Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Study
2.1.1. Baseline Data Collection
2.1.2. Case Ascertainment
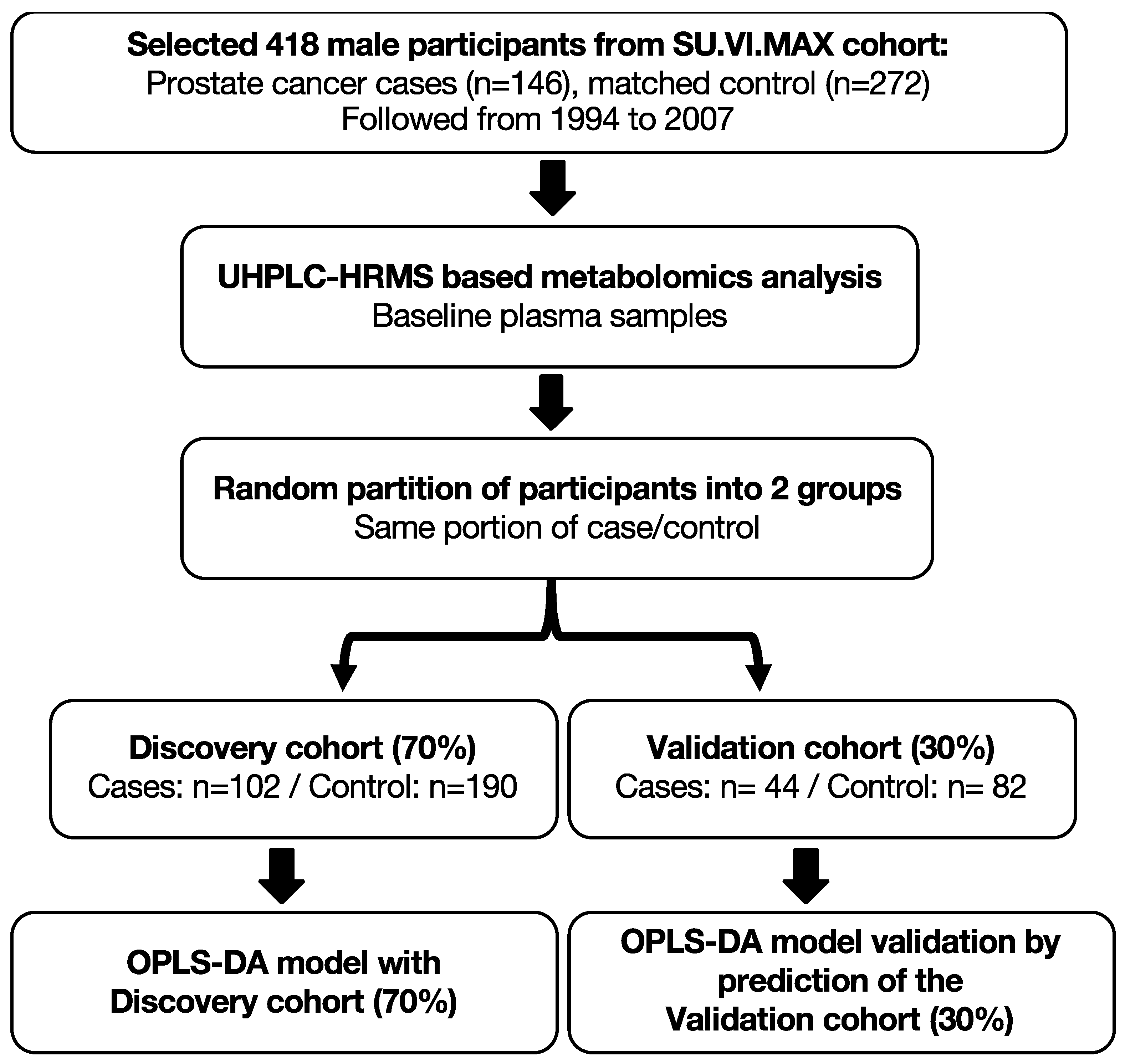
2.1.3. Nested Case–Control Study
2.2. UHPLC-HRMS Metabolomic Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of PCa Cases and Matched Controls
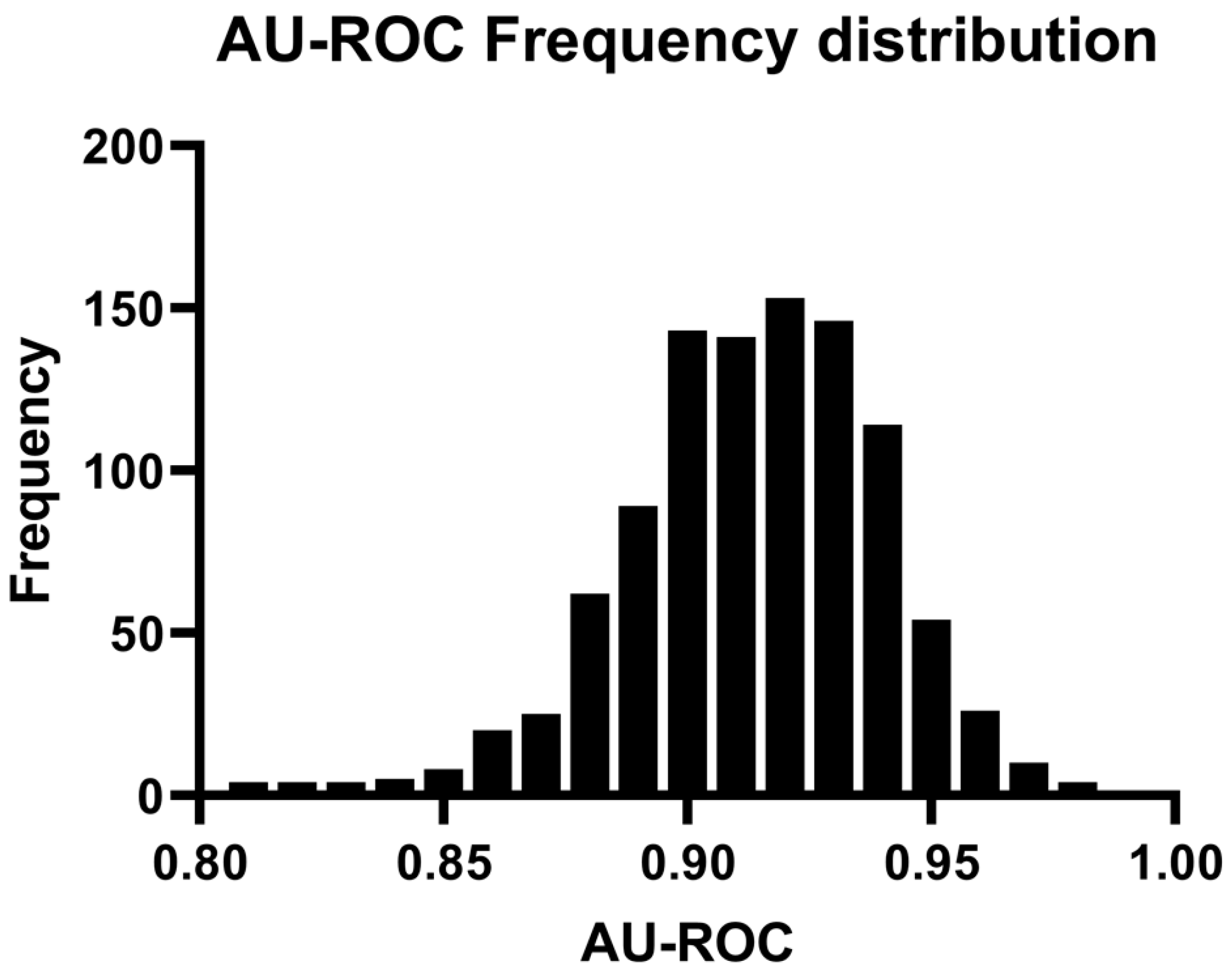
3.2. Discrimination of PCa Cases from Controls Using OPLS-DA Model
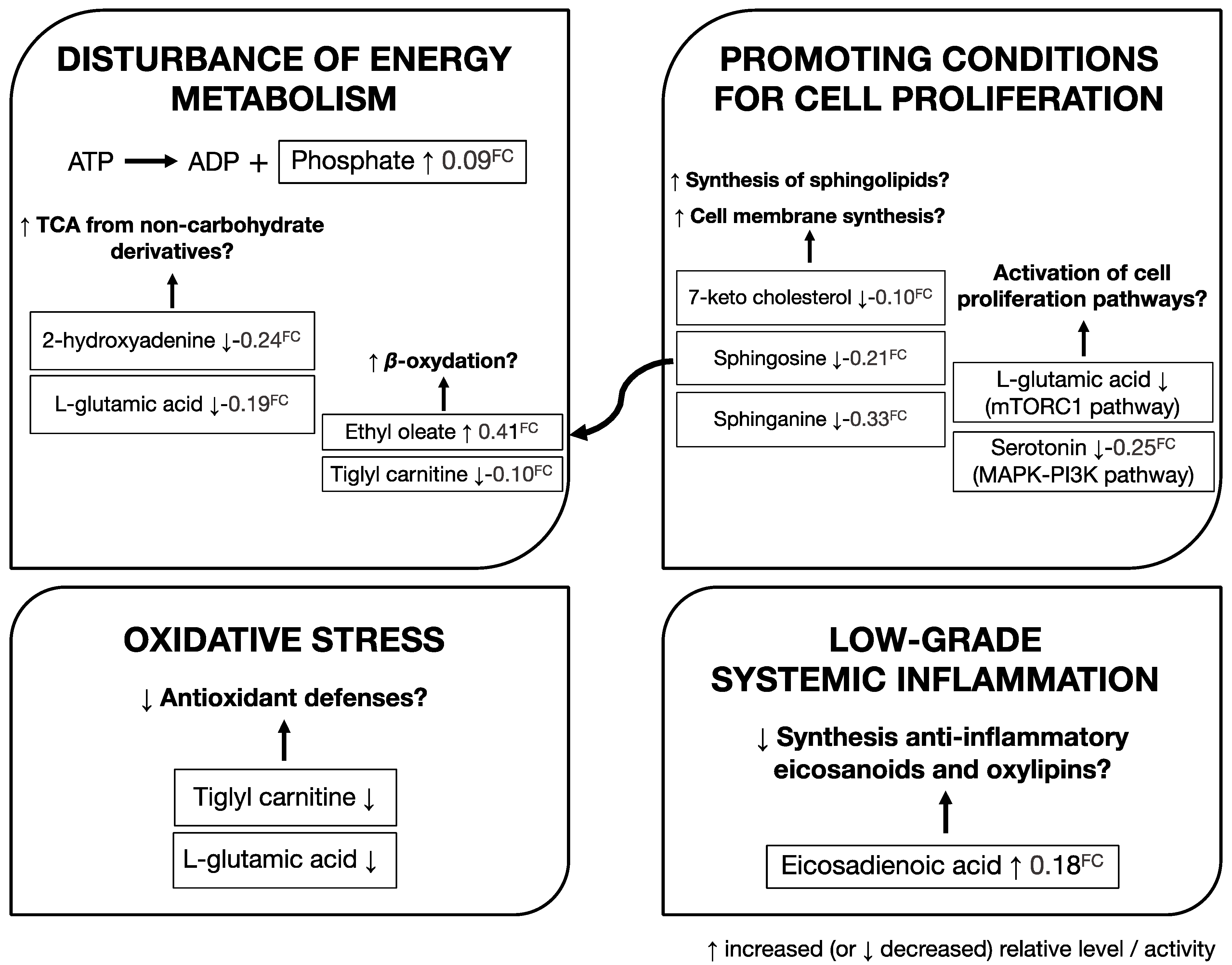
3.3. Identification of Metabolites Associated with Risk of Developing PCa
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.; Mahon, J.; Boland, A.; Beale, S.; Dwan, K.; Fleeman, N.; Hockenhull, J.; Dundar, Y. The Clinical Effectiveness and Cost-Effectiveness of the PROGENSA® Prostate Cancer Antigen 3 Assay and the Prostate Health Index in the Diagnosis of Prostate Cancer: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2015, 19, i–xxxi, 1–191. [Google Scholar] [CrossRef] [Green Version]
- Zadra, G.; Loda, M. Metabolic Vulnerabilities of Prostate Cancer: Diagnostic and Therapeutic Opportunities. Cold Spring Harb. Perspect. Med. 2018, 8, a030569. [Google Scholar] [CrossRef]
- Draisma, G.; Boer, R.; Otto, S.J.; van der Cruijsen, I.W.; Damhuis, R.A.M.; Schröder, F.H.; de Koning, H.J. Lead Times and Overdetection due to Prostate-Specific Antigen Screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J. Natl. Cancer Inst. 2003, 95, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Etzioni, R.; Penson, D.F.; Legler, J.M.; di Tommaso, D.; Boer, R.; Gann, P.H.; Feuer, E.J. Overdiagnosis due to Prostate-Specific Antigen Screening: Lessons from U.S. Prostate Cancer Incidence Trends. J. Natl. Cancer Inst. 2002, 94, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Al Olama, A.A.; Kote-Jarai, Z.; Berndt, S.I.; Conti, D.V.; Schumacher, F.; Han, Y.; Benlloch, S.; Hazelett, D.J.; Wang, Z.; Saunders, E.; et al. A Meta-Analysis of 87,040 Individuals Identifies 23 New Susceptibility Loci for Prostate Cancer. Nat. Genet. 2014, 46, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Leon, A.F.; Chau, C.H.; Price, D.K.; Figg, W.D. Diversity on Demand: Multi-Ancestry Meta-Analysis Improves Genetic Risk Prediction in Prostate Cancer. Am. J. Clin. Exp. Urol. 2021, 9, 189–193. [Google Scholar]
- Penney, K.L.; Sinnott, J.A.; Fall, K.; Pawitan, Y.; Hoshida, Y.; Kraft, P.; Stark, J.R.; Fiorentino, M.; Perner, S.; Finn, S.; et al. MRNA Expression Signature of Gleason Grade Predicts Lethal Prostate Cancer. J. Clin. Oncol. 2011, 29, 2391–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madu, C.O.; Lu, Y. Novel Diagnostic Biomarkers for Prostate Cancer. J. Cancer 2010, 1, 150–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, R.J.; van Oort, I.M.; Schalken, J.A. Blood-Based and Urinary Prostate Cancer Biomarkers: A Review and Comparison of Novel Biomarkers for Detection and Treatment Decisions. Prostate Cancer Prostatic Dis. 2017, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Zargar-Shoshtari, K.; Pow-Sang, J.M. Biomarkers for Prostate Cancer: Present Challenges and Future Opportunities. Future Sci. OA 2016, 2, FSO72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahmad, H.F.; Jalloul, M.; Azar, J.; Moubarak, M.M.; Samad, T.A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Tumor Microenvironment in Prostate Cancer: Toward Identification of Novel Molecular Biomarkers for Diagnosis, Prognosis, and Therapy Development. Front. Genet. 2021, 12, 652747. [Google Scholar] [CrossRef] [PubMed]
- Frantzi, M.; Hupe, M.C.; Merseburger, A.S.; Schanstra, J.P.; Mischak, H.; Latosinska, A. Omics Derived Biomarkers and Novel Drug Targets for Improved Intervention in Advanced Prostate Cancer. Diagnostics 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Galleggiante, V.; Giglio, A.; Palazzo, S.; Ferro, M.; Simone, C.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Metabolomic Profiling for the Identification of Novel Diagnostic Markers in Prostate Cancer. Expert Rev. Mol. Diagn. 2015, 15, 1211–1224. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; Rojas-Benedicto, A.; Albors-Vaquer, A.; López-Guerrero, J.A.; Pineda-Lucena, A.; Puchades-Carrasco, L. Metabolomics Contributions to the Discovery of Prostate Cancer Biomarkers. Metabolites 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- McDunn, J.E.; Stirdivant, S.M.; Ford, L.A.; Wolfert, R.L. Metabolomics and Its Application to the Development of Clinical Laboratory Tests for Prostate Cancer. EJIFCC 2015, 26, 92–104. [Google Scholar]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-Fat Diet Fuels Prostate Cancer Progression by Rewiring the Metabolome and Amplifying the MYC Program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef] [Green Version]
- Cerrato, A.; Bedia, C.; Capriotti, A.L.; Cavaliere, C.; Gentile, V.; Maggi, M.; Montone, C.M.; Piovesana, S.; Sciarra, A.; Tauler, R.; et al. Untargeted Metabolomics of Prostate Cancer Zwitterionic and Positively Charged Compounds in Urine. Anal. Chim. Acta 2021, 1158, 338381. [Google Scholar] [CrossRef]
- Penney, K.L.; Tyekucheva, S.; Rosenthal, J.; El Fandy, H.; Carelli, R.; Borgstein, S.; Zadra, G.; Fanelli, G.N.; Stefanizzi, L.; Giunchi, F.; et al. Metabolomics of Prostate Cancer Gleason Score in Tumor Tissue and Serum. Mol. Cancer Res. 2021, 19, 475–484. [Google Scholar] [CrossRef]
- Puhka, M.; Takatalo, M.; Nordberg, M.-E.; Valkonen, S.; Nandania, J.; Aatonen, M.; Yliperttula, M.; Laitinen, S.; Velagapudi, V.; Mirtti, T.; et al. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics 2017, 7, 3824–3841. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Koutros, S.; Derkach, A.; Karoly, E.; Sampson, J.N.; Moore, S.C.; Berndt, S.I.; Albanes, D. Serum Metabolomic Profiling of Prostate Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br. J. Cancer 2016, 115, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rambla, C.; Puchades-Carrasco, L.; García-Flores, M.; Rubio-Briones, J.; López-Guerrero, J.A.; Pineda-Lucena, A. Non-Invasive Urinary Metabolomic Profiling Discriminates Prostate Cancer from Benign Prostatic Hyperplasia. Metabolomics 2017, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Giunchi, F.; Fiorentino, M.; Loda, M. The Metabolic Landscape of Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 28–36. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Amaro, F.; Bastos, M.D.L.; Carvalho, M.; Guedes de Pinho, P. Advances and Perspectives in Prostate Cancer Biomarker Discovery in the Last 5 Years through Tissue and Urine Metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Gelzo, M.; Stornaiuolo, M.; Ruoppolo, M.; Castaldo, G. The Evolving Landscape of Untargeted Metabolomics. Nutr. Metab. Cardiovasc. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna-Silva, A.C.B.; Perez-Valencia, J.A.; Sciacovelli, M.; Lalou, C.; Sarlak, S.; Tronci, L.; Nikitopoulou, E.; Meszaros, A.T.; Frezza, C.; Rossignol, R.; et al. Succinate Anaplerosis Has an Onco-Driving Potential in Prostate Cancer Cells. Cancers 2021, 13, 1727. [Google Scholar] [CrossRef]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Metabolomic Analysis of Prostate Cancer Risk in a Prospective Cohort: The Alpha-Tocolpherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int. J. Cancer 2015, 137, 2124–2132. [Google Scholar] [CrossRef]
- Koutros, S.; Meyer, T.E.; Fox, S.D.; Issaq, H.J.; Veenstra, T.D.; Huang, W.-Y.; Yu, K.; Albanes, D.; Chu, L.W.; Andriole, G.; et al. Prospective Evaluation of Serum Sarcosine and Risk of Prostate Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis 2013, 34, 2281–2285. [Google Scholar] [CrossRef] [Green Version]
- Ankerst, D.P.; Liss, M.; Zapata, D.; Hoefler, J.; Thompson, I.M.; Leach, R.J. A Case Control Study of Sarcosine as an Early Prostate Cancer Detection Biomarker. BMC Urol. 2015, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Röhnisch, H.E.; Kyrø, C.; Olsen, A.; Thysell, E.; Hallmans, G.; Moazzami, A.A. Identification of Metabolites Associated with Prostate Cancer Risk: A Nested Case-Control Study with Long Follow-up in the Northern Sweden Health and Disease Study. BMC Med. 2020, 18, 187. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Favier, A.; Briançon, S. The SU.VI.MAX Study: A Randomized, Placebo-Controlled Trial of the Health Effects of Antioxidant Vitamins and Minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercberg, S.; Preziosi, P.; Briançon, S.; Galan, P.; Triol, I.; Malvy, D.; Roussel, A.M.; Favier, A. A Primary Prevention Trial Using Nutritional Doses of Antioxidant Vitamins and Minerals in Cardiovascular Diseases and Cancers in a General Population: The SU.VI.MAX Study—Design, Methods, and Participant Characteristics. Supplementation En Vitamines et Minéraux AntioXydants. Control. Clin. Trials 1998, 19, 336–351. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Diseases and Related Health Problems, 10th Revision. Available online: http://www.who.int/classifications/apps/icd/icd10online (accessed on 2 January 2020).
- Wise, B.M.; Gallagher, N.B.; Bro, R.; Shaver, J.M. PLS_Toolbox for Use with MATLAB, Version 3.0; Eigenvector Research, Inc.: Manson, WA, USA, 2002. [Google Scholar]
- Wise, B.M.; Ricker, N.L. Recent Advances in Multivariate Statistical Process Control: Improving Robustness and Sensitivity; IFAC Symposium on Advanced Control of Chemical Processes: Toulouse, France, 1991; pp. 125–130. [Google Scholar]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.M.; Ludwig, C.; Günther, U.L.; Viant, M.R. Improved Classification Accuracy in 1- and 2-Dimensional NMR Metabolomics Data Using the Variance Stabilising Generalised Logarithm Transformation. BMC Bioinform. 2007, 8, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.C.Y.; Pasikanti, K.K.; Nicholson, J.K. Global Urinary Metabolic Profiling Procedures Using Gas Chromatography–Mass Spectrometry. Nat. Protoc. 2011, 6, 1483–1499. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS Models in Metabolomics: The Impact of Permutation of Dataset Rows on the K-Fold Cross-Validation Quality Parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-Check: Validation of Diagnostic Statistics for PLS-DA Models in Metabolomics Studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Tukey, J.W. Exploratory Data Analysis. In Reading, Mass; Addison-Wesley Pub. Co.: Boston, MA, USA, 1977; ISBN 978-0-201-07616-5. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS Discriminant Analysis: Biologically Relevant Feature Selection and Graphical Displays for Multiclass Problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS.; Statistics and Computing, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef]
- Pawlik-Sobecka, L.; Sołkiewicz, K.; Kokot, I.; Kiraga, A.; Płaczkowska, S.; Schlichtinger, A.M.; Kratz, E.M. The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study. Diagnostics 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Lécuyer, L.; Victor Bala, A.; Demidem, A.; Rossary, A.; Bouchemal, N.; Triba, M.N.; Galan, P.; Hercberg, S.; Partula, V.; Srour, B.; et al. NMR Metabolomic Profiles Associated with Long-Term Risk of Prostate Cancer. Metabolomics 2021, 17, 32. [Google Scholar] [CrossRef]
- Stracka, D.; Jozefczuk, S.; Rudroff, F.; Sauer, U.; Hall, M.N. Nitrogen Source Activates TOR (Target of Rapamycin) Complex 1 via Glutamine and Independently of Gtr/Rag Proteins. J. Biol. Chem. 2014, 289, 25010–25020. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yi, D.; Hou, Y.; Ding, B.; Li, K.; Li, B.; Zhu, H.; Liu, Y.; Wu, G. Dietary Supplementation with α-Ketoglutarate Activates MTOR Signaling and Enhances Energy Status in Skeletal Muscle of Lipopolysaccharide-Challenged Piglets. J. Nutr. 2016, 146, 1514–1520. [Google Scholar] [CrossRef]
- Dang, C.V. Glutaminolysis: Supplying Carbon or Nitrogen or Both for Cancer Cells? Cell Cycle 2010, 9, 3884–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Chong, N.; Lewis, N.E.; Jia, W.; Xie, G.; Garmire, L.X. Novel Personalized Pathway-Based Metabolomics Models Reveal Key Metabolic Pathways for Breast Cancer Diagnosis. Genome Med. 2016, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.-H.; Cheng, M.-L.; Lo, C.-J.; Lin, Y.-H.; Lai, M.-W.; Lin, W.-R.; Yeh, C.-T. Plasma Phenylalanine and Glutamine Concentrations Correlate with Subsequent Hepatocellular Carcinoma Occurrence in Liver Cirrhosis Patients: An Exploratory Study. Sci. Rep. 2020, 10, 10926. [Google Scholar] [CrossRef]
- Kamiya, H. Mutagenicities of 8-Hydroxyguanine and 2-Hydroxyadenine Produced by Reactive Oxygen Species. Biol. Pharm. Bull. 2004, 27, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome, W.G.; O’Connor, M.S. 7-Ketocholesterol in Disease and Aging. Redox Biol. 2020, 29, 101380. [Google Scholar] [CrossRef]
- Sedić, M.; Grbčić, P.; Pavelić, S.K. Bioactive Sphingolipids as Biomarkers Predictive of Disease Severity and Treatment Response in Cancer: Current Status and Translational Challenges. Anticancer Res. 2019, 39, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Murdica, V.; Mancini, G.; Loberto, N.; Bassi, R.; Giussani, P.; Di Muzio, N.; Deantoni, C.; Prinetti, A.; Aureli, M.; Sonnino, S. Abiraterone and Ionizing Radiation Alter the Sphingolipid Homeostasis in Prostate Cancer Cells. Adv. Exp. Med. Biol. 2018, 1112, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. The Role of Cholesterol and Cholesterol-Driven Membrane Raft Domains in Prostate Cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Hannun, Y.A. Biologically Active Sphingolipids in Cancer Pathogenesis and Treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A. The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer. Mediat. Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N. Endogenous Pro-Resolving and Anti-Inflammatory Lipid Mediators: A New Pharmacologic Genus. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S200–S215. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Piao, H.; Wang, F.; Yin, P.; Xu, C.; Lu, X.; Ye, G.; Shao, Y.; Yan, M.; et al. Integration of Lipidomics and Transcriptomics Unravels Aberrant Lipid Metabolism and Defines Cholesteryl Oleate as Potential Biomarker of Prostate Cancer. Sci. Rep. 2016, 6, 20984. [Google Scholar] [CrossRef] [Green Version]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Cascini, C.; Gallazzi, M.; Mortara, L.; Sessa, F.; Pelosi, G.; Albini, A.; Noonan, D.M. Acetyl-L-Carnitine Downregulates Invasion (CXCR4/CXCL12, MMP-9) and Angiogenesis (VEGF, CXCL8) Pathways in Prostate Cancer Cells: Rationale for Prevention and Interception Strategies. J. Exp. Clin. Cancer Res. 2019, 38, 464. [Google Scholar] [CrossRef]
- Dizeyi, N.; Hedlund, P.; Bjartell, A.; Tinzl, M.; Austild-Taskén, K.; Abrahamsson, P.-A. Serotonin Activates MAP Kinase and PI3K/Akt Signaling Pathways in Prostate Cancer Cell Lines. Urol. Oncol. 2011, 29, 436–445. [Google Scholar] [CrossRef]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases from Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef]
- Brown, R.B.; Razzaque, M.S. Phosphate Toxicity and Tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Tong, H.; Luo, P.; Kong, H.; Xu, Z.; Yin, P.; Xu, G. A High Throughput Metabolomics Method and Its Application in Female Serum Samples in a Normal Menstrual Cycle Based on Liquid Chromatography-Mass Spectrometry. Talanta 2018, 185, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Carayol, M.; Licaj, I.; Achaintre, D.; Sacerdote, C.; Vineis, P.; Key, T.J.; Onland Moret, N.C.; Scalbert, A.; Rinaldi, S.; Ferrari, P. Reliability of Serum Metabolites over a Two-Year Period: A Targeted Metabolomic Approach in Fasting and Non-Fasting Samples from EPIC. PLoS ONE 2015, 10, e0135437. [Google Scholar] [CrossRef] [Green Version]
- Floegel, A.; Drogan, D.; Wang-Sattler, R.; Prehn, C.; Illig, T.; Adamski, J.; Joost, H.-G.; Boeing, H.; Pischon, T. Reliability of Serum Metabolite Concentrations over a 4-Month Period Using a Targeted Metabolomic Approach. PLoS ONE 2011, 6, e21103. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Controls (n = 272) | Cases (n = 146) | |||
|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | p-Value * | |
| Age at baseline (years) | 54.3 | 4.6 | 54.7 | 4.8 | 0.09 |
| Age at baseline (categories) | / | ||||
| <45 years | 6 | 2.2 | 3 | 2.1 | |
| ≥45–<50 years | 53 | 19.5 | 27 | 18.5 | |
| ≥50–<55 years | 71 | 26.1 | 38 | 26.0 | |
| ≥55–<60 years | 113 | 41.5 | 62 | 42.5 | |
| ≥60–<65 years | 29 | 10.7 | 16 | 11.0 | |
| Age at diagnosis (years) | 63.0 | 5.0 | / | / | / |
| Time between blood collection and diagnosis (years) | 8.3 | 3.0 | / | / | / |
| Gleason ≥ 7 ** | 62 | 42.5 | / | / | / |
| BMI (kg/m2) | 25.0 | 3.0 | 25.4 | 3.0 | 0.07 |
| BMI (categories) | / | ||||
| Underweight (<18.5 kg/m2) | 2 | 0.7 | 1 | 0.7 | |
| Normal weight (≥18.5–<25 kg/m2) | 133 | 48.9 | 72 | 49.3 | |
| Overweight (>25 kg/m2) | 137 | 50.4 | 73 | 50.0 | |
| Season of blood draw | / | ||||
| March–May (Spring) | 102 | 37.5 | 56 | 38.4 | |
| October–November (Fall) | 36 | 13.2 | 21 | 14.4 | |
| December–January (Winter) | 134 | 49.3 | 69 | 47.3 | |
| Smoking status | / | ||||
| Non smokers | 242 | 89.0 | 130 | 89.0 | |
| Smokers | 30 | 11.0 | 16 | 11.0 | |
| SU.VI.MAX intervention group | / | ||||
| Supplementation | 123 | 45.2 | 65 | 44.5 | |
| Placebo | 149 | 54.8 | 81 | 55.5 | |
| Family history of prostate cancer | 0.01 | ||||
| No | 261 | 96.0 | 130 | 89.0 | |
| Yes | 11 | 4.0 | 16 | 11.0 | |
| Prostate-specific antigen (ng/mL) | 1.3 | 1.2 | 3.4 | 3.4 | <0.0001 |
| Prostate-specific antigen (categories) | <0.0001 | ||||
| <3 ng/mL | 256 | 94.1 | 97 | 66.4 | |
| ≥3 ng/mL | 16 | 5.9 | 49 | 33.6 | |
| Physical activity | 0.7 | ||||
| Irregular | 68 | 25.0 | 32 | 21.9 | |
| <1 h walk or equivalent | 57 | 21.0 | 35 | 24.0 | |
| ≥1 h walk or equivalent | 147 | 54.0 | 79 | 54.1 | |
| Educational level | 0.9 | ||||
| Primary school | 63 | 23.2 | 31 | 21.2 | |
| Secondary school | 101 | 37.1 | 56 | 38.4 | |
| ≥High-school degree | 108 | 39.7 | 59 | 40.4 | |
| Alcohol intake (g/day) | 29.8 | 22.5 | 26.9 | 21.4 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Lécuyer, L.; Liu, X.; Triba, M.N.; Deschasaux-Tanguy, M.; Demidem, A.; Liu, Z.; Palama, T.; Rossary, A.; Vasson, M.-P.; et al. Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Cancers 2021, 13, 3140. https://doi.org/10.3390/cancers13133140
Lin X, Lécuyer L, Liu X, Triba MN, Deschasaux-Tanguy M, Demidem A, Liu Z, Palama T, Rossary A, Vasson M-P, et al. Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Cancers. 2021; 13(13):3140. https://doi.org/10.3390/cancers13133140
Chicago/Turabian StyleLin, Xiangping, Lucie Lécuyer, Xinyu Liu, Mohamed N. Triba, Mélanie Deschasaux-Tanguy, Aïcha Demidem, Zhicheng Liu, Tony Palama, Adrien Rossary, Marie-Paule Vasson, and et al. 2021. "Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry" Cancers 13, no. 13: 3140. https://doi.org/10.3390/cancers13133140
APA StyleLin, X., Lécuyer, L., Liu, X., Triba, M. N., Deschasaux-Tanguy, M., Demidem, A., Liu, Z., Palama, T., Rossary, A., Vasson, M.-P., Hercberg, S., Galan, P., Savarin, P., Xu, G., & Touvier, M. (2021). Plasma Metabolomics for Discovery of Early Metabolic Markers of Prostate Cancer Based on Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Cancers, 13(13), 3140. https://doi.org/10.3390/cancers13133140








