Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Preparation and Microbiota Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
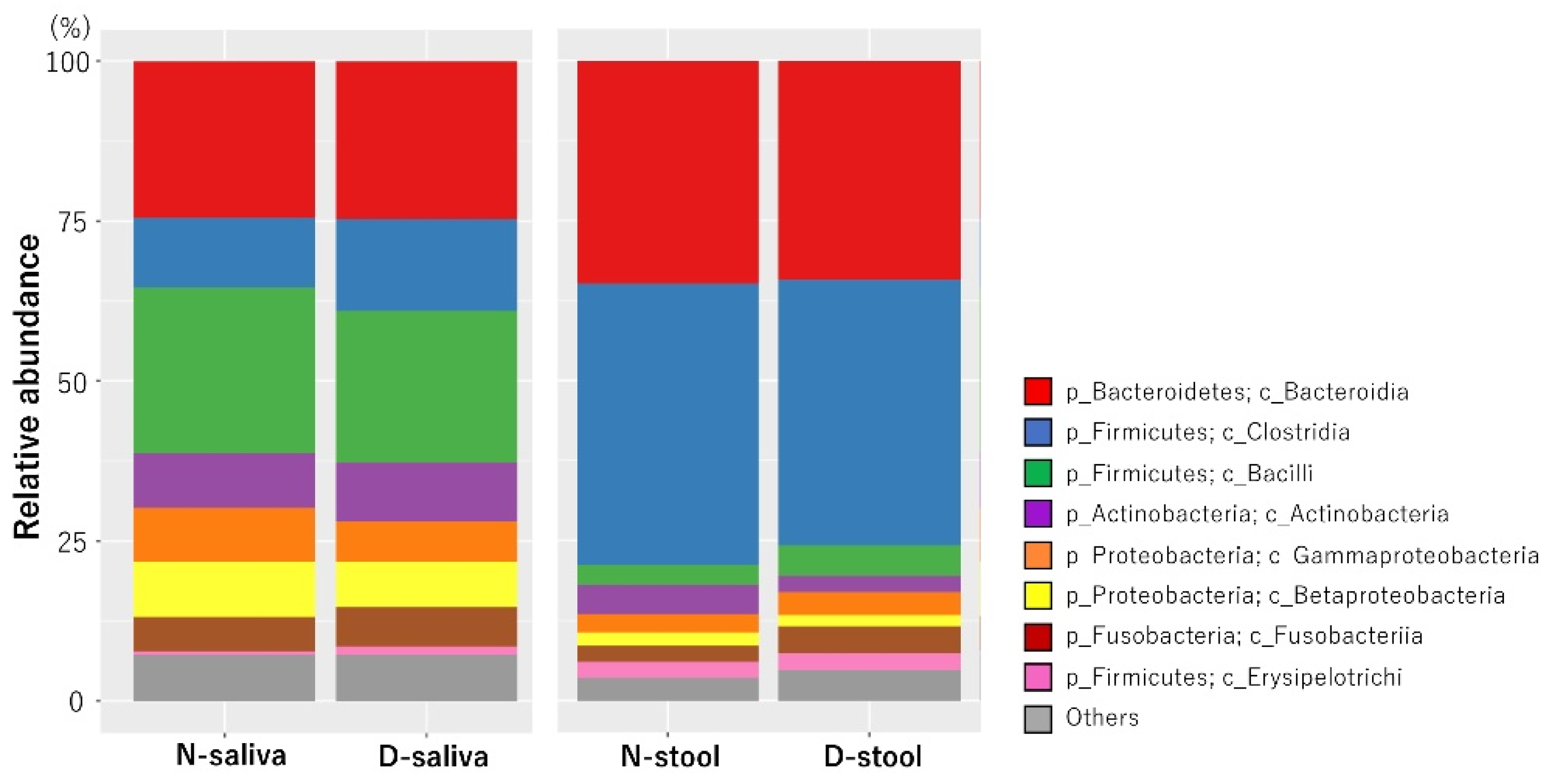
3.2. Saliva and Stool Microbiota Differences between CRC Patients and Controls
3.3. Potential Biomarker Bacterial Species Based on Lefse Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; DeSantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA: A Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Komiya, Y.; Shimomura, Y.; Higurashi, T.; Sugi, Y.; Arimoto, J.; Umezawa, S.; Uchiyama, S.; Matsumoto, M.; Nakajima, A. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 2018, 68, 1335–1337. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Bacci, G.; Chiellini, C.; Fagorzi, C.; Niccolai, E.; Taddei, A.; Ricci, F.; Ringressi, M.N.; Borrelli, R.; Melli, F.; et al. Preliminary Comparison of Oral and Intestinal Human Microbiota in Patients with Colorectal Cancer: A Pilot Study. Front. Microbiol. 2018, 8, 2699. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, R.; Shu, R.; Yu, J.; Li, H.; Long, H.; Jin, S.; Li, S.; Hu, Q.; Yao, F.; et al. Study of the Relationship between Microbiome and Colorectal Cancer Susceptibility Using 16SrRNA Sequencing. BioMed. Res. Int. 2020, 2020, 7828392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Gao, H.; Mihindukulasuriya, K.A.; La Rosa, P.S.; Wylie, K.M.; Vishnivetskaya, T.; Podar, M.; Warner, B.; Tarr, P.I.; Nelson, D.E.; et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013, 14, R1. [Google Scholar] [CrossRef] [Green Version]
- Huse, S.M.; Ye, Y.; Zhou, Y.; Fodor, A.A. A Core Human Microbiome as Viewed through 16S rRNA Sequence Clusters. PLoS ONE 2012, 7, e34242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H.; et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016, 22, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.-O.; Sugai, T.; An, B.; et al. Fusobacterium in Colonic Flora and Molecular Features of Colorectal Carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellarin, M.; Warren, R.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2011, 22, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.; Ojesina, A.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2011, 22, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobin, C. Colorectal cancer: CRC--all about microbial products and barrier function? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 694–696. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Cai, Q.; Shu, X.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Long, J. Prospective study of oral microbiome and colorectal cancer risk in low-income and African American populations. Int. J. Cancer 2019, 144, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Babic, A.; Tworoger, S.S.; Zhang, L.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Meyerhardt, J.; Giovannucci, E.; et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int. J. Cancer 2017, 140, 646–652. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Qian, Y.; Xie, Y.; Jiang, S.; Kang, Z.; Chen, Y.; Chen, Z.; Fang, J. Alterations in the oral and gut microbiome of colorectal cancer patients and association with host clinical factors. Int. J. Cancer 2021, 149, 925–935. [Google Scholar] [CrossRef]
- Pedersen, R.M.; Holt, H.M.; Justesen, U.S. Solobacterium moorei Bacteremia: Identification, Antimicrobial Susceptibility, and Clinical Characteristics: Table 1. J. Clin. Microbiol. 2011, 49, 2766–2768. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.K.; Park, S.-N.; Shin, J.H.; Ji, S.; Jo, E.; Chang, Y.-H.; Shin, Y.; Paek, J.; Kim, H.; Kook, J.-K. Streptococcus koreensis sp. nov., Isolated from Human Subgingival Dental Plaque of Periodontitis Lesion. Curr. Microbiol. 2019, 76, 1531–1536. [Google Scholar] [CrossRef]
- Masood, U.; Sharma, A.; Lowe, D.; Khan, R.; Manocha, D. Colorectal Cancer Associated with Streptococcus anginosus Bacteremia and Liver Abscesses. Case Rep. Gastroenterol. 2016, 10, 769–774. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immunity, Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and Functional Features of the Gastrointestinal Microbiome and Their Effects on Human Health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.G.; Fitzgerald, G.F.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4586–4591. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mizoue, T.; Yamaji, T.; Tabata, S.; Yamaguchi, K.; Shimizu, E.; Mineshita, M.; Ogawa, S.; Kono, S. Dietary Patterns and Colorectal Adenomas in Japanese Men: The Self-Defense Forces Health Study. Am. J. Epidemiology 2005, 161, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.; Chan, A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterol. 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef] [Green Version]
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, I.; Dhar, R.; Pethusamy, K.; Seethy, A.; Srivastava, T.; Sah, R.; Sharma, J.; Karmakar, S. Exploring the Role of Gut Microbiome in Colon Cancer. Appl. Biochem. Biotechnol. 2021, 193, 1780–1799. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Fang, L.; Lee, M.-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | Controls (Group N) | CRC (Group D) | p Value | |
|---|---|---|---|---|
| Samples (n) | 51 | 52 | ||
| Gender | Male | 26 (51.0%) | 33 (63.5%) | 0.2004 |
| Female | 25 (49.0%) | 19 (36.5%) | ||
| Age (mean±SD) | 54.49 (±10.6) | 68.52 (±10.6) | <0.01 ** | |
| Medical history | Hypertension | 12 (23.5%) | 23 (44.2%) | 0.0256 * |
| Diabetes | 4 (7.8%) | 9 (17.3%) | 0.1481 | |
| Teeth | Average number of teeth | 24.92 (±9.3) | 17.7 (±5.2) | <0.01 ** |
| No decayed teeth | 43 (84.3%) | 33 (63.5%) | <0.01 ** | |
| With decayed teeth | 6 (11.8%) | 18 (34.6%) | ||
| Denture | None | 47 (92.2%) | 27 (51.9%) | <0.01 ** |
| Using | 4 (7.8%) | 25 (48.1%) | ||
| Gingival plaque | <1/3 of tooth surface | 30 (58.8%) | 7 (13.5%) | <0.01 ** |
| ≥1/3 of tooth surface | 18 (35.3%) | 41 (78.8%) | ||
| Alcohol | None | 21 (41.2%) | 32 (61.5%) | 0.0662 |
| ≤3 days/week | 12 (23.5%) | 5 (9.6%) | ||
| ≥4 days/week | 18 (35.3%) | 15 (28.8%) | ||
| Smoking | Never | 24 (47.1%) | 24 (46.2%) | 0.5875 |
| Experienced | 19 (37.3%) | 24 (46.2%) | ||
| Current | 8 (15.7%) | 4 (7.7%) | ||
| Number of teeth brushing (/day) | ≤2 | 14 (27.5%) | 33 (63.5%) | <0.01 ** |
| ≥3 | 37 (72.5%) | 18 (34.6%) | ||
| Dental examination in 3M | None | 36 (70.6%) | 35 (67.3%) | 0.8296 |
| Yes | 15 (29.4%) | 16 (30.8%) |
| Characteristics | n | (%) | |
|---|---|---|---|
| Region | Cecum | 7 | 13.5 |
| Ascending colon | 7 | 13.5 | |
| Transverse colon | 2 | 3.8 | |
| Descending colon | 5 | 9.6 | |
| Sigmoid colon | 7 | 13.5 | |
| Rectum | 24 | 46.2 | |
| T | T1 | 7 | 13.5 |
| T2≤ | 45 | 86.5 | |
| N | N0 | 29 | 55.8 |
| N1≤ | 23 | 44.2 | |
| M | M0 | 43 | 82.7 |
| M1 | 9 | 17.3 | |
| Stage | Early (Ⅰ, Ⅱ) | 26 | 50.0 |
| Advanced (Ⅲ, Ⅳ) | 26 | 50.0 | |
| Treatment | Surgery | 34 | 65.4 |
| Chemotherapy | 4 | 7.7 | |
| Neoadjuvant chemotherapy + surgery | 12 | 23.1 | |
| None | 2 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchino, Y.; Goto, Y.; Konishi, Y.; Tanabe, K.; Toda, H.; Wada, M.; Kita, Y.; Beppu, M.; Mori, S.; Hijioka, H.; et al. Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects. Cancers 2021, 13, 3332. https://doi.org/10.3390/cancers13133332
Uchino Y, Goto Y, Konishi Y, Tanabe K, Toda H, Wada M, Kita Y, Beppu M, Mori S, Hijioka H, et al. Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects. Cancers. 2021; 13(13):3332. https://doi.org/10.3390/cancers13133332
Chicago/Turabian StyleUchino, Yoshinori, Yuichi Goto, Yusuke Konishi, Kan Tanabe, Hiroko Toda, Masumi Wada, Yoshiaki Kita, Mahiro Beppu, Shinichiro Mori, Hiroshi Hijioka, and et al. 2021. "Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects" Cancers 13, no. 13: 3332. https://doi.org/10.3390/cancers13133332
APA StyleUchino, Y., Goto, Y., Konishi, Y., Tanabe, K., Toda, H., Wada, M., Kita, Y., Beppu, M., Mori, S., Hijioka, H., Otsuka, T., Natsugoe, S., Hara, E., & Sugiura, T. (2021). Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects. Cancers, 13(13), 3332. https://doi.org/10.3390/cancers13133332








