Translational Utility of Liquid Biopsies in Thyroid Cancer Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Modalities Used in Clinical Practice to Monitor Recurrence in Thyroid Cancer
2.1. Tumour Markers
2.2. Imaging
3. The Need for New Screening Tools in Thyroid Cancer: Identifying Molecular Markers
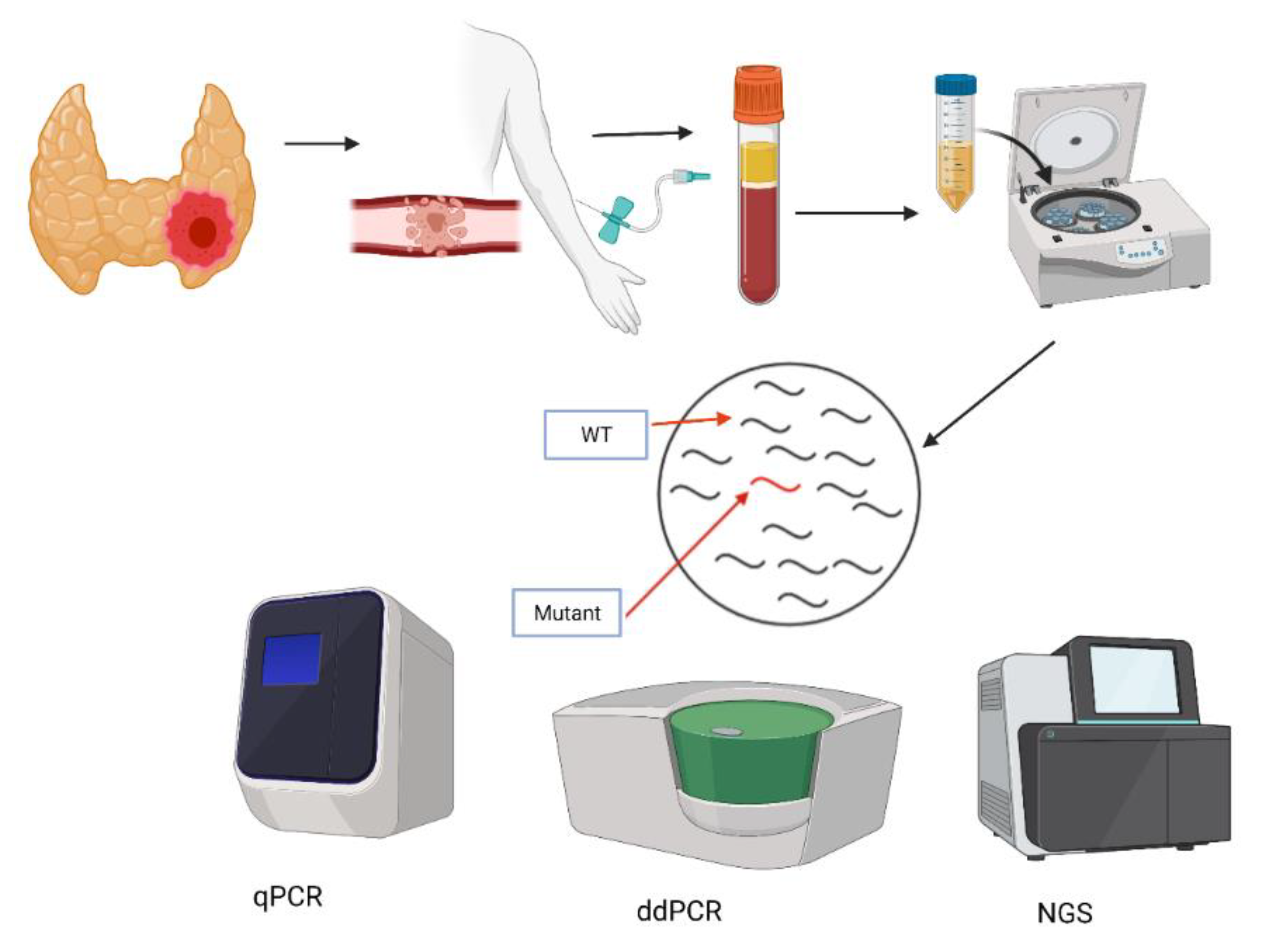
4. Genesis of Liquid Biopsy
5. Methods of ctDNA Detection
6. Liquid Biopsy in Thyroid Cancer
7. Clinical Applications
7.1. Mutational Screening
7.2. Monitoring Treatment Response
7.3. Diagnostic Applications: Assessment of Suspicious Nodules
8. Liquid Biopsies of Epigenetic Markers
8.1. Circulating miRNA
8.2. Methylation
8.3. Future Directions
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azadnajafabad, S.; Moghaddam, S.S.; Mohammadi, E.; Rezaei, N.; Ghasemi, E.; Fattahi, N.; Aminorroaya, A.; Azadnajafabad, R.; Aryannejad, A.; Rezaei, N.; et al. Global, regional, and national burden and quality of care index (QCI) of thyroid cancer: A systematic analysis of the Global Burden of Disease Study 1990–2017. Cancer Med. 2021, 10, 2496–2508. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Maso, L.D. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Sebastian, S.O.; Gonzalez, J.M.; Paricio, P.P.; Perez, J.S.; Flores, D.P.; Madrona, A.P.; Romero, P.R.; Tebar, F.J. Papillary thyroid carcinoma: Prognostic index for survival including the histological variety. Arch. Surg. 2000, 135, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Van Velsen, E.F.; Stegenga, M.T.; Van Kemenade, F.J.; Kam, B.L.; Van Ginhoven, T.M.; Visser, W.E.; Peeters, R.P. Evaluating the 2015 American Thyroid Association Risk Stratification System in High-Risk Papillary and Follicular Thyroid Cancer Patients. Thyroid 2019, 29, 1073–1079. [Google Scholar] [CrossRef]
- Rousset, B.; Dupuy, C.; Miot, F.; Dumont, J. Chapter 2 Thyroid Hormone Synthesis and Secretion; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Giovanella, L.; Suriano, S.; Ceriani, L.; Verburg, F.A. Undetectable Thyroglobulin in Patients with Differentiated Thyroid Carcinoma and Residual Radioiodine Uptake on a Postablation Whole-Body Scan. Clin. Nucl. Med. 2011, 36, 109–112. [Google Scholar] [CrossRef]
- Robenshtok, E.; Grewal, R.K.; Fish, S.; Sabra, M.; Tuttle, R.M. A Low Postoperative Nonstimulated Serum Thyroglobulin Level Does Not Exclude the Presence of Radioactive Iodine Avid Metastatic Foci in Intermediate-Risk Differentiated Thyroid Cancer Patients. Thyroid 2013, 23, 436–442. [Google Scholar] [CrossRef]
- De Rosário, P.W.S.; Guimarães, V.C.; Maia, F.F.R.; Fagundes, T.A.; Purisch, S.; Padrao, E.L.; Rezende, L.L.; Barroso, A.L. Thyroglobulin before Ablation and Correlation with Posttreatment Scanning. Laryngoscope 2005, 115, 264–267. [Google Scholar] [CrossRef]
- Spencer, C.A. Clinical Utility of Thyroglobulin Antibody (TgAb) Measurements for Patients with Differentiated Thyroid Cancers (DTC). J. Clin. Endocrinol. Metab. 2011, 96, 3615–3627. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jeon, M.J.; Oh, H.-S.; Lee, Y.-M.; Sung, T.-Y.; Han, M.; Han, J.M.; Kim, T.Y.; Chung, K.-W.; Kim, W.B.; et al. Changes in Serum Thyroglobulin Levels After Lobectomy in Patients with Low-Risk Papillary Thyroid Cancer. Thyroid 2018, 28, 997–1003. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Toledo, S.P.; Lourenço, D.M.; Santos, M.A.; Tavares, M.R.; Toledo, R.A.; Correia-Deur, J.E.D.M. Hypercalcitoninemia is not pathognomonic of medullary thyroid carcinoma. Clinics 2009, 64, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Rondeau, G.; Fish, S.; Hann, L.E.; Fagin, J.A.; Tuttle, R.M. Ultrasonographically Detected Small Thyroid Bed Nodules Identified After Total Thyroidectomy for Differentiated Thyroid Cancer Seldom Show Clinically Significant Structural Progression. Thyroid 2011, 21, 845–853. [Google Scholar] [CrossRef]
- Lin, E.C. Radiation Risk from Medical Imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricarte-Filho, J.C.; Ryder, M.; Chitale, D.; Rivera, M.; Heguy, A.; Ladanyi, M.; Janakiraman, M.; Solit, D.; Knauf, J.; Tuttle, R.M.; et al. Mutational Profile of Advanced Primary and Metastatic Radioactive Iodine-Refractory Thyroid Cancers Reveals Distinct Pathogenetic Roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009, 69, 4885–4893. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Nutting, C.M.; Jarząb, B.; Elisei, R.; Siena, S.; Bastholt, L.; DE LA Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients With Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [Green Version]
- Mandel, P.; Metais, P. Nuclear Acids in Human Blood Plasma. Comptes Rendus Seances Soc. Biol. Ses Fil. 1948, 142, 241–243. [Google Scholar]
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of Circulating Tumour DNA in the Blood (Plasma/Serum) of Cancer Patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.V.; Capri, M.; Morsiani, C.; Pizza, G.; Faria, A.M.; Franceschi, C.; Neretti, N. Cell-free DNA as a biomarker of aging. Aging Cell 2019, 18, e12890. [Google Scholar] [CrossRef] [Green Version]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C.C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped by Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, G.C.; Petrone, A.B.; Tennant, C.S.; Lucke-Wold, N.; Kabbani, Y.; Tarabishy, A.R.; Chantler, P.D.; Barr, T.L. Circulating extracellular DNA levels are acutely elevated in ischaemic stroke and associated with innate immune system activation. Brain Inj. 2017, 31, 1369–1375. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.S.; Lee, Y.K.; Norton, J.A.; Jeffrey, S.S. Liquid biopsy in pancreatic ductal adenocarcinoma: Current status of circulating tumor cells and circulating tumorDNA. Mol. Oncol. 2019, 13, 1623–1650. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Eng, P.; Sali, A. Screening for Circulating Tumour Cells Allows Early Detection of Cancer and Monitoring of Treatment Effectiveness: An Observational Study. Asian Pac. J. Cancer Prev. 2017, 18, 2275–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passiglia, F.; Rizzo, S.; Di Maio, M.; Galvano, A.; Badalamenti, G.; Listì, A.; Gulotta, L.; Castiglia, M.; Fulfaro, F.; Bazan, V.; et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Li, S.; Cheng, H. Circulating DNA in EGFR-mutated lung cancer. Ann. Transl. Med. 2017, 5, 379. [Google Scholar] [CrossRef] [Green Version]
- Thierry, A.R.; Mouliere, F.; El Messaoudi, S.; Mollevi, C.; Crapez, E.; Rolet, F.; Gillet, B.; Gongora, C.; Dechelotte, P.; Robert, B.; et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 2014, 20, 430–435. [Google Scholar] [CrossRef]
- Jones, R.P.; Pugh, S.A.; Graham, J.; Primrose, J.N.; Barriuso, J. Circulating tumour DNA as a biomarker in resectable and irresectable stage IV colorectal cancer; a systematic review and meta-analysis. Eur. J. Cancer 2021, 144, 368–381. [Google Scholar] [CrossRef]
- Dawson, S.-J.; Tsui, D.W.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.-F.; Dunning, M.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.A.; Jacob, S.; Gerratana, L.; Shah, A.N.; Wehbe, F.; Katam, N.; Zhang, Q.; Flaum, L.; Siziopikou, K.P.; Platanias, L.C.; et al. Landscape of circulating tumour DNA in metastatic breast cancer. EBioMedicine 2020, 58, 102914. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Siravegna, G.; Blaszkowsky, L.S.; Corti, G.; Crisafulli, G.; Ahronian, L.G.; Mussolin, B.; Kwak, E.L.; Buscarino, M.; Lazzari, L.; et al. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016, 6, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nat. Cell Biol. 2012, 486, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Barbano, R.; Pasculli, B.; Coco, M.; Fontana, A.; Copetti, M.; Rendina, M.; Valori, V.M.; Graziano, P.; Maiello, E.; Fazio, V.M.; et al. Competitive allele-specific TaqMan PCR (Cast-PCR) is a sensitive, specific and fast method for BRAF V600 mutation detection in Melanoma patients. Sci. Rep. 2015, 5, 18592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinde, I.; Wu, J.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 9530–9535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef]
- Newman, A.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.W.; Modlin, L.A.; Liu, C.L.; Neal, J.W.; Wakelee, H.A.; Merritt, R.E.; et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014, 20, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.W.; Kennedy, S.R.; Salk, J.J.; Fox, E.; Hiatt, J.B.; Loeb, L.A. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 14508–14513. [Google Scholar] [CrossRef] [Green Version]
- Paweletz, C.P.; Sacher, A.; Raymond, C.K.; Alden, R.S.; O’Connell, A.; Mach, S.L.; Kuang, Y.; Gandhi, L.; Kirschmeier, P.; English, J.M.; et al. Bias-Corrected Targeted Next-Generation Sequencing for Rapid, Multiplexed Detection of Actionable Alterations in Cell-Free DNA from Advanced Lung Cancer Patients. Clin. Cancer Res. 2016, 22, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Liu, Y.; Fontana, R.; Makrigiorgos, A.; Mamon, H.; Kulke, M.H.; Makrigiorgos, G.M. Elimination of unaltered DNA in mixed clinical samples via nuclease-assisted minor-allele enrichment. Nucleic Acids Res. 2016, 44, e146. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Yu, J.; Hwang, G.-H.; Kim, S.; Kim, H.S.; Ye, S.; Kim, K.; Park, J.; Park, D.Y.; Cho, Y.-K.; et al. CUT-PCR: CRISPR-mediated, ultrasensitive detection of target DNA using PCR. Oncogene 2017, 36, 6823–6829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladas, I.; Yu, F.; Leong, K.W.; Fitarelli-Kiehl, M.; Song, C.; Ashtaputre, R.; Kulke, M.; Mamon, H.; Makrigiorgos, G.M. Enhanced detection of microsatellite instability using pre-PCR elimination of wild-type DNA homo-polymers in tissue and liquid biopsies. Nucleic Acids Res. 2018, 46, e74. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Song, C.; Ladas, I.; Fitarelli-Kiehl, M.; Makrigiorgos, G.M. Methylation-sensitive enrichment of minor DNA alleles using a double-strand DNA-specific nuclease. Nucleic Acids Res. 2016, 45, e39. [Google Scholar] [CrossRef] [Green Version]
- Salvianti, F.; Giuliani, C.; Petrone, L.; Mancini, I.; Vezzosi, V.; Pupilli, C.; Pinzani, P. Integrity and Quantity of Total Cell-Free DNA in the Diagnosis of Thyroid Cancer: Correlation with Cytological Classification. Int. J. Mol. Sci. 2017, 18, 1350. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. Review the Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Współczesna Onkol. 2015, 1A, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Ruggeri, R.M.; Giuffrè, G.; Siracusa, M.; Alibrandi, A.; Cardile, D.; La Torre, F.; Lanzafame, H.; Giacoppo, G.; Ieni, A.; et al. BRAFV600E mutation is associated with increased prevalence of contralateral lymph-node metastases in low and low-to-intermediate risk papillary thyroid cancer. Nucl. Med. Commun. 2021, 42, 611–618. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, O.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef] [Green Version]
- Elisei, R.; Viola, D.; Torregrossa, L.; Giannini, R.; Romei, C.; Ugolini, C.; Molinaro, E.; Agate, L.; Biagini, A.; Lupi, C.; et al. The BRAFV600E Mutation Is an Independent, Poor Prognostic Factor for the Outcome of Patients with Low-Risk Intrathyroid Papillary Thyroid Carcinoma: Single-Institution Results from a Large Cohort Study. J. Clin. Endocrinol. Metab. 2012, 97, 4390–4398. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. J. Clin. Oncol. 2015, 33, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients with Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Melillo, R.M.; Fusco, A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur. J. Endocrinol. 2006, 155, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. RET Gene Fusions in Malignancies of the Thyroid and Other Tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.A.; Pacini, F.; Robinson, B.G.; Santoro, M. Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update. J. Clin. Endocrinol. Metab. 2013, 98, 3149–3164. [Google Scholar] [CrossRef]
- Condello, V.; Macerola, E.; Ugolini, C.; De Napoli, L.; Romei, C.; Materazzi, G.; Elisei, R.; Basolo, F. Analysis of circulating tumor DNA does not improve the clinical management of patients with locally advanced and metastatic papillary thyroid carcinoma. Head Neck 2018, 40, 1752–1758. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Jeong, J.J.; Kang, S.-W.; Park, S.; Choi, J.R.; Park, S.-J.; Kim, E.K.; Chung, W.Y. Study of peripheral BRAFV600E mutation as a possible novel marker for papillary thyroid carcinomas. Head Neck 2012, 35, 1630–1633. [Google Scholar] [CrossRef]
- Jensen, K.; Thakur, S.; Patel, A.; Mendonca-Torres, M.C.; Costello, J.; Gomes-Lima, C.J.; Walter, M.; Wartofsky, L.; Burman, K.D.; Bikas, A.; et al. Detection of BRAFV600E in Liquid Biopsy from Patients with Papillary Thyroid Cancer Is Associated with Tumor Aggressiveness and Response to Therapy. J. Clin. Med. 2020, 9, 2481. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, I.J.; Lee, B.J.; Lee, J.C.; Kim, S.-J.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Kim, Y.K.; Kim, I.S. Detection of Plasma BRAFV600E Mutation is Associated with Lung Metastasis in Papillary Thyroid Carcinomas. Yonsei Med. J. 2015, 56, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Almubarak, H.; Qassem, E.; Alghofaili, L.; Alzahrani, A.S.; Karakas, B. Non-invasive Molecular Detection of Minimal Residual Disease in Papillary Thyroid Cancer Patients. Front. Oncol. 2020, 9. [Google Scholar] [CrossRef]
- Cote, G.J.; Evers, C.; Hu, M.I.; Grubbs, E.G.; Williams, M.D.; Hai, T.; Duose, D.Y.; Houston, M.R.; Bui, J.H.; Mehrotra, M.; et al. Prognostic Significance of Circulating RET M918T Mutated Tumor DNA in Patients with Advanced Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 3591–3599. [Google Scholar] [CrossRef]
- Allin, D.M.; Shaikh, R.; Carter, P.; Thway, K.; Sharabiani, M.T.A.; Gonzales-de-Castro, D. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur. J. Cancer 2018, 103, 165–175. [Google Scholar] [CrossRef]
- Sandulache, V.C.; Williams, M.D.; Lai, S.; Lu, C.; William, W.N.; Busaidy, N.L.; Cote, G.J.; Singh, R.R.; Luthra, R.; Cabanillas, M.E. Real-Time Genomic Characterization Utilizing Circulating Cell-Free DNA in Patients with Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, J.R.; Wang, Y.; Iyer, P.C.; Cote, G.J.; Busaidy, N.L.; Dadu, R.; Zafereo, M.; Williams, M.D.; Ferrarotto, R.; et al. Clinical Utility of Circulating Cell-Free DNA Mutations in Anaplastic Thyroid Carcinoma. Thyroid 2021. [Google Scholar] [CrossRef]
- Suh, Y.; Kwon, M.; Noh, H.-M.; Lee, H.; Ra, Y.; Kim, N. Limited Clinical and Diagnostic Utility of Circulating Tumor DNA Detection in Patients with Early-Stage Well-Differentiated Thyroid Cancer: Comparison with Benign Thyroid Nodules and Healthy Individuals. Healthcare 2021, 9, 386. [Google Scholar] [CrossRef]
- Pupilli, C.; Pinzani, P.; Salvianti, F.; Fibbi, B.; Rossi, M.; Petrone, L.; Perigli, G.; De Feo, M.L.; Vezzosi, V.; Pazzagli, M.; et al. CirculatingBRAFV600Ein the Diagnosis and Follow-Up of Differentiated Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 3359–3365. [Google Scholar] [CrossRef] [Green Version]
- Konda, B.; Shah, M.H.; Wei, L.; Espinosa, A.V.; Busaidy, N.L.; Wirth, L.J.; Daniels, G.A.; De Souza, J.A.; Sexton, J.L.; Beshara, M.; et al. Evaluation of BRAFV600E levels in cell free DNA (CFDNA) as a biomarker of response in BRAF V600E mutated radioactive iodine refractory (RAIR) differentiated thyroid cancer (DTC) treated with dabrafenib alone or in combination with trametinib. Thyroid 2017, 27 (Suppl. 1), A171–A172. [Google Scholar] [CrossRef]
- Besse, B.; Subbiah, V.; Drilon, A.; Shah, M.; Wirth, L.; Bauer, T.; Velcheti, V.; Lakhani, N.; Boni, V.; Solomon, B.; et al. Detection and clearance of RET variants in plasma cell free DNA (cfDNA) from patients (pts) treated with LOXO-292. Ann. Oncol. 2018, 29, viii33. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Cabanillas, M.E.; Sherman, S.I.; Habra, M.; Dadu, R.; Hu, M.I.; Jimenez, C.; Waguespack, S.E.; Subbiah, V.; Ying, A.; et al. Emergence of V804M resistance gatekeeper mutation in sporadic medullary thyroid carcinoma patients treated with TKI tyrosine kinase inhibitors. Thyroid 2017, 27 (Suppl. 1), A168. [Google Scholar] [CrossRef]
- Lupo, M.; Guttler, R.; Geck, Z.; Tonozzi, T.R.; Kammesheidt, A.; Braunstein, G.D. Is measurement of circulating tumor dna of diagnostic use in patients with thyroid nodules? Endocr. Pract. 2018, 24, 453–459. [Google Scholar] [CrossRef]
- Cao, S.; Yu, S.; Yin, Y.; Su, L.; Hong, S.; Gong, Y.; Lv, W.; Li, Y.; Xiao, H. Genetic alterations in cfDNA of benign and malignant thyroid nodules based on amplicon-based next-generation sequencing. Ann. Transl. Med. 2020, 8, 1225. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [Green Version]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Jin, Y.; Liu, M.; Ruan, M.; Chen, L. HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget 2017, 8, 19843–19854. [Google Scholar] [CrossRef] [Green Version]
- Gild, M.L.; Topliss, D.; Learoyd, D.; Parnis, F.; Tie, J.; Hughes, B.; Walsh, J.; McLeod, D.; Clifton-Bligh, R.J.; Robinson, B.G. Clinical guidance for radioiodine refractory differentiated thyroid cancer. Clin. Endocrinol. 2017, 88, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Wang, Y.; Chen, L.-L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Chou, C.-K.; Chen, R.-F.; Chou, F.-F.; Chang, H.-W.; Chen, Y.-J.; Lee, Y.-F.; Yang, K.D.; Cheng, J.-T.; Huang, C.-C.; Liu, R.-T. miR-146b is Highly Expressed in Adult Papillary Thyroid Carcinomas with High Risk Features Including Extrathyroidal Invasion and the BRAFV600E Mutation. Thyroid 2010, 20, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, C.; Luo, D.-C.; Ding, J.-W.; Zhang, W.; Pan, G. Expression Profile and Clinical Significance of MicroRNAs in Papillary Thyroid Carcinoma. Molecules 2014, 19, 11586–11599. [Google Scholar] [CrossRef] [Green Version]
- Acibucu, F.; Dökmetaş, H.S.; Tutar, Y.; Elagoz, Ş.; Kilicli, F. Correlations between the Expression Levels of Micro-RNA146b, 221, 222 and p27Kip1 protein mRNA and the Clinicopathologic Parameters in Papillary Thyroid Cancers. Exp. Clin. Endocrinol. Diabetes 2014, 122, 137–143. [Google Scholar] [CrossRef]
- Guo, Z.; Hardin, H.; Montemayor-Garcia, C.; Asioli, S.; Righi, A.; Maletta, F.; Sapino, A.; Lloyd, R.V. In Situ Hybridization Analysis of miR-146b-5p and miR-21 in Thyroid Nodules: Diagnostic Implications. Endocr. Pathol. 2015, 26, 157–163. [Google Scholar] [CrossRef]
- Sondermann, A.; Andreghetto, F.M.; Moulatlet, A.C.B.; Victor, E.D.S.; De Castro, M.G.; Nunes, F.D.; Brandão, L.G.; Severino, P. MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin. Exp. Metastasis 2015, 32, 521–530. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Chen, L.; Zheng, J.; Li, J.; Wu, X. MiR-221, a potential prognostic biomarker for recurrence in papillary thyroid cancer. World J. Surg. Oncol. 2017, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Wang, C.; Lu, H.; Chen, X.; Ba, Y.; Zhang, C.; Zhang, C.-Y. Altered Serum MicroRNA Profile May Serve as an Auxiliary Tool for Discriminating Aggressive Thyroid Carcinoma from Nonaggressive Thyroid Cancer and Benign Thyroid Nodules. Dis. Markers 2019, 2019, 3717683. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Song, Q.; Li, H.; Lou, Y.; Wang, L. Circulating miR-25-3p and miR-451a May Be Potential Biomarkers for the Diagnosis of Papillary Thyroid Carcinoma. PLoS ONE 2015, 10, e0132403. [Google Scholar] [CrossRef]
- Graham, M.E.R.; Hart, R.D.; Douglas, S.E.; Makki, F.M.; Pinto, D.M.; Butler, A.L.; Bullock, M.; Rigby, M.H.; Trites, J.R.B.; Taylor, S.M.; et al. Serum microRNA profiling to distinguish papillary thyroid cancer from benign thyroid masses. J. Otolaryngol. Head Neck Surg. 2015, 44, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosignolo, F.; Sponziello, M.; Giacomelli, L.; Russo, D.; Pecce, V.; Biffoni, M.; Bellantone, R.; Lombardi, C.P.; Lamartina, L.; Grani, G.; et al. Identification of Thyroid-Associated Serum microRNA Profiles and Their Potential Use in Thyroid Cancer Follow-Up. J. Endocr. Soc. 2017, 1, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Liu, Y.; Wang, J.; Guo, Z.; Zhang, Q.; Yu, F.; Zhang, Y.; Huang, K.; Li, Y.; Song, E.; et al. Circulating MicroRNA Profiles as Potential Biomarkers for Diagnosis of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 2084–2092. [Google Scholar] [CrossRef] [Green Version]
- Kondrotienė, A.; Daukša, A.; Pamedytytė, D.; Kazokaitė, M.; Žvirblienė, A.; Daukšienė, D.; Simanavičienė, V.; Klimaitė, R.; Golubickaitė, I.; Stakaitis, R.; et al. Plasma-Derived miRNA-222 as a Candidate Marker for Papillary Thyroid Cancer. Int. J. Mol. Sci. 2020, 21, 6445. [Google Scholar] [CrossRef] [PubMed]
- Yoruker, E.E.; Terzioglu, D.; Teksoz, S.; Uslu, F.E.; Gezer, U.; Dalay, N. MicroRNA Expression Profiles in Papillary Thyroid Carcinoma, Benign Thyroid Nodules and Healthy Controls. J. Cancer 2016, 7, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Gao, F.; Wang, Z.-Y.; Zhang, H.; Liu, Q.-X.; Jiang, L.; Zhou, X.; Zhu, W. A three-microRNA panel in serum as novel biomarker for papillary thyroid carcinoma diagnosis. Chin. Med. J. 2020, 133, 2543–2551. [Google Scholar] [CrossRef]
- Ferracin, M.; Lupini, L.; Salamon, I.; Saccenti, E.; Zanzi, M.V.; Rocchi, A.; Da Ros, L.; Zagatti, B.; Musa, G.; Bassi, C.; et al. Absolute quantification of cell-free microRNAs in cancer patients. Oncotarget 2015, 6, 14545–14555. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Lim, Y.S.; Lee, J.-C.; Wang, S.-G.; Park, H.-Y.; Kim, S.Y.; Lee, B.-J. Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncol. 2015, 51, 77–83. [Google Scholar] [CrossRef]
- Grawenda, A.M.; O’Neill, E. Clinical utility of RASSF1A methylation in human malignancies. Br. J. Cancer 2015, 113, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Oxnard, G.; Klein, E.; Swanton, C.; Seiden, M.; Smith, D.; Richards, D.; Yeatman, T.J.; Cohn, A.L.; Lapham, R.; et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Ihara, M.; Ashizawa, K.; Shichijo, K.; Kudo, T. Expression of the DNA-dependent protein kinase catalytic subunit is associated with the radiosensitivity of human thyroid cancer cell lines. J. Radiat. Res. 2019, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ewertz, M.; Tufano, R.P.; Brait, M.; Carvalho, A.L.; Liu, D.; Tufaro, A.P.; Basaria, S.; Cooper, D.S.; Sidransky, D.; et al. Detection of Serum Deoxyribonucleic Acid Methylation Markers: A Novel Diagnostic Tool for Thyroid Cancer. J. Clin. Endocrinol. Metab. 2006, 91, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Zane, M.; Agostini, M.; Enzo, M.V.; Ide, E.C.; Del Bianco, P.; Torresan, F.; Boschin, I.M.; Pennelli, G.; Saccani, A.; Rubello, D.; et al. Circulating cell-free DNA, SLC5A8 and SLC26A4 hypermethylation, BRAFV600E: A non-invasive tool panel for early detection of thyroid cancer. Biomed. Pharmacother. 2013, 67, 723–730. [Google Scholar] [CrossRef]

| Study | Subjects | Mutation | Detection Technique | Concordance of ctDNA to Tissue | Comments |
|---|---|---|---|---|---|
| Mutational Screening | |||||
| Condello et al. (2015) [59] | 22 PTC (n = 4 distant metastasis) | BRAFV600E | ddPCR qPCR | 0% | |
| Kwak et al. (2013) [60] | 94 PTC (n = 43; stage 3/4 disease) | BRAFV600E | qPCR | 0% | |
| Jensen et al. (2020) [61] | 57 PTC | BRAFV600E | ddPCR COLD PCR | 42.1% | Tumor size, gross ETE, pulmonary micro-metastases associated with increases |
| Kim et al. (2015) [62] | 49 PTC (n = 3 lung metastasis) | BRAFV600E | PCR | 6.1% | 100% concordance in 3 patients with lateral LN and lung metastasis |
| Almubarak et al. (2020) [63] | 28 DTC | BRAFV600E | ddPCR | 100% | |
| Cote et al. (2017) [64] | 50 MTC | RETM918T | ddPCR | 32% | Presence of RETm918T associated with worse clinical outcomes and calcitonin doubling in time |
| Allin et al. (2018) [65] | 42 advanced thyroid caner (15 PTC, 14 MTC, 10 FTC, 2 PDTC & 1 ATC) | BRAFV600E NRAS RETM918T | ddPCR | 67% (combined) | |
| Sandulache et al ( 2017) [66] | 23 ATC | BRAFV600E NRAS | NGS | 51.4% (combined) | Concordance higher with persistent disease |
| Qin et al. (2021) [67] | 87 ATC | BRAFV600E TERTp | NGS | 92.9% (BRAFV600E naïve) 83.7% (BRAFV600E previous treatment 5.8% (TERTp) | Higher concordance in treatment naive than previously treated |
| Suh et al. (2021) [68] | 62 early stage DTC | BRAFV600E TERTp NRAS KRAS | qPCR | 0% (combined) | |
| Treatment | |||||
| Pupilli et al. (2013) [69] | N = 19 patients tested pre and post operatively | BRAFV600E | qPCR | NA | ctDNA levels reduced 3–6 months post-operatively (p < 0.001) |
| Konda et al. (2017) [70] | 7 DTC | BRAFV600E | ddPCR | NA | Undetectable ctDNA following treatment with dabrafenib |
| Besse et al. (2018) [71] | 29 MTC 9 PTC | RET | NGS | 76% | Selpercatinib resulted in 50% reduction in variant allele frequency (VAF) in 79% of sampled patients following treatment |
| Busaidy et al. (2017) [72] | 16 MTC | RETM918T | ddPCR | 61.5% | 13 patients treated with TKI–8 with progressive disease developed new RET V804M mutations |
| Suspicious Nodules | |||||
| Salvianti et al. (2017) [49] | Bethesda 4 and 5 (n = 28) Bethesda 2 and 3 (n = 69) Healthy control | cfDNA | qPCR | NA | AUC of 0.765 (p < 0.001), 0.982 (p < 0.001) and 0.796 (p < 0.001) for cfDNA quantity by 67 bp amplicon, cfDNA quantity by 180 bp amplicon and integrity index, respectively |
| Lupo et al. (2018) [73] | 56 nodular diseases (13 malignant, 43 benign) | 96 gene Panel | NGS | NA | 1/13 malignant and 2/43 benign had positive ctDNA |
| Pupilli et al. (2013) [69] | 103 nodular gotire (n = 19 screened with bloods post operatively) | BRAFV600E | qPCR | NA | AUC = 0.797, p < 0.001 |
| Cao et al. (2020) [74] | 10 benign nodules 10 malignant nodules | 50 amplicon library panel | NGS | 0% |
| Study | Subjects | Sample | Sampling Time | Normalisation | Analysis Method | Differentially Expressed miRNA | AUC | |
| Yoruker et al., 2016 [95] | 31 PTC 31 MNG 24 HC | Serum | Pre-operative 5 weeks post-op | miR-16 EC | RT-qPCR | ↓ in PTC and MNG relative to HC ↓ in PTC postop relative to preop | miR-21 miR-31 miR-151-5p miR-221 miR-222 | |
| Zou et al., 2020 [96] | 100 PTC 30 MNG 96 HC | Serum exosomes | Pre-operative | C. elegans miR-39 spike-in control | 20 PTC, 20 MNG, 10 HC Screening cohort microarray Training, testing and Validation cohort RT-qPCR | ↑ in PTC relative to HC | miR-25-3p miR-296-5p miR-92a-3p Combined panel | 0.623 0.621 0.702 0.775 |
| Zhang et al., 2019 [89] | 100 PTC 15 MTC 91 BN 89 HC | Serum | Pre-operative | let-7d/g/I EC | TLDA screening RT-qPCR validation | ↑ in PTC and BN relative to HC ↓ in PTC and BN relative to HC ↑ in MTC relative to BN * and HC | miR-222-3p miR-17-5p miR-451a miR-146a-5p miR-132-3p miR-183-3p miR-222-3p miR-17-5p combined panel | 0.858 * 0.840 * 0.907 * |
| Graham et al., 2015 [91] | 18 PTC 13 BN | Serum | Pre-operative | MS2 bacteriophage RNA spike-in control | RT-qPCR | ↓ in PTC relative to BN ↑ in PTC relative to BN | miR-146a-5p miR-199b-3p miR-10a-5p let-7b-5p | |
| Rosignolo et al., 2017 [92] | 44 PTC 19 BN 20 HC | Serum | Pre-operative 30 days post-op | C. elegans miR-39 spike-in control | 11 PTC screening cohort TLDA RT-qPCR validation | ↓ in PTC postop relative to preop | miR-146a-5p miR-221-3p miR-222-3p | 0.653 * 0.730 * 0.587 * |
| Yu et al., 2012 [93] | 106 PTC 95 BN 44 HC | Serum | Pre-operative 5-15 days post-op | miR-16 EC | Solexa sequencing screening RT-qPCR validation | ↑ in PTC relative to BN and HC ↓ in PTC postop relative to preop | let-7e miR-151-5p miR-222 combined panel | 0.782 * 0.780 * 0.906 * 0.917 * |
| Ferracin et al., 2015 [97] | 27 TC 60 HC | Plasma | Pre-operative | C. elegans miR-39 spike-in control | ddPCR | ↑ in TC relative to HC | miR-181a-5p | 0.870 |
| Li et al., 2015 [90] | 56 PTC 95 BN 10 HC | Plasma | Pre-operative 4-7 days post-op | U6 RNA EC | microarray screening RT-qPCR validation | ↑ in PTC relative to BN and HC ↓ in PTC postop relative to preop α | miR-25-3pα miR-451aα miR-140-3p let-7i | 0.835 * 0.857 * |
| Lee et al., 2015 [98] | 70 PTC 19 BN | Plasma | Pre-operative | C. elegans miR-39 spike-in control | RT-qPCR | ↑ in PTC relative to BN | miR-146b miR-155 | 0.649 * 0.695 * |
| Kondrotiene et al., 2020 [94] | 49 PTC 23 MNG 57 HC | Plasma | Pre-operative 1 month post-op | C. elegans miR-39-3p spike-in control | RT-qPCR | ↑ in PTC relative to HC ↓ in PTC postop relative to preop | miR-221 miR-222 miR-146b miR-21 miR-181b miR-21 miR-221 miR-146b miR-181b | 0.711 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijewardene, A.A.; Chehade, M.; Gild, M.L.; Clifton-Bligh, R.J.; Bullock, M. Translational Utility of Liquid Biopsies in Thyroid Cancer Management. Cancers 2021, 13, 3443. https://doi.org/10.3390/cancers13143443
Wijewardene AA, Chehade M, Gild ML, Clifton-Bligh RJ, Bullock M. Translational Utility of Liquid Biopsies in Thyroid Cancer Management. Cancers. 2021; 13(14):3443. https://doi.org/10.3390/cancers13143443
Chicago/Turabian StyleWijewardene, Ayanthi A., Marthe Chehade, Matti L. Gild, Roderick J. Clifton-Bligh, and Martyn Bullock. 2021. "Translational Utility of Liquid Biopsies in Thyroid Cancer Management" Cancers 13, no. 14: 3443. https://doi.org/10.3390/cancers13143443
APA StyleWijewardene, A. A., Chehade, M., Gild, M. L., Clifton-Bligh, R. J., & Bullock, M. (2021). Translational Utility of Liquid Biopsies in Thyroid Cancer Management. Cancers, 13(14), 3443. https://doi.org/10.3390/cancers13143443







