.C. PFK1 glycosylation is a key regulator of cancer cell growth and central metabolic pathways. Science 2012, 337, 975–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, S.J.; Pan, J.; Lee, M.-H. Roles of P53, Myc and HIF-1 in regulating glycolysis—The seventh hallmark of cancer. Cell. Mol. Life Sci. 2008, 65, 3981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Lei, Q.-Y. Metabolic recoding of epigenetics in cancer. Cancer Commun. 2018, 38, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasmann, G.; Smolle, E.; Olschewski, H.; Leithner, K. Gluconeogenesis in cancer cells—Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 24–36. [Google Scholar] [CrossRef]
- Engelking, L.R. Gluconeogenesis. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Engelking, L.R., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 225–230. ISBN 978-0-12-391909-0. [Google Scholar]
- Wang, Z.; Dong, C. Gluconeogenesis in cancer: Function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; An, S.; Liu, Y.; Liu, J.; Wang, F. PCK1 regulates glycolysis and tumor progression in clear cell renal cell carcinoma through LDHA. OncoTargets Ther. 2020, 13, 2613–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, N.; Weinberg, E.M.; Nguyen, A.; Liberti, M.V.; Goodarzi, H.; Janjigian, Y.Y.; Paty, P.B.; Saltz, L.B.; Kingham, T.P.; Loo, J.M.; et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. Elife 2019, 8, e52135. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Fan, T.W.M.; Hou, S.X. Glycolytic reprogramming through PCK2 regulates tumor initiation of prostate cancer cells. Oncotarget 2017, 8, 83602–83618. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-M.; Zhang, Y.-M. Targeting FBPase is an emerging novel approach for cancer therapy. Cancer Cell Int. 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yuan, T.; Wu, Y.; Wang, Y.; Fan, T.W.M.; Miriyala, S.; Lin, Y.; Yao, J.; Shi, J.; Kang, T.; et al. Loss of FBP1 by snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013, 23, 316–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Qiu, B.; Lee, D.S.M.; Walton, Z.E.; Ochocki, J.D.; Mathew, L.K.; Mancuso, A.; Gade, T.P.F.; Keith, B.; Nissim, I.; et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 2014, 513, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipp, F.V.; Scott, D.A.; Ronai, Z.A.; Osterman, A.L.; Smith, J.W. Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res. 2012, 25, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, A.; Mohiuddin, S.S. Biochemistry, citric acid cycle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cardaci, S.; Ciriolo, M.R. TCA Cycle Defects and Cancer: When Metabolism Tunes Redox State. Available online: https://www.hindawi.com/journals/ijcb/2012/161837/ (accessed on 6 February 2021).
- King, A.; Selak, M.A.; Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene 2006, 25, 4675–4682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennant, D.A.; Frezza, C.; MacKenzie, E.D.; Nguyen, Q.D.; Zheng, L.; Selak, M.A.; Roberts, D.L.; Dive, C.; Watson, D.G.; Aboagye, E.O.; et al. Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene 2009, 28, 4009–4021. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Choi, J.; Kim, E.-S.; Koo, J.S. Expression of Pentose Phosphate Pathway-Related Proteins in Breast Cancer. Available online: https://www.hindawi.com/journals/dm/2018/9369358/ (accessed on 7 February 2021).
- Cossu, V.; Bonanomi, M.; Bauckneht, M.; Ravera, S.; Righi, N.; Miceli, A.; Morbelli, S.; Orengo, A.M.; Piccioli, P.; Bruno, S.; et al. Two high-rate pentose-phosphate pathways in cancer cells. Sci. Rep. 2020, 10, 22111. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Stine, Z.E.; Altman, B.J.; Hsieh, A.L.; Gouw, A.M.; Dang, C.V. Deregulation of the cellular energetics of cancer cells. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 444–455. ISBN 978-0-12-386457-4. [Google Scholar]
- Jin, L.; Zhou, Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol. Lett. 2019, 17, 4213–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. 20.3 the pentose phosphate pathway generates NADPH and synthesizes five-carbon sugars. In Biochemistry, 5th ed.; NCBI: Bethesda, MD, USA, 2002. [Google Scholar]
- Zhang, Q.; Yi, X.; Yang, Z.; Han, Q.; Di, X.; Chen, F.; Wang, Y.; Yi, Z.; Kuang, Y.; Zhu, Y. Overexpression of G6PD represents a potential prognostic factor in clear cell renal cell carcinoma. J. Cancer 2017, 8, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Pu, H.; Zhang, Q.; Zhao, C.; Shi, L.; Wang, Y.; Wang, J.; Zhang, M. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World J. Surg. Oncol. 2015, 13, 323. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Liang, Q.; Wang, J.; Wang, K.; Gao, J.; Zhang, J.; Zeng, Y.; Chiu, P.W.Y.; Ng, E.K.W.; Sung, J.J.Y. REC8 Functions as a tumor suppressor and is epigenetically downregulated in gastric cancer, especially in EBV-positive subtype. Oncogene 2017, 36, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Li, Y.; Shao, Y.; Xiao, J.; Zhu, G.; Li, F. PAK4 regulates G6PD activity by P53 degradation involving colon cancer cell growth. Cell Death Dis. 2017, 8, e2820. [Google Scholar] [CrossRef]
- Lu, M.; Lu, L.; Dong, Q.; Yu, G.; Chen, J.; Qin, L.; Wang, L.; Zhu, W.; Jia, H. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim. Biophy. Sin. 2018, 50, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.; Jung, W.; Koo, J. Differential site-based expression of pentose phosphate pathway-related proteins among breast cancer metastases. Dis. Markers 2017, 2017, 7062517. [Google Scholar] [CrossRef] [PubMed]
- Simabuco, F.M.; Morale, M.G.; Pavan, I.C.B.; Morelli, A.P.; Silva, F.R.; Tamura, R.E. P53 and metabolism: From mechanism to therapeutics. Oncotarget 2018, 9, 23780–23823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The tumor suppressor P53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef] [Green Version]
- Tsouko, E.; Khan, A.S.; White, M.A.; Han, J.J.; Shi, Y.; Merchant, F.A.; Sharpe, M.A.; Xin, L.; Frigo, D.E. Regulation of the pentose phosphate pathway by an androgen receptor-MTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis 2014, 3, e103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Chen, J.; Liu, X.-M.; Zhao, R.; Zhe, H. Nrf2-mediated metabolic reprogramming in cancer. Oxid. Med. Cell. Longev. 2018, 2018, 9304091. [Google Scholar] [CrossRef] [Green Version]
- Santana-Codina, N.; Roeth, A.A.; Zhang, Y.; Yang, A.; Mashadova, O.; Asara, J.M.; Wang, X.; Bronson, R.T.; Lyssiotis, C.A.; Ying, H.; et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat. Commun. 2018, 9, 4945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, E.; Samuel, S.M.; Líšková, A.; Samec, M.; Kubatka, P.; Büsselberg, D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers 2020, 12, 2252. [Google Scholar] [CrossRef]
- Chen, X.-S.; Li, L.-Y.; Guan, Y.; Yang, J.-M.; Cheng, Y. Anticancer strategies based on the metabolic profile of tumor cells: Therapeutic targeting of the warburg effect. Acta Pharmacol. Sin. 2016, 37, 1013–1019. [Google Scholar] [CrossRef]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Montrose, D.C.; Galluzzi, L. Drugging cancer metabolism: Expectations vs. reality. Int. Rev. Cell Mol. Biol. 2019, 347, 1–26. [Google Scholar] [CrossRef]
- Cortés-Hernández, L.E.; Eslami-S, Z.; Dujon, A.M.; Giraudeau, M.; Ujvari, B.; Thomas, F.; Alix-Panabières, C. Do malignant cells sleep at night? Genome Biol. 2020, 21, 276. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.K.; Dhanesha, N.; Doddapattar, P.; Rodriguez, O.; Sonkar, V.K.; Dayal, S.; Chauhan, A.K. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018, 2, 2029–2038. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.; Acuna-Castroviejo, D.; Escames, G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019, 2, 105–119. [Google Scholar] [CrossRef]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Sharma, R.; Pires de Campos Zuccari, D.A.; de Almeida Chuffa, L.G.; Manucha, W.; Rodriguez, C. Melatonin synthesis in and uptake by mitochondria: Implications for diseased cells with dysfunctional mitochondria. Future Med. Chem. 2021, 13, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell. Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Gonzalez-Menendez, P.; Fernandez-Fernandez, M.; Cueto, S.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Mayo, J.C.; Sainz, R.M. Melatonin decreases glucose metabolism in prostate cancer cells: A 13C stable isotope-resolved metabolomic study. Int. J. Mol. Sci. 2017, 18, 1620. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sanchez, A.M.; Antolin, I.; Puente-Moncada, N.; Suarez, S.; Gomez-Lobo, M.; Rodriguez, C.; Martin, V. Melatonin cytotoxicity is associated to warburg effect inhibition in ewing sarcoma cells. PLoS ONE 2015, 10, e0135420. [Google Scholar] [CrossRef]
- Kim, W.; Cho, Y.S.; Wang, X.; Park, O.; Ma, X.; Kim, H.; Gan, W.; Jho, E.; Cha, B.; Jeung, Y.; et al. Hippo signaling is intrinsically regulated during cell cycle progression by APC/CCdh1. Proc. Natl. Acad. Sci. USA 2019, 116, 9423–9432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.B.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y. Analysis of the role of the hippo pathway in cancer. J. Transl. Med. 2019, 17, 116. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Kuang, H. Melatonin regulates cisplatin resistance and glucose metabolism through hippo signaling in hepatocellular carcinoma cells. Cancer Manag. Res. 2020, 12, 1863–1874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
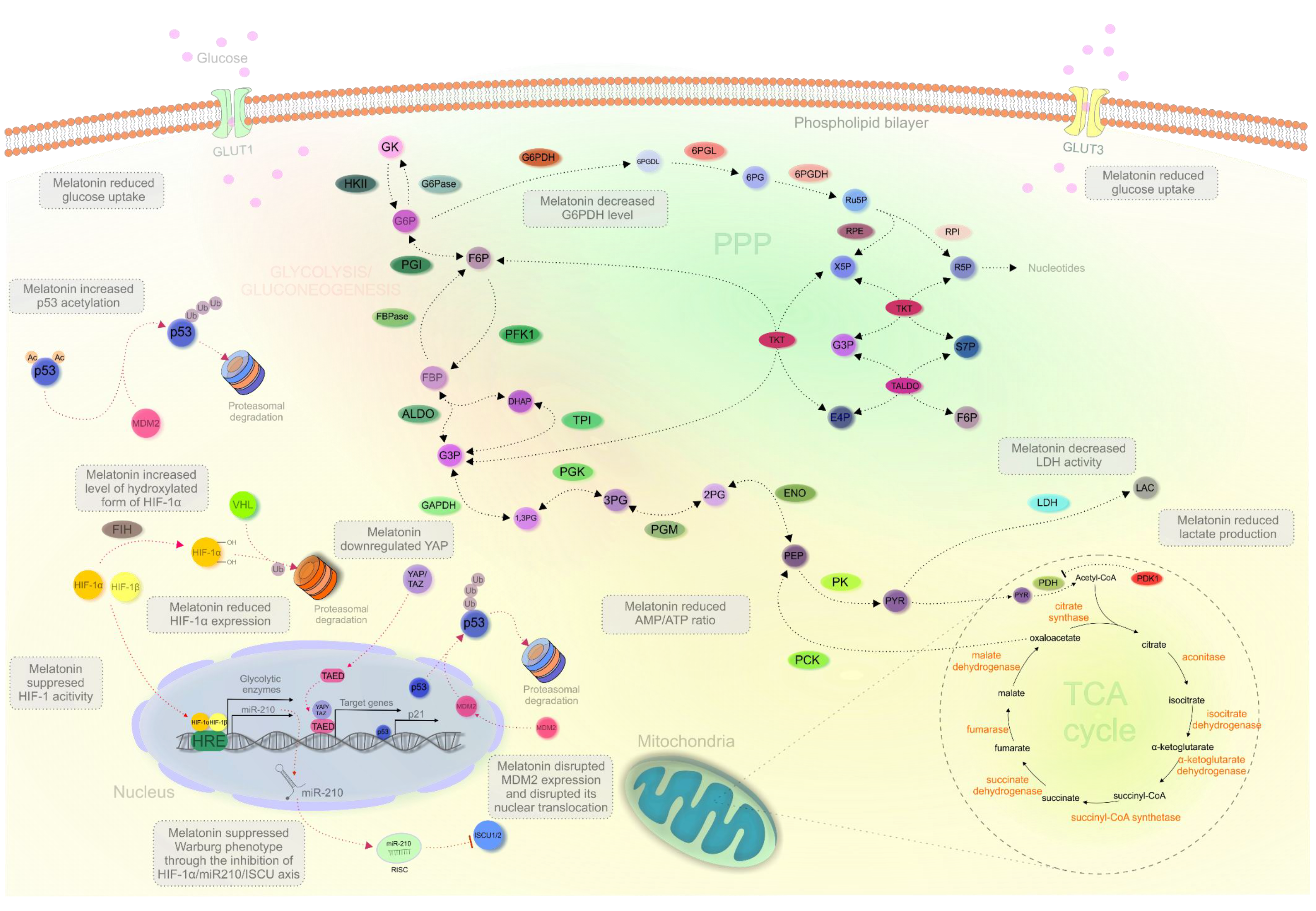
- He, M.; Zhou, C.; Lu, Y.; Mao, L.; Xi, Y.; Mei, X.; Wang, X.; Zhang, L.; Yu, Z.; Zhou, Z. Melatonin antagonizes nickel-induced aerobic glycolysis by blocking ROS-mediated HIF-1 α/MiR210/ISCU axis activation. Oxid. Med. Cell. Longev. 2020, 2020, 5406284. [Google Scholar] [CrossRef]
- Yunus, N.M.; Johan, M.F.; Ali Nagi Al-Jamal, H.; Husin, A.; Hussein, A.R.; Hassan, R. Characterisation and clinical significance of FLT3-ITD and Non-ITD in acute myeloid leukaemia patients in Kelantan, northeast peninsular Malaysia. Asian Pac. J. Cancer Prev. 2015, 16, 4869–4872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puente-Moncada, N.; Turos-Cabal, M.; Sánchez-Sánchez, A.M.; Antolín, I.; Herrera, F.; Rodriguez-Blanco, J.; Duarte-Olivenza, C.; Rodriguez, C.; Martín, V. Role of glucose metabolism in the differential antileukemic effect of melatonin on wild-type and FLT3-ITD mutant cells. Oncol. Rep. 2020, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Martinez-Ruiz, L.; Rodríguez-Santana, C.; Shen, Y.-Q.; García-Verdugo, J.M.; López-Rodríguez, A.; Rusanova, I.; Quiñones-Hinojosa, A.; et al. Melatonin targets metabolism in head and neck cancer cells by regulating mitochondrial structure and function. Antioxidants 2021, 10, 603. [Google Scholar] [CrossRef]
- Hevia, D.; González-Menéndez, P.; Quiros-González, I.; Miar, A.; Rodríguez-García, A.; Tan, D.-X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Hoffman, A.E.; Wren-Dail, M.A.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Xiang, S.; Yuan, L.; Hill, S.M.; Belancio, V.P.; et al. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comp. Med. 2015, 65, 473–485. [Google Scholar] [PubMed]
- Xiang, S.; Dauchy, R.T.; Hauch, A.; Mao, L.; Yuan, L.; Wren, M.A.; Belancio, V.P.; Mondal, D.; Frasch, T.; Blask, D.E.; et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015, 59, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef] [Green Version]
- Chuffa, L.G.A.; Lupi Júnior, L.A.; Seiva, F.R.F.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Dauchy, E.M.; Slakey, L.M.; Brimer, S.; Yuan, L.; Xiang, S.; Hauch, A.; Smith, K.; et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J. Pineal Res. 2016, 60, 167–177. [Google Scholar] [CrossRef]
- Puzio-Kuter, A.M. The role of P53 in metabolic regulation. Genes Cancer 2011, 2, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.; Ramos, H.; Soares, J.; Saraiva, L. P53 and glucose metabolism: An orchestra to be directed in cancer therapy. Pharmacol. Res. 2018, 131, 75–86. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itahana, Y.; Itahana, K. Emerging roles of P53 family members in glucose metabolism. Int. J. Mol. Sci. 2018, 19, 776. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Arora, R.; Kaur, P.; Singh, B.; Mannan, R.; Arora, S. Overexpression of hypoxia-inducible factor and metabolic pathways: Possible targets of cancer. Cell Biosci. 2017, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Martín-Renedo, J.; Mauriz, J.L.; Jorquera, F.; Ruiz-Andrés, O.; González, P.; González-Gallego, J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 2008, 45, 532–540. [Google Scholar] [CrossRef]
- Amin, A.H.; El-Missiry, M.A.; Othman, A.I.; Ali, D.A.; Gouida, M.S.; Ismail, A.H. Ameliorative effects of melatonin against solid ehrlich carcinoma progression in female mice. J. Pineal Res. 2019, 67, e12585. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Mandel, S.; Lorenz, M.; Ruegg, U.; Wanker, E.E.; Boschmann, M.; Paul, F. Epigallocatechin-3-Gallate: A useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases? EPMA J. 2013, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Biringer, K.; Petras, M.; Blahutova, D.; Smejkal, K.; et al. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Makvandi, P.; Zarrabi, A.; Farkhondeh, T.; Samarghandian, S. Versatile role of curcumin and its derivatives in lung cancer therapy. J. Cell. Physiol. 2020, 235, 9241–9268. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Najafi, M.; Orouei, S.; Zabolian, A.; Saleki, H.; Azami, N.; Sharifi, N.; Hushmandi, K.; Zarrabi, A.; Ahn, K.S. Resveratrol modulates transforming growth factor-beta (TGF-β) signaling pathway for disease therapy: A new insight into its pharmacological activities. Biomedicines 2020, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in cancer therapy: How this plant derived-natural compound targets various oncogene and onco-suppressor pathways. Biomedicines 2020, 8, 110. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Pec, M.; Zhai, K.; Smejkal, K.; Mirzaei, S.; et al. Flavonoids targeting HIF-1: Implications on cancer metabolism. Cancers 2021, 13, 130. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in cancer metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Ashrafizadeh, M.; Taeb, S.; Haghi-Aminjan, H.; Afrashi, S.; Moloudi, K.; Musa, A.E.; Najafi, M.; Farhood, B. Resveratrol as an enhancer of apoptosis in cancer: A mechanistic review. Anticancer Agents Med. Chem. 2020. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Mestanova, V.; Franekova, M.; Kassayova, M.; Bojkova, B.; Uramova, S.; Zubor, P.; Janikova, K.; et al. Fluctuations of histone chemical modifications in breast, prostate, and colorectal cancer: An implication of phytochemicals as defenders of chromatin equilibrium. Biomolecules 2019, 9, 829. [Google Scholar] [CrossRef] [Green Version]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of mirna expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA methylation status in cancer disease: Modulations by plant-derived natural compounds and dietary interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; He, Y.; Wu, X.; Zhao, G.; Zhang, K.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin and (-)-epigallocatechin-3-gallate: Partners in fighting cancer. Cells 2019, 8, 745. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid intake in european adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Wang, T.; Wan, X.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin attenuates (-)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. J. Pineal Res. 2015, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Xing, J.; Li, W.; Li, H.; Ke, X.; Zhang, J.; Ren, T.; Shang, Y.; Yang, H.; et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the P53-dependent signaling pathway. J. Hepatol. 2014, 61, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, S.-J.; Luo, J.-H.; Zhang, H.; Wang, R.-X.; Liu, H.; Li, L.; Zhang, Z.-G.; Zhou, R.-X. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol. Rep. 2018, 39, 1975–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; Dinicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin down-regulates MDM2 gene expression and enhances P53 acetylation in MCF-7 cells. J. Pineal Res. 2014, 57, 120–129. [Google Scholar] [CrossRef]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and HIF-1 alpha induced by CoCl2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef]
- Kim, K.-J.; Choi, J.-S.; Kang, I.; Kim, K.-W.; Jeong, C.-H.; Jeong, J.-W. Melatonin suppresses tumor progression by reducing angiogenesis stimulated by HIF-1 in a mouse tumor model. J. Pineal Res. 2013, 54, 264–270. [Google Scholar] [CrossRef]
- Colombo, J.; Maciel, J.M.W.; Ferreira, L.C.; DA Silva, R.F.; Zuccari, D.A.P. Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol. Lett. 2016, 12, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-W.; Hwang, M.-S.; Suh, S.-I.; Baek, W.-K. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 2009, 46, 415–421. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wang, F.; Ling, E.-A.; Liu, S.; Wang, L.; Yang, Y.; Yao, L.; Chen, X.; Wang, F.; et al. Melatonin antagonizes hypoxia-mediated glioblastoma cell migration and invasion via inhibition of HIF-1α. J. Pineal Res. 2013, 55, 121–130. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhu, L.; Fan, X.; Luo, T.; Liu, D.; Liang, Z.; Hu, X.; Shi, T.; Tan, W.; Wang, Z. Melatonin derivatives combat with inflammation-related cancer by targeting the main culprit STAT3. Eur. J. Med. Chem. 2021, 211, 113027. [Google Scholar] [CrossRef]
- Abolhasanpour, N.; Alihosseini, S.; Golipourkhalili, S.; Badalzadeh, R.; Mahmoudi, J.; Hosseini, L. Insight into the effects of melatonin on endoplasmic reticulum, mitochondrial function, and their cross-talk in the stroke. Arch. Med. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J.; Polivka, J.; Pesta, M.; Rohan, V.; Celedova, L.; Mahajani, S.; Topolcan, O.; Golubnitschaja, O. Risks associated with the stroke predisposition at young age: Facts and hypotheses in light of individualized predictive and preventive approach. EPMA J. 2019, 10, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [Green Version]
- Abdelgadir, I.S.; Gordon, M.A.; Akobeng, A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Arch. Dis. Child. 2018, 103, 1155–1162. [Google Scholar] [CrossRef]
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 93, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Minini, M.; Bizzarri, M. Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 2017, 74, 4015–4025. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.-X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.-F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.; Varma, N.R.S.; Shankar, A.; Coimbra, V.B.; et al. Melatonin decreases breast cancer metastasis by modulating rho-associated kinase protein-1 expression. J. Pineal Res. 2016, 60, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Glenister, R.; McDaniel, K.; Francis, H.; Venter, J.; Jensen, K.; Dusio, G.; Glaser, S.; Meng, F.; Alpini, G. Therapeutic actions of melatonin on gastrointestinal cancer development and progression. Transl. Gastrointest. Cancer 2013, 2, 110–120. [Google Scholar]
- Wang, S.-W.; Tai, H.-C.; Tang, C.-H.; Lin, L.-W.; Lin, T.-H.; Chang, A.-C.; Chen, P.-C.; Chen, Y.-H.; Wang, P.-C.; Lai, Y.-W.; et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J. Cell. Physiol. 2021, 236, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin increases overall survival of prostate cancer patients with poor prognosis after combined hormone radiation treatment. Oncotarget 2020, 11, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Ju, L.; Zhou, F.; Yu, M.; Ma, H.; Zhang, Y.; Liu, T.; Xiao, Y.; Wang, X.; Qian, K. The inhibitory effect of melatonin on human prostate cancer. Cell. Commun. Signal. 2021, 19, 34. [Google Scholar] [CrossRef]
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O. Paradigm change from curative to predictive medicine: Novel strategic trends in Europe. Croat. Med. J. 2009, 50, 596–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liskova, A.; Samec, M.; Koklesova, L.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Liquid biopsy is instrumental for 3PM dimensional solutions in cancer management. J. Clin. Med. 2020, 9, 2749. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, X.; Zhan, X. Multi-parameter systematic strategies for predictive, preventive and personalised medicine in cancer. EPMA J. 2013, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crigna, A.T.; Samec, M.; Koklesova, L.; Liskova, A.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Cell-free nucleic acid patterns in disease prediction and monitoring—Hype or hope? EPMA J. 2020, 11, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Kunin, A.; Sargheini, N.; Birkenbihl, C.; Moiseeva, N.; Fröhlich, H.; Golubnitschaja, O. Voice perturbations under the stress overload in young individuals: Phenotyping and suboptimal health as predictors for cascading pathologies. EPMA J. 2020, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, V.; Bubnov, R.; Polivka, J.; Zubor, P.; Biringer, K.; Bielik, T.; Kuhn, W.; Golubnitschaja, O. Vaginal dryness: Individualised patient profiles, risks and mitigating measures. EPMA J. 2019, 10, 73–79. [Google Scholar] [CrossRef]
| Study Design | Effects of Melatonin | Mechanism | Reference |
|---|---|---|---|
| In Vitro | |||
| PC3, LNCaP cells | ↓ Glycolysis ↓ TCA ↓ PPP | Reduced the ATP/AMP ratio, lactate labeling, LDH activity and G6PDH | [103] |
| TC-71, A-673 and A-4573 cells | ↓ Glycolysis | Inhibited LDH activity; reduced glucose uptake; upregulated the hydroxylated (inactivated) form of HIF-1α | [104] |
| HepG2, Hep3B cells | ↓ Glucose uptake ↓ Resistance to cisplatin ↓ Proliferation | Downregulated YAP and subsequently regulated the Hippo signaling pathway; suppressed Bcl-2 and GLUT3 expression | [108] |
| BEAS-2B cells | ↓ Glycolysis | Melatonin suppressed the nickel-induced Warburg effect by inhibiting the HIF-1α/miR210/ISCU axis | [110] |
| MV-4-11, MOLM-13, OCI-AML3, U-937 cells | ↓ Glycolysis ↓ Proliferation ↑ Apoptosis | Melatonin regulated glucose metabolism by attenuating glucose uptake, LDH activity, lactate secretion and HIF-1α activation | [112] |
| Cal-27, SCC-9 cells | ↓ Glycolysis ↓ Proliferation ↑ Apoptosis ↑ Mitophagy ↑ ROS production | Melatonin intervention increased the level of acetyl CoA succinyl CoA, citric acid, NADH and reduced the level of pyruvate. Melatonin also increased OXPHOS level leading to suppression of aerobic glycolysis. Melatonin stimulated mitochondrial function, resulting in oxidative stress and subsequent apoptosis and mitophagy | [113] |
| In Vivo | |||
| PC3, LNCaP cells; TRAMP mice | ↓ Glucose uptake ↓ Tumor progression | Reduced glucose uptake via GLUT1 in prostate cancer cells; inhibited glucose-induced tumor progression in mice | [114] |
| PC3 xenografts in male nude rats | ↓ Glycolysis ↓ Proliferation ↓ Growth | Daytime blue light exposure amplified supraphysiologic nocturnal melatonin release, resulting in the suppression of cancer progression | [115] |
| MCF-7 xenografts in Weanling female nude rats | ↓ Glycolysis ↓ Lipid signaling ↓ Proliferation | Dim light at night disrupted the circadian cycle associated with melatonin release, leading to the promotion of cancer processes such as aerobic glycolysis, proliferation and lipid signaling | [96] |
| MCF-7 xenografts in female nude rats | ↓ Glycolysis ↑ Sensitivity to doxorubicin | Disruption of the circadian release of melatonin due to dim light exposure at night affected cancer metabolism and doxorubicin resistance. Conversely, melatonin suppressed lactate release and glucose uptake and restored the sensitivity of cancer cells to doxorubicin | [116] |
| MCF-7 xenografts in female nude rats | ↑ Sensitivity to tamoxifen ↓ Glycolysis | Disruption of melatonin release due to dim light exposure at night led to tamoxifen resistance and enhanced cancer metabolism. These characteristics were not identified in animals without circadian rhythm disruption or after supplementation with melatonin | [117] |
| DMBA-induced ovarian carcinogenesis in Fisher 344 rats | ↓ Proteins associated with cancer metabolism pathways | Melatonin administration for 60 days decreased the levels of proteins related to metabolic cascades, including proteins contributing to mitochondrial systems, HIF-1 signaling and generation of metabolites | [118] |
| SK-LMS-1 xenografts | ↓ Glycolysis | Melatonin suppressed the Warburg effect by decreasing glucose uptake and lactate production | [119] |
| Study Design | Effects | Mechanism | Reference |
|---|---|---|---|
| HepG2 cells | ↑ Apoptosis → Cell cycle arrest | Melatonin exhibited oncostatic abilities through the upregulation of caspase-3, -8 and -9, p53 and Bax; cytochrome C release; and the activation of Poly (ADP-ribose) polymerase (PARP) proteolysis | [125] |
| Ehrlich ascites carcinoma cells inoculated into BALB/c mice | ↓ Growth ↓ Proliferation ↓ Angiogenesis | Melatonin downregulated Bcl-2 and upregulated p53 and caspase-3 and -9 | [126] |
| HepG2 cells | ↑ Sensitization of cancer cells to EGCG toxicity ↓ Risk of EGCG-induced hepatoxicity | Melatonin downregulated p21 and subsequently sensitized cancer cells to EGCG toxicity | [141] |
| SGC-7901 cells | ↓ Proliferation ↑ Apoptosis | Melatonin blocked the Akt/MDM2 cascade, resulting in p53 activation | [145] |
| MCF-7 cells | ↓ Growth ↑ Apoptosis | Melatonin inhibited MDM2 expression and disrupted MDM2 nuclear translocation Melatonin induced p53 activation and increased p53 acetylation/stabilization | [146] |
| PANC-1, HeLa and A549 cells | ↓ Angiogenesis | Melatonin decreased VEGF and HIF-1α in cancer cells | [147] |
| HUVEC | ↓ Angiogenesis under hypoxia | Melatonin suppressed the HIF-1/ROS/VEGF cascade | [41] |
| RENCA cells inoculated into BALB/c mice | ↓ Growth ↓ Angiogenesis | Melatonin reduced HIF-1 activity in the animal model | [148] |
| HepG2 cells | ↓ Invasiveness ↓ Proliferation ↓ Angiogenesis | Melatonin downregulated HIF-1α and VEGF | [149] |
| DU145, PC-3 and LNCaP cells | ↓ Angiogenesis | Melatonin inhibited HIF-1α protein synthesis through the dephosphorylation of p70S6K and RPS6 | [150] |
| U251 and U87 cells | ↓ Migration ↓ Invasion | Melatonin downregulated HIF-1α, MMP2 and VEGF | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samec, M.; Liskova, A.; Koklesova, L.; Zhai, K.; Varghese, E.; Samuel, S.M.; Šudomová, M.; Lucansky, V.; Kassayova, M.; Pec, M.; et al. Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers 2021, 13, 3018. https://doi.org/10.3390/cancers13123018
Samec M, Liskova A, Koklesova L, Zhai K, Varghese E, Samuel SM, Šudomová M, Lucansky V, Kassayova M, Pec M, et al. Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers. 2021; 13(12):3018. https://doi.org/10.3390/cancers13123018
Chicago/Turabian StyleSamec, Marek, Alena Liskova, Lenka Koklesova, Kevin Zhai, Elizabeth Varghese, Samson Mathews Samuel, Miroslava Šudomová, Vincent Lucansky, Monika Kassayova, Martin Pec, and et al. 2021. "Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects" Cancers 13, no. 12: 3018. https://doi.org/10.3390/cancers13123018
APA StyleSamec, M., Liskova, A., Koklesova, L., Zhai, K., Varghese, E., Samuel, S. M., Šudomová, M., Lucansky, V., Kassayova, M., Pec, M., Biringer, K., Brockmueller, A., Kajo, K., Hassan, S. T. S., Shakibaei, M., Golubnitschaja, O., Büsselberg, D., & Kubatka, P. (2021). Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers, 13(12), 3018. https://doi.org/10.3390/cancers13123018












