Arginine Signaling and Cancer Metabolism
Abstract
:Simple Summary
Abstract
1. Introduction
2. Arginine and Cancer Metabolism
3. Arginine and Signal Transduction
3.1. Arginine Mediated Signals
3.2. Arginine Deprivation Induced Signals
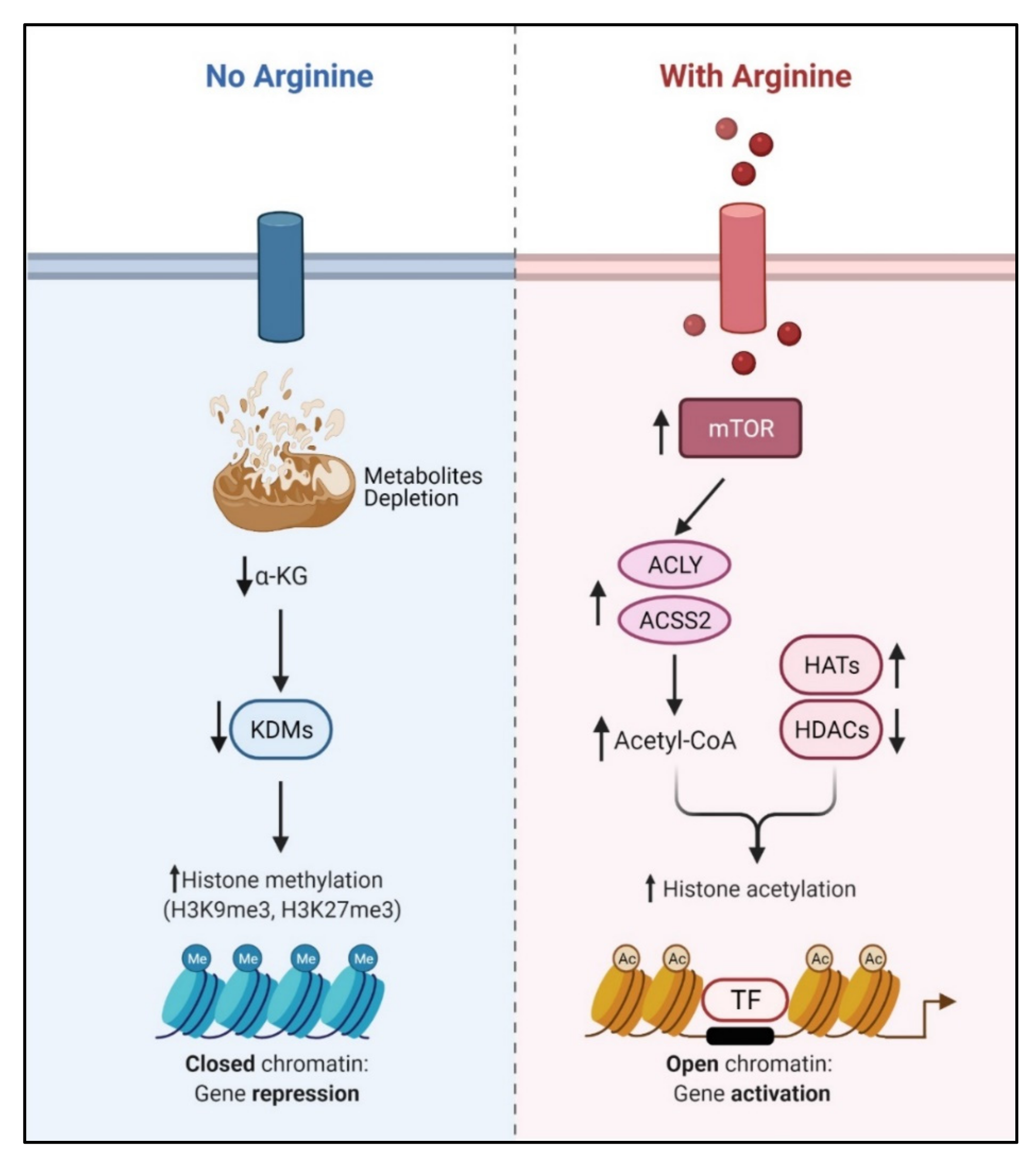
4. Arginine and Epigenetic Regulation
5. Arginine and Genome Integrity
6. Arginine and Immunomodulation
7. Arginine Deprivation and Cell Killing
7.1. Caspase-Dependent Apoptosis
7.2. Caspase-Independent Apoptosis
7.3. Caspase-Independent Autophagic Death
7.4. Necroptosis
8. Arginine Deprivation and Cancer Therapy
8.1. Preclinical Studies
8.2. Clinical Trials
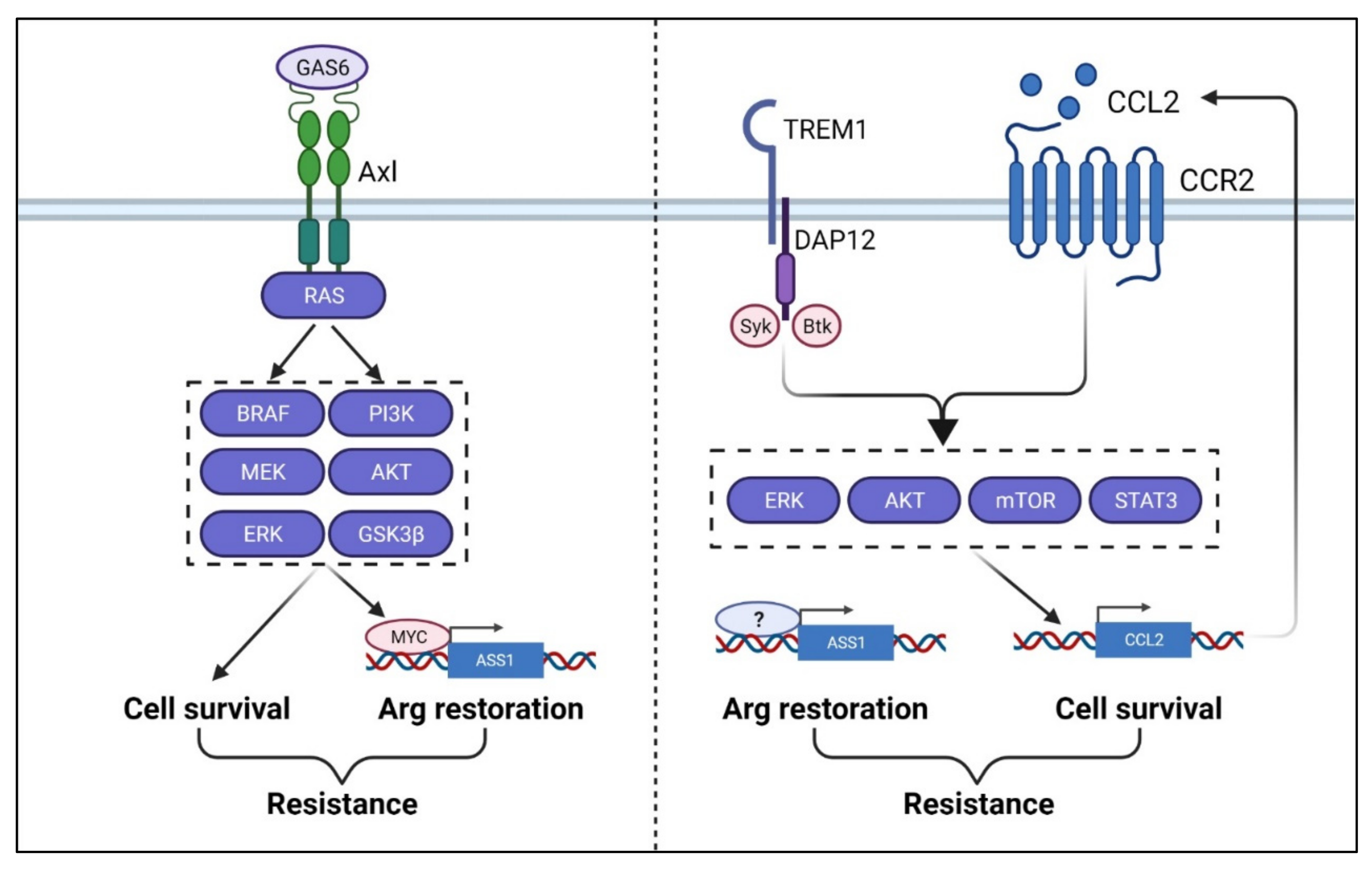
9. Arginine Deprivation and Therapy Resistance
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Bermudez, J.; Williams, R.T.; Guarecuco, R.; Birsoy, K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020, 33, 67–82. [Google Scholar] [CrossRef]
- Zou, S.; Wang, X.; Liu, P.; Ke, C.; Xu, S. Arginine metabolism and deprivation in cancer therapy. Biomed. Pharmacother. 2019, 118, 109210. [Google Scholar] [CrossRef] [PubMed]
- Riess, C.; Shokraie, F.; Classen, C.F.; Kreikemeyer, B.; Fiedler, T.; Junghanss, C.; Maletzki, C. Arginine-depleting enzymes–An increasingly recognized treatment strategy for therapy-refractory malignancies. Cell. Physiol. Biochem. 2018, 51, 854–870. [Google Scholar] [CrossRef]
- Rogers, L.C.; Van Tine, B.A. Innate and adaptive resistance mechanisms to arginine deprivation therapies in sarcoma and other cancers. Cancer Drug Resist. 2019, 2, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Fultang, L.; Vardon, A.; De Santo, C.; Mussai, F. Molecular basis and current strategies of therapeutic arginine depletion for cancer. Int. J. Cancer 2016, 139, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, M.D.; Bhaumik, J.; Babykutty, S.; Banerjee, U.C.; Fukumura, D. Arginine dependence of tumor cells: Targeting a chink in cancer’s armor. Oncogene 2016, 35, 4957–4972. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shaibe, E.; Metzer, E.; Halpern, Y.S. Control of utilization of L-arginine, L-ornithine, agmatine, and putrescine as nitrogen sources in Escherichia coli K-12. J. Bacteriol. 1985, 163, 938–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 2003, 270, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.P.; Grisolia, S. The intermediate role of carbamyl-L-glutamic acid in citrulline synthesis. J. Biol. Chem. 1948, 174, 389. [Google Scholar] [CrossRef]
- Hamilton, P.B. Proline: Synthesis from ornithine, citrulline, or arginine. J. Biol. Chem. 1952, 198, 587–597. [Google Scholar] [CrossRef]
- Levenberg, B. Role of L-glutamine as donor of carbamyl nitrogen for the enzymatic synthesis of citruline in Agaricus bisporus. J. Biol. Chem. 1962, 237, 2590–2598. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.R.; Liu, X.; Chu, C.Y.; Shen, L.J.; Xu, J.; Gaur, S.; Forman, H.J.; Zhang, H.; Zheng, S.; et al. Arginine starvation impairs mitochondrial respiratory function in ASS1-deficient breast cancer cells. Sci. Signal. 2014, 7, ra31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changou, C.A.; Chen, Y.R.; Xing, L.; Yen, Y.; Chuang, F.Y.; Cheng, R.H.; Bold, R.J.; Ann, D.K.; Kung, H.J. Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 14147–14152. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.T.; Qi, Y.; Wang, Y.C.; Chi, K.K.; Chung, Y.; Ouyang, C.; Chen, Y.R.; Oh, M.E.; Sheng, X.; Tang, Y.; et al. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun. Biol. 2018, 1, 178. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.Y.; Lee, Y.C.; Hsieh, C.H.; Yeh, C.T.; Chao, T.Y.; Chen, P.H.; Lin, I.H.; Hsieh, T.H.; Shih, J.W.; Cheng, C.H.; et al. Genome-wide CRISPR/Cas9 knockout screening uncovers a novel inflammatory pathway critical for resistance to arginine-deprivation therapy. Theranostics 2021, 11, 3624–3641. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, S.C.; Chung, T.Y.; Chu, C.Y.; Wang, H.J.; Hsiao, P.W.; Yeh, S.D.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine is an epigenetic regulator targeting TEAD4 to modulate OXPHOS in prostate cancer cells. Nat. Commun. 2021, 12, 2398. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Chen, C.-L.; Cheng, M.-L.; Chu, C.-Y.; Changou, C.A.; Yu, Y.-L.; Yeh, S.-D.; Kuo, T.-C.; Kuo, C.-C.; Chuu, C.-P.; et al. Arginine starvation elicits chromatin leakage and cGAS-STING activation via epigenetic silencing of metabolic and DNA-repair genes. Theranostics 2021, 11, 7527–7545. [Google Scholar] [CrossRef]
- Cheng, C.T.; Kuo, C.Y.; Ouyang, C.; Li, C.F.; Chung, Y.; Chan, D.C.; Kung, H.J.; Ann, D.K. Metabolic Stress-Induced Phosphorylation of KAP1 Ser473 Blocks Mitochondrial Fusion in Breast Cancer Cells. Cancer Res. 2016, 76, 5006–5018. [Google Scholar] [CrossRef] [Green Version]
- Kremer, J.C.; Prudner, B.C.; Lange, S.E.S.; Bean, G.R.; Schultze, M.B.; Brashears, C.B.; Radyk, M.D.; Redlich, N.; Tzeng, S.C.; Kami, K.; et al. Arginine Deprivation Inhibits the Warburg Effect and Upregulates Glutamine Anaplerosis and Serine Biosynthesis in ASS1-Deficient Cancers. Cell Rep. 2017, 18, 991–1004. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Tsai, W.B.; Wangpaichitr, M.; Tsukamoto, T.; Savaraj, N.; Feun, L.G.; Kuo, M.T. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol. Cancer Ther. 2013, 12, 2581–2590. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.B.; Aiba, I.; Long, Y.; Lin, H.K.; Feun, L.; Savaraj, N.; Kuo, M.T. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012, 72, 2622–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brashears, C.B.; Barlin, M.; Ehrhardt, W.R.; Rathore, R.; Schultze, M.; Tzeng, S.C.; Van Tine, B.A.; Held, J.M. Systems level profiling of arginine starvation reveals MYC and ERK adaptive metabolic reprogramming. Cell Death Dis. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tchernyshyov, I.; Chang, T.C.; Lee, Y.S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjarnahor, S.; Rodionov, R.N.; Konig, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zou, W. Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy. Mol. Cell 2020, 80, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.C.; Chen, Y.R.; Kensicki, E.; Li, A.Y.; Kong, M.; Li, Y.; Mohney, R.P.; Shen, H.M.; Stiles, B.; Mizushima, N.; et al. Autophagy: Resetting glutamine-dependent metabolism and oxygen consumption. Autophagy 2012, 8, 1477–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishton, R.J.; Sukumar, M.; Restifo, N.P. Arginine Arms T Cells to Thrive and Survive. Cell Metab. 2016, 24, 647–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.; Peng, F.; Xiao, X.; Gong, X.; Jiang, Y.; Zhang, M.; Tian, Y.; Xu, Y.; Ma, J.; Li, M.; et al. Hepatitis B virus X protein-induced upregulation of CAT-1 stimulates proliferation and inhibits apoptosis in hepatocellular carcinoma cells. Oncotarget 2017, 8, 60962–60974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Wang, W.; Wang, J.; Yang, C.; Mao, H.; Fu, X.; Wu, Y.; Cai, J.; Han, J.; Xu, Z.; et al. Overexpression of arginine transporter CAT-1 is associated with accumulation of L-arginine and cell growth in human colorectal cancer tissue. PLoS ONE 2013, 8, e73866. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.A.; Rickard, J.A.; McDonald, W.J.; Thomas, L.N.; Too, C.K. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J. Cell Biochem. 2011, 112, 1084–1092. [Google Scholar] [CrossRef]
- Werner, A.; Pieh, D.; Echchannaoui, H.; Rupp, J.; Rajalingam, K.; Theobald, M.; Closs, E.I.; Munder, M. Cationic Amino Acid Transporter-1-Mediated Arginine Uptake Is Essential for Chronic Lymphocytic Leukemia Cell Proliferation and Viability. Front. Oncol. 2019, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Amann, E.; Schnitzius, V.; Habermeier, A.; Luckner-Minden, C.; Leuchtner, N.; Rupp, J.; Closs, E.I.; Munder, M. Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur. J. Immunol. 2016, 46, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta 2005, 1741, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Prasad, P.D.; Ghamande, S.; Moore-Martin, P.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Mager, S.; Ganapathy, M.E.; Ganapathy, V. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol. Oncol. 2006, 100, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.L.; Corchete, L.; Teodosio, C.; Sarasquete, M.E.; del Mar Abad, M.; Iglesias, M.; Esteban, C.; Sayagues, J.M.; Orfao, A.; Munoz-Bellvis, L. Identification and characterization of the gene expression profiles for protein coding and non-coding RNAs of pancreatic ductal adenocarcinomas. Oncotarget 2015, 6, 19070–19086. [Google Scholar] [CrossRef] [Green Version]
- Babu, E.; Bhutia, Y.D.; Ramachandran, S.; Gnanaprakasam, J.P.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem. J. 2015, 469, 17–23. [Google Scholar] [CrossRef]
- Mossner, J.; Hammermann, R.; Racke, K. Concomitant down-regulation of L-arginine transport and nitric oxide (NO) synthesis in rat alveolar macrophages by the polyamine spermine. Pulm. Pharmacol. Ther. 2001, 14, 297–305. [Google Scholar] [CrossRef]
- Lowman, X.H.; Hanse, E.A.; Yang, Y.; Ishak Gabra, M.B.; Tran, T.Q.; Li, H.; Kong, M. p53 Promotes Cancer Cell Adaptation to Glutamine Deprivation by Upregulating Slc7a3 to Increase Arginine Uptake. Cell Rep. 2019, 26, 3051–3060.e4. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Cao, Y.; Wang, Y.; Li, W.; Liu, X.; Lv, Y.; Li, X.; Mi, J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 2017, 7, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Han, T.; Bian, Y.; Tong, H.; Wen, X.; Li, Y.; Wan, X. Knockdown of SLCO4C1 inhibits cell proliferation and metastasis in endometrial cancer through inactivating the PI3K/Akt signaling pathway. Oncol. Rep. 2020, 43, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Qian, Y.; Li, X.; Xu, J.; Kang, W.; Tong, J.H.; To, K.F.; Jin, Y.; Li, W.; Chen, H.; et al. SLC25A22 Promotes Proliferation and Survival of Colorectal Cancer Cells with KRAS Mutations and Xenograft Tumor Progression in Mice via Intracellular Synthesis of Aspartate. Gastroenterology 2016, 151, 945–960.e6. [Google Scholar] [CrossRef] [Green Version]
- Ban, H.; Shigemitsu, K.; Yamatsuji, T.; Haisa, M.; Nakajo, T.; Takaoka, M.; Nobuhisa, T.; Gunduz, M.; Tanaka, N.; Naomoto, Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int. J. Mol. Med. 2004, 13, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, J.L.; Guan, K.L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazian, D.; Roux, P.P.; Mieulet, V.; Cohen, M.S.; Raught, B.; Taunton, J.; Hershey, J.W.; Blenis, J.; Pende, M.; Sonenberg, N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006, 25, 2781–2791. [Google Scholar] [CrossRef]
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. eLife 2016, 5, e11058. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Tan, B.; Yin, Y.; Gao, H.; Li, X.; Jaeger, L.A.; Bazer, F.W.; Wu, G. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 2012, 23, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, B.; Wellendorph, P.; Brauner-Osborne, H. Known regulators of nitric oxide synthase and arginase are agonists at the human G-protein-coupled receptor GPRC6A. Br. J. Pharm. 2006, 147, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-arginine stimulates fibroblast proliferation through the GPRC6A-ERK1/2 and PI3K/Akt pathway. PLoS ONE 2014, 9, e92168. [Google Scholar]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. GPRC6A: Jack of all metabolism (or master of none). Mol. Metab. 2017, 6, 185–193. [Google Scholar] [CrossRef]
- Pi, M.; Quarles, L.D. GPRC6A regulates prostate cancer progression. Prostate 2012, 72, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Keshet, R.; Erez, A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis. Model. Mech. 2018, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef]
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signalling by thrombospondin 1: Implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Ambs, S.; Ogunfusika, M.O.; Merriam, W.G.; Bennett, W.P.; Billiar, T.R.; Harris, C.C. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc. Natl. Acad. Sci. USA 1998, 95, 8823–8828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisavljevic, Z. Inactivated tumor suppressor Rb by nitric oxide promotes mitosis in human breast cancer cells. J. Cell Biochem. 2004, 92, 1–5. [Google Scholar] [CrossRef]
- Chen, C.N.; Hsieh, F.J.; Cheng, Y.M.; Chang, K.J.; Lee, P.H. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J. Surg. Oncol. 2006, 94, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, S.G.; Song, S.H.; Heo, D.S.; Park, B.J.; Lee, D.W.; Kim, K.H.; Sung, M.W. The effect of nitric oxide on cyclooxygenase-2 (COX-2) overexpression in head and neck cancer cell lines. Int. J. Cancer 2003, 107, 729–738. [Google Scholar] [CrossRef]
- Rahman, M.A.; Dhar, D.K.; Yamaguchi, E.; Maruyama, S.; Sato, T.; Hayashi, H.; Ono, T.; Yamanoi, A.; Kohno, H.; Nagasue, N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: Possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin. Cancer Res. 2001, 7, 1325–1332. [Google Scholar] [PubMed]
- Garrido, P.; Shalaby, A.; Walsh, E.M.; Keane, N.; Webber, M.; Keane, M.M.; Sullivan, F.J.; Kerin, M.J.; Callagy, G.; Ryan, A.E.; et al. Impact of inducible nitric oxide synthase (iNOS) expression on triple negative breast cancer outcome and activation of EGFR and ERK signaling pathways. Oncotarget 2017, 8, 80568–80588. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rivera, E.; Jayaraman, P.; Parikh, F.; Davies, M.A.; Ekmekcioglu, S.; Izadmehr, S.; Milton, D.R.; Chipuk, J.E.; Grimm, E.A.; Estrada, Y.; et al. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res. 2014, 74, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Poillet-Perez, L.; Xie, X.; Zhan, L.; Yang, Y.; Sharp, D.W.; Hu, Z.S.; Su, X.; Maganti, A.; Jiang, C.; Lu, W.; et al. Autophagy maintains tumour growth through circulating arginine. Nature 2018, 563, 569–573. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.F.; Chow, H.Y.; Choi, S.C.; Leung, Y.C. Arginine deprivation inhibits pancreatic cancer cell migration, invasion and EMT via the down regulation of Snail, Slug, Twist, and MMP1/9. J. Physiol. Biochem. 2020, 76, 73–83. [Google Scholar] [CrossRef]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.; Bold, R.J.; Kung, H.J. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009, 69, 700–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef] [Green Version]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Chen, X.; Zang, A.; Li, T.; Hu, Y.; Ma, S.; Lu, M.; Yin, H.; Wang, H.; Zhang, X.; et al. TSC1/mTOR-controlled metabolic-epigenetic cross talk underpins DC control of CD8+ T-cell homeostasis. PLoS Biol. 2019, 17, e3000420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef] [Green Version]
- Robitaille, A.M.; Christen, S.; Shimobayashi, M.; Cornu, M.; Fava, L.L.; Moes, S.; Prescianotto-Baschong, C.; Sauer, U.; Jenoe, P.; Hall, M.N. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013, 339, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Kim, S.S.; Xu, S.; Cui, J.; Poddar, S.; Le, T.M.; Hayrapetyan, H.; Li, L.; Wu, N.; Moore, A.M.; Zhou, L.; et al. Histone deacetylase inhibition is synthetically lethal with arginine deprivation in pancreatic cancers with low argininosuccinate synthetase 1 expression. Theranostics 2020, 10, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Su, G.C.; Huang, W.Y.; Ko, M.Y.; Yeh, H.Y.; Chang, G.D.; Lin, S.J.; Chi, P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019, 10, 65. [Google Scholar] [CrossRef]
- Snyder, R.D.; Lachmann, P.J. Hyperthermia, polyamine depletion, and inhibition of X-ray-induced DNA strand break repair. Radiat. Res. 1989, 120, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.D.; Sunkara, P.S. Effect of polyamine depletion on DNA damage and repair following UV irradiation of HeLa cells. Photochem. Photobiol. 1990, 52, 525–532. [Google Scholar] [CrossRef]
- Locke, M.; Ghazaly, E.; Freitas, M.O.; Mitsinga, M.; Lattanzio, L.; Lo Nigro, C.; Nagano, A.; Wang, J.; Chelala, C.; Szlosarek, P.; et al. Inhibition of the Polyamine Synthesis Pathway Is Synthetically Lethal with Loss of Argininosuccinate Synthase 1. Cell Rep. 2016, 16, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Arginine: Master and commander in innate immune responses. Sci. Signal. 2010, 3, pe27. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [Green Version]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Zea, A.H.; DeSalvo, J.; Culotta, K.S.; Zabaleta, J.; Quiceno, D.G.; Ochoa, J.B.; Ochoa, A.C. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 2003, 171, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008, 68, 5439–5449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [Green Version]
- Steggerda, S.M.; Bennett, M.K.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.R.; Li, W.; MacKinnon, A.L.; Makkouk, A.; Marguier, G.; et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 2017, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Miranda, K.M.; Thomas, D.D.; Xavier, S.; Citrin, D.; Vitek, M.P.; Wink, D.A. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen oxide species. Ann. N. Y. Acad. Sci. 2002, 962, 195–206. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Gonzalez-Cotto, M.; Baseler, W.A.; Davies, L.C.; Ghesquiere, B.; Maio, N.; Rice, C.M.; Rouault, T.A.; Cassel, T.; Higashi, R.M.; et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020, 11, 698. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef] [Green Version]
- Qualls, J.E.; Neale, G.; Smith, A.M.; Koo, M.S.; DeFreitas, A.A.; Zhang, H.; Kaplan, G.; Watowich, S.S.; Murray, P.J. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 2010, 3, ra62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, N.D.; Asim, M.; Barry, D.P.; de Sablet, T.; Singh, K.; Piazuelo, M.B.; Gobert, A.P.; Chaturvedi, R.; Wilson, K.T. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J. Immunol. 2011, 186, 3632–3641. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, U.; Paduch, K.; Debus, A.; Obermeyer, S.; Konig, T.; Kling, J.C.; Ribechini, E.; Dudziak, D.; Mougiakakos, D.; Murray, P.J.; et al. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016, 15, 1062–1075. [Google Scholar] [CrossRef] [Green Version]
- Brin, E.; Wu, K.; Lu, H.T.; He, Y.; Dai, Z.; He, W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget 2017, 8, 58948–58963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Hu, H. Arginine Deiminase Induces Immunogenic Cell Death and Is Enhanced by N-acetylcysteine in Murine MC38 Colorectal Cancer Cells and MDA-MB-231 Human Breast Cancer Cells In Vitro. Molecules 2021, 26, 511. [Google Scholar] [CrossRef]
- Werner, A.; Koschke, M.; Leuchtner, N.; Luckner-Minden, C.; Habermeier, A.; Rupp, J.; Heinrich, C.; Conradi, R.; Closs, E.I.; Munder, M. Reconstitution of T Cell Proliferation under Arginine Limitation: Activated Human T Cells Take Up Citrulline via L-Type Amino Acid Transporter 1 and Use It to Regenerate Arginine after Induction of Argininosuccinate Synthase Expression. Front. Immunol. 2017, 8, 864. [Google Scholar] [CrossRef] [Green Version]
- Moynihan, K.D.; Irvine, D.J. Roles for Innate Immunity in Combination Immunotherapies. Cancer Res. 2017, 77, 5215–5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Klabatsa, A.; Pallaska, A.; Sheaff, M.; Smith, P.; Crook, T.; Grimshaw, M.J.; Steele, J.P.; Rudd, R.M.; Balkwill, F.R.; et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin. Cancer Res. 2006, 12, 7126–7131. [Google Scholar] [CrossRef] [Green Version]
- Delage, B.; Luong, P.; Maharaj, L.; O'Riain, C.; Syed, N.; Crook, T.; Hatzimichael, E.; Papoudou-Bai, A.; Mitchell, T.J.; Whittaker, S.J.; et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012, 3, e342. [Google Scholar] [CrossRef]
- Bowles, T.L.; Kim, R.; Galante, J.; Parsons, C.M.; Virudachalam, S.; Kung, H.J.; Bold, R.J. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int. J. Cancer 2008, 123, 1950–1955. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.X.; Cochrane, D.R.; Tessier-Cloutier, B.; Chen, S.Y.; Ho, G.; Pathak, K.V.; Alcazar, I.N.; Farnell, D.; Leung, S.; Cheng, A.; et al. Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase-Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type. Clin. Cancer Res. 2020, 26, 4402–4413. [Google Scholar] [CrossRef]
- Bean, G.R.; Kremer, J.C.; Prudner, B.C.; Schenone, A.D.; Yao, J.C.; Schultze, M.B.; Chen, D.Y.; Tanas, M.R.; Adkins, D.R.; Bomalaski, J.; et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016, 7, e2406. [Google Scholar] [CrossRef]
- Noh, E.J.; Kang, S.W.; Shin, Y.J.; Choi, S.H.; Kim, C.G.; Park, I.S.; Wheatley, D.N.; Min, B.H. Arginine deiminase enhances dexamethasone-induced cytotoxicity in human T-lymphoblastic leukemia CCRF-CEM cells. Int. J. Cancer 2004, 112, 502–508. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, S.; Xiao, D.; Bian, X.; Wang, J.; Wang, Y.; Zhou, H.; Cai, J.; Zheng, Z. Arginine deiminase expressed in vivo, driven by human telomerase reverse transcriptase promoter, displays high hepatoma targeting and oncolytic efficiency. Oncotarget 2017, 8, 37694–37704. [Google Scholar] [CrossRef] [Green Version]
- Savaraj, N.; Wu, C.; Kuo, M.T.; You, M.; Wangpaichitr, M.; Robles, C.; Spector, S.; Feun, L. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target. Insights 2007, 2, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Syed, N.; Langer, J.; Janczar, K.; Singh, P.; Lo Nigro, C.; Lattanzio, L.; Coley, H.M.; Hatzimichael, E.; Bomalaski, J.; Szlosarek, P.; et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013, 4, e458. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.P.; Jungbluth, A.A.; Wu, B.W.; Bomalaski, J.; Old, L.J.; Ritter, G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br. J. Cancer 2012, 106, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Bian, X.; Liu, X.; Wang, Y.; Zhou, H.; Ma, X.; Quan, C.; Yao, Y.; Zheng, Z. Intracellular expression of arginine deiminase activates the mitochondrial apoptosis pathway by inhibiting cytosolic ferritin and inducing chromatin autophagy. BMC Cancer 2020, 20, 665. [Google Scholar] [CrossRef]
- Khalil, N.; Abi-Habib, R.J. [HuArgI (co)-PEG5000]-induced arginine deprivation leads to autophagy dependent cell death in pancreatic cancer cells. Investig. New Drugs 2020, 38, 1236–1246. [Google Scholar] [CrossRef]
- Gaude, E.; Frezza, C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat. Commun. 2016, 7, 13041. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Lo, P.H.Y.; Saichi, N.; Ueda, K.; Hirata, M.; Tanikawa, C.; Matsuda, K. Argininosuccinate synthase 1 is an intrinsic Akt repressor transactivated by p53. Sci. Adv. 2017, 3, e1603204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Wu, W.R.; Wang, Y.H.; Wang, J.W.; Fang, F.M.; Tsai, J.W.; Li, S.H.; Hung, H.C.; Yu, S.C.; Lan, J.; et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: Aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin. Cancer Res. 2013, 19, 2861–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, T.; Cheng, P.N.; Chan, P.; Chan, W.; Chen, L.; Yuen, J.; Pang, R.; Fan, S.T.; Poon, R.T. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Investig. New Drugs 2013, 31, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar] [PubMed]
- Ott, P.A.; Carvajal, R.D.; Pandit-Taskar, N.; Jungbluth, A.A.; Hoffman, E.W.; Wu, B.W.; Bomalaski, J.S.; Venhaus, R.; Pan, L.; Old, L.J.; et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Investig. New Drugs 2013, 31, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Gupta, S.; Hau, A.M.; Nakashima, K.; Leivo, M.Z.; Searles, S.C.; Elson, P.; Bomalaski, J.S.; Casteel, D.E.; Boss, G.R.; et al. Argininosuccinate Synthetase 1 Loss in Invasive Bladder Cancer Regulates Survival through General Control Nonderepressible 2 Kinase-Mediated Eukaryotic Initiation Factor 2alpha Activity and Is Targetable by Pegylated Arginine Deiminase. Am. J. Pathol. 2017, 187, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Roeksomtawin, S.; Navasumrit, P.; Waraprasit, S.; Parnlob, V.; Sricharunrat, T.; Bhudhisawasdi, V.; Savaraj, N.; Ruchirawat, M. Decreased argininosuccinate synthetase expression in Thai patients with cholangiocarcinoma and the effects of ADI-PEG20 treatment in CCA cell lines. Oncol. Lett. 2018, 16, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Burrows, N.; Cane, G.; Robson, M.; Gaude, E.; Howat, W.J.; Szlosarek, P.W.; Pedley, R.B.; Frezza, C.; Ashcroft, M.; Maxwell, P.H. Hypoxia-induced nitric oxide production and tumour perfusion is inhibited by pegylated arginine deiminase (ADI-PEG20). Sci. Rep. 2016, 6, 22950. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tsai, S.T.; Kuo, C.C.; Chang, J.S.; Jin, Y.T.; Chang, J.Y.; Hsiao, J.R. Arginine deprivation as a new treatment strategy for head and neck cancer. Oral. Oncol. 2012, 48, 1227–1235. [Google Scholar] [CrossRef]
- Tsai, W.B.; Aiba, I.; Lee, S.Y.; Feun, L.; Savaraj, N.; Kuo, M.T. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol. Cancer 2009, 8, 3223–3233. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.B.; Long, Y.; Park, J.R.; Chang, J.T.; Liu, H.; Rodriguez-Canales, J.; Savaraj, N.; Feun, L.G.; Davies, M.A.; Wistuba, I.I.; et al. Gas6/Axl is the sensor of arginine-auxotrophic response in targeted chemotherapy with arginine-depleting agents. Oncogene 2016, 35, 1632–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Tsai, W.B.; Wang, D.; Hawke, D.H.; Savaraj, N.; Feun, L.G.; Hung, M.C.; Chen, H.H.; Kuo, M.T. Argininosuccinate synthetase 1 (ASS1) is a common metabolic marker of chemosensitivity for targeted arginine- and glutamine-starvation therapy. Cancer Lett. 2017, 388, 54–63. [Google Scholar] [CrossRef]
- Cheon, D.J.; Walts, A.E.; Beach, J.A.; Lester, J.; Bomalaski, J.S.; Walsh, C.S.; Ruprecht Wiedemeyer, W.; Karlan, B.Y.; Orsulic, S. Differential expression of argininosuccinate synthetase in serous and non-serous ovarian carcinomas. J. Pathol. Clin. Res. 2015, 1, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Deorukhkar, A.A.; Venkatesulu, B.P.; Li, X.; Tailor, R.; Bomalaski, J.S.; Krishnan, S. Exploiting Arginine Auxotrophy with Pegylated Arginine Deiminase (ADI-PEG20) to Sensitize Pancreatic Cancer to Radiotherapy via Metabolic Dysregulation. Mol. Cancer Ther. 2019, 18, 2381–2393. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; MacKenzie, E.D.; Karim, S.A.; Hedley, A.; Blyth, K.; Kalna, G.; Watson, D.G.; Szlosarek, P.; Frezza, C.; Gottlieb, E. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 2013, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Chalishazar, M.D.; Wait, S.J.; Huang, F.; Ireland, A.S.; Mukhopadhyay, A.; Lee, Y.; Schuman, S.S.; Guthrie, M.R.; Berrett, K.C.; Vahrenkamp, J.M.; et al. MYC-Driven Small-Cell Lung Cancer is Metabolically Distinct and Vulnerable to Arginine Depletion. Clin. Cancer Res. 2019, 25, 5107–5121. [Google Scholar] [CrossRef] [Green Version]
- Yeon, A.; You, S.; Kim, M.; Gupta, A.; Park, M.H.; Weisenberger, D.J.; Liang, G.; Kim, J. Rewiring of cisplatin-resistant bladder cancer cells through epigenetic regulation of genes involved in amino acid metabolism. Theranostics 2018, 8, 4520–4534. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.A.; Lu, H.T.; Wu, K.C.; Knowles, S.K.; Thomson, J.A. Down-regulation of argininosuccinate synthetase is associated with cisplatin resistance in hepatocellular carcinoma cell lines: Implications for PEGylated arginine deiminase combination therapy. BMC Cancer 2014, 14, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savaraj, N.; Wu, C.; Li, Y.Y.; Wangpaichitr, M.; You, M.; Bomalaski, J.; He, W.; Kuo, M.T.; Feun, L.G. Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 2015, 6, 6295–6309. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Tsai, W.B.; Chang, J.T.; Estecio, M.; Wangpaichitr, M.; Savaraj, N.; Feun, L.G.; Chen, H.H.; Kuo, M.T. Cisplatin-induced synthetic lethality to arginine-starvation therapy by transcriptional suppression of ASS1 is regulated by DEC1, HIF-1alpha, and c-Myc transcription network and is independent of ASS1 promoter DNA methylation. Oncotarget 2016, 7, 82658–82670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudner, B.C.; Rathore, R.; Robinson, A.M.; Godec, A.; Chang, S.F.; Hawkins, W.G.; Hirbe, A.C.; Van Tine, B.A. Arginine Starvation and Docetaxel Induce c-Myc-Driven hENT1 Surface Expression to Overcome Gemcitabine Resistance in ASS1-Negative Tumors. Clin. Cancer Res. 2019, 25, 5122–5134. [Google Scholar] [CrossRef] [Green Version]
- Thongkum, A.; Wu, C.; Li, Y.Y.; Wangpaichitr, M.; Navasumrit, P.; Parnlob, V.; Sricharunrat, T.; Bhudhisawasdi, V.; Ruchirawat, M.; Savaraj, N. The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma. Int. J. Mol. Sci. 2017, 18, 1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daylami, R.; Muilenburg, D.J.; Virudachalam, S.; Bold, R.J. Pegylated arginine deiminase synergistically increases the cytotoxicity of gemcitabine in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2014, 33, 102. [Google Scholar] [CrossRef]
- Alexandrou, C.; Al-Aqbi, S.S.; Higgins, J.A.; Boyle, W.; Karmokar, A.; Andreadi, C.; Luo, J.L.; Moore, D.A.; Viskaduraki, M.; Blades, M.; et al. Sensitivity of Colorectal Cancer to Arginine Deprivation Therapy is Shaped by Differential Expression of Urea Cycle Enzymes. Sci. Rep. 2018, 8, 12096. [Google Scholar] [CrossRef]
- Przystal, J.M.; Hajji, N.; Khozoie, C.; Renziehausen, A.; Zeng, Q.; Abaitua, F.; Hajitou, A.; Suwan, K.; Want, E.; Bomalaski, J.; et al. Efficacy of arginine depletion by ADI-PEG20 in an intracranial model of GBM. Cell Death Dis. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Wangpaichitr, M.; Wu, C.; Bigford, G.; Theodoropoulos, G.; You, M.; Li, Y.Y.; Verona-Santos, J.; Feun, L.G.; Nguyen, D.M.; Savaraj, N. Combination of arginine deprivation with TRAIL treatment as a targeted-therapy for mesothelioma. Anticancer Res. 2014, 34, 6991–6999. [Google Scholar] [PubMed]
- You, M.; Savaraj, N.; Kuo, M.T.; Wangpaichitr, M.; Varona-Santos, J.; Wu, C.; Nguyen, D.M.; Feun, L. TRAIL induces autophagic protein cleavage through caspase activation in melanoma cell lines under arginine deprivation. Mol. Cell Biochem. 2013, 374, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.B.; Long, Y.; Chang, J.T.; Savaraj, N.; Feun, L.G.; Jung, M.; Chen, H.H.W.; Kuo, M.T. Chromatin remodeling system p300-HDAC2-Sin3A is involved in Arginine Starvation-Induced HIF-1alpha Degradation at the ASS1 promoter for ASS1 Derepression. Sci. Rep. 2017, 7, 10814. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.T.; Long, Y.; Tsai, W.B.; Li, Y.Y.; Chen, H.H.W.; Feun, L.G.; Savaraj, N. Collaboration Between RSK-EphA2 and Gas6-Axl RTK Signaling in Arginine Starvation Response That Confers Resistance to EGFR Inhibitors. Transl. Oncol. 2020, 13, 355–364. [Google Scholar] [CrossRef]
- Dillon, B.J.; Holtsberg, F.W.; Ensor, C.M.; Bomalaski, J.S.; Clark, M.A. Biochemical characterization of the arginine degrading enzymes arginase and arginine deiminase and their effect on nitric oxide production. Med. Sci Monit. 2002, 8, BR248–BR253. [Google Scholar]
- Agrawal, V.; Woo, J.H.; Mauldin, J.P.; Jo, C.; Stone, E.M.; Georgiou, G.; Frankel, A.E. Cytotoxicity of human recombinant arginase I (Co)-PEG5000 in the presence of supplemental L-citrulline is dependent on decreased argininosuccinate synthetase expression in human cells. Anticancer Drugs 2012, 23, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Basu, A.; Palm, T.; Hua, J.; Youngster, S.; Hwang, L.; Liu, H.C.; Li, X.; Peng, P.; Zhang, Y.; et al. Engineering an arginine catabolizing bioconjugate: Biochemical and pharmacological characterization of PEGylated derivatives of arginine deiminase from Mycoplasma arthritidis. Bioconjug. Chem. 2006, 17, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Qin, S.; Ryoo, B.Y.; Lu, S.N.; Yen, C.J.; Feng, Y.H.; Lim, H.Y.; Izzo, F.; Colombo, M.; Sarker, D.; et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018, 29, 1402–1408. [Google Scholar] [CrossRef]
- Hall, P.E.; Ready, N.; Johnston, A.; Bomalaski, J.S.; Venhaus, R.R.; Sheaff, M.; Krug, L.; Szlosarek, P.W. Phase II Study of Arginine Deprivation Therapy With Pegargiminase in Patients With Relapsed Sensitive or Refractory Small-cell Lung Cancer. Clin. Lung Cancer 2020, 21, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Hsiao, H.H.; Hsu, Y.T.; Liu, Y.C.; Kao, H.W.; Liu, T.C.; Cho, S.F.; Feng, X.; Johnston, A.; Bomalaski, J.S.; et al. Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer Med. 2021, 10, 2946–2955. [Google Scholar] [CrossRef]
- Tsai, H.J.; Jiang, S.S.; Hung, W.C.; Borthakur, G.; Lin, S.F.; Pemmaraju, N.; Jabbour, E.; Bomalaski, J.S.; Chen, Y.P.; Hsiao, H.H.; et al. A Phase II Study of Arginine Deiminase (ADI-PEG20) in Relapsed/Refractory or Poor-Risk Acute Myeloid Leukemia Patients. Sci. Rep. 2017, 7, 11253. [Google Scholar] [CrossRef] [Green Version]
- Lowery, M.A.; Yu, K.H.; Kelsen, D.P.; Harding, J.J.; Bomalaski, J.S.; Glassman, D.C.; Covington, C.M.; Brenner, R.; Hollywood, E.; Barba, A.; et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer 2017, 123, 4556–4565. [Google Scholar] [CrossRef]
- Harding, J.J.; Do, R.K.; Dika, I.E.; Hollywood, E.; Uhlitskykh, K.; Valentino, E.; Wan, P.; Hamilton, C.; Feng, X.; Johnston, A.; et al. A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother. Pharmacol. 2018, 82, 429–440. [Google Scholar] [CrossRef]
- Hall, P.E.; Lewis, R.; Syed, N.; Shaffer, R.; Evanson, J.; Ellis, S.; Williams, M.; Feng, X.; Johnston, A.; Thomson, J.A.; et al. A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-grade Glioma. Clin. Cancer Res. 2019, 25, 2708–2716. [Google Scholar] [CrossRef] [Green Version]
- Beddowes, E.; Spicer, J.; Chan, P.Y.; Khadeir, R.; Corbacho, J.G.; Repana, D.; Steele, J.P.; Schmid, P.; Szyszko, T.; Cook, G.; et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers. J. Clin. Oncol. 2017, 35, 1778–1785. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Janku, F.; Subbiah, V.; Stewart, J.; Patel, S.P.; Kaseb, A.; Westin, S.N.; Naing, A.; Tsimberidou, A.M.; Hong, D.; et al. Phase 1 trial of ADI-PEG20 plus cisplatin in patients with pretreated metastatic melanoma or other advanced solid malignancies. Br. J. Cancer 2021, 124, 1533–1539. [Google Scholar] [CrossRef]
- Tomlinson, B.K.; Thomson, J.A.; Bomalaski, J.S.; Diaz, M.; Akande, T.; Mahaffey, N.; Li, T.; Dutia, M.P.; Kelly, K.; Gong, I.Y.; et al. Phase I Trial of Arginine Deprivation Therapy with ADI-PEG 20 Plus Docetaxel in Patients with Advanced Malignant Solid Tumors. Clin. Cancer Res. 2015, 21, 2480–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlosarek, P.W.; Steele, J.P.; Nolan, L.; Gilligan, D.; Taylor, P.; Spicer, J.; Lind, M.; Mitra, S.; Shamash, J.; Phillips, M.M.; et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Marini, A.; Walker, G.; Elgart, G.; Moffat, F.; Rodgers, S.E.; Wu, C.J.; You, M.; Wangpaichitr, M.; Kuo, M.T.; et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br. J. Cancer 2012, 106, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- De Santo, C.; Cheng, P.; Beggs, A.; Egan, S.; Bessudo, A.; Mussai, F. Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J. Hematol. Oncol. 2018, 11, 68. [Google Scholar] [CrossRef]
- Yau, T.; Cheng, P.N.; Chan, P.; Chen, L.; Yuen, J.; Pang, R.; Fan, S.T.; Wheatley, D.N.; Poon, R.T. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Investig. New Drugs 2015, 33, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Khadeir, R.; Szyszko, T.; Szlosarek, P.W. Optimizing arginine deprivation for hard-to-treat cancers. Oncotarget 2017, 8, 96468–96469. [Google Scholar] [CrossRef]
- Jalili, M.; Scharm, M.; Wolkenhauer, O.; Damaghi, M.; Salehzadeh-Yazdi, A. Exploring the Metabolic Heterogeneity of Cancers: A Benchmark Study of Context-Specific Models. J. Pers. Med 2021, 11, 496. [Google Scholar]




| Cancer | Immune Cells | ||
|---|---|---|---|
| SLC | Type | SLC | Type |
| SLC7A1 | Hepatocellular carcinoma [33], colorectal cancer [34], breast cancer [35], leukemia [36] | SLC7A1 | memory CD4(+) T cells and CD8(+) T cells [37] |
| SLC6A14 | Colorectal cancer [38], cervical cancer [39], pancreatic ductal adenocarcinomas [40], breast cancer [41] | SLC7A2 | Macrophage [42] |
| SLC7A3 | Osteosarcoma [43] | - | - |
| SLC7A9/SCL3A1 | Breast cancer [44] | - | - |
| SLC214C1 | Endometrial cancer [45] | - | - |
| SLC25A2 | Colorectal cancer [46] | - | - |
| Comparison of the Enzymatic Properties | Pegylated Arginine Deiminase (ADI-PEG20) | Pegylated Recombinant Human Arginase I (PEG-BCT-100) |
|---|---|---|
| Reaction products | L-citrulline + ammonia | L-ornithine + urea |
| Arginine affinity | High (Km~0.1–1 mM) | Low (Km~2.9mM) [125] |
| Half-life | 7 days [126] | 2~3 days [125] |
| Time requires to maximal arginine depletion in plasma | 4 days [127] | 4 h [125] |
| Origin of enzyme [4] | Mycoplasma | Human |
| Immunogenicity [128] | Antigenic (requires pegylation) | No |
| Clinical trials (Clinicaltrials.gov) | 25 trials Phase I, II, III | 10 trails Phase I, II |
| ADI as a Single Agent | ||||
|---|---|---|---|---|
| Type of Cancer | Cell Line | Remarks | Ref | |
| Bladder cancer | T24, J82, UM-UC-3, 5637, RT112, and RT4 | ADI-PEG20 reduces the colony formation and cell viability by caspase-independent apoptotic cell death in ASS1-deficient cell lines. | [129] | |
| Breast | MDA-MD-231, ZR-75, T47D, MCF-7, SK-BR-3, MCF-10A | ADI-PEG20 induces the autophagy-dependent cell death, leading to mitochondrial dysfunction and growth inhibition. | [15] | |
| Cholangiocarcinoma | HuCCA, RmCCA-1, BJ-1 | ADI-PEG20 treatment reduces cholangiocarcinoma cell viability and proliferation. | [130] | |
| Colon carcinoma, Bladder carcinoma | HCT116, UMUC3 | ADI-PEG20 reduces hypoxia-induced NO pathway and vascular perfusion. | [131] | |
| Head and neck cancer | FaDu, HONE-1, KB, OEC-M1, UMSCC-1, SCC-4, SCC-15, SCC-25 | ADI-PEG20 inhibits the proliferation of head and neck cancer cells. | [132] | |
| Lymphomas | NcNc, Karpas-422, MyLa | ADI-PEG20 induces the caspase-dependent apoptosis in ASS1-methylated lymphoma cell lines. | [111] | |
| Melanoma | A2058, SK-Mel-2, A375 | ADI resistant cell lines are preferentially sensitive to glycolytic inhibitors and glutaminase inhibitors | [23] | |
| Melanoma | A375, A2058, SK-MEL-2 | ADI-resistance is due to the induction of ASS1 expression via c-Myc/HIF-1α/Sp4 pathway | [133] | |
| Melanoma, Breast cancer | UCSD354L, UACC62, UACC257, MEL1220, A20558, A375, SK-MEL-2, SK-MEL-5, SK-MEL-624, WM35, WM46, WM1799, WM2664, WM3248, SB-2, MDA-MB-231, SKOV3 | ADI-resistance is due to the induction of ASS1 expression via Gas6/Axl/Shp2 signal. | [134] | |
| Melanoma, Breast cancer | A2058, A375, BJ-1, WM2664, BT20, BT549, Hs578T, MDA-MB-157, MDA-MB-231, MDA-MB-436, MDA-MB-453, MDA-MB-468, HCC70, HCC38, HCC1806 | Knockdown of GLS increase the sensitivity to ADI-PEG20 | [135] | |
| Myxofibrosarcoma | OH931, NMFH-1, and NMFH-2 | ADI-PEG20 attenuates the cell viability in ASS1-deficient myxofibrosarcoma cells | [124] | |
| Ovarian cancer | OVCAR3, CAOV3, OVCAR4, IGROV1, TOV112D, OVCAR8, OV90, ES2, TOV21G | The ASS1 expression levels in ovarian cancer cell lines are inversely correlated with the susceptibility to ADI-PEG20 | [136] | |
| Pancreatic cancer | MiaPaCa-2, AsPc-1, BxPc-3, Capan1, HPAC, SW1990, L3.6pl, Panc-1 | ADI-PEG20 enhances the radio-sensitization by triggering the ER stress pathway, resulting in apoptosis in pancreatic tumor cells. | [137] | |
| Pancreatic cancer | BxPC-3, Capan-I, HPAC, HFAF-II, L3.3, MIA-PaCa-2, Panc-1 | ADI-PEG20 inhibits the pancreatic cancer cell growth via induction of apoptosis | [112] | |
| Prostate cancer | CWR22 | ADI-PEG20 induces the mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy | [16] | |
| Renal cell carcinoma | UOK262 | ADI-PEG 20 inhibits the cellular proliferation in fumarate hydratase-deficient cells | [138] | |
| Small-cell lung cancer | GLC1, GLC8, NCI-H1092, NCI-H2141, SBC4, NCI-H82, NCI-H524, NCI-H446, NCI-H889, NCI-H69, NCI-H1963, H1048, DMS53 | MYC-driven human SCLC is preferentially sensitive to ADI-PEG20 in vivo | [139] | |
| Small-cell lung cancer | SW1222, SK-LC-13, SE1271, NCI-H82, NCI-H146, NCI-H209, SHP-77, NCI-H740, NCI-H889, NCI-H526, NCI-H69 | ADI-PEG20 induces apoptosis and autophagy in ASS1-negative SCLC cell lines | [119] | |
| Combination treatment with ADI-PEG20 | ||||
| Co-targeting reagent(s) | Type of cancer | Cell line | Remarks | Ref |
| Chloroquine | Glioblastoma | DBTRG, GAMG, SNB19, U87, U118, CCF, LN229, 8MG, T87G, MO59J, MO59K, 42MG | Combination of chloroquine inhibits autophagy and accelerates ADI-PEG20 induced cell death | [118] |
| Chloroquine | Sarcoma | Osteosarcoma (U-2 OS, MNNG/HOS, MG-63, NOS-1, HuO 9N2), Leiomyosarcoma (SK-LMS-1, SK-UT-1, SK-UT-1B), Synovial sarcoma (SYO-1, Fuji), Chondrosarcoma (HCH-1), Ewing’s sarcoma (LUPI, RD-ES, SK-ES), Alveolar soft part sarcoma (ASPS-1) | The combination of chloroquine with ADI-PEG20 causes synthetic lethality via necroptosis in sarcoma cell lines | [114] |
| Cisplatin | Bladder cancer | T24, J82, RT4 | Ass1 is down-regulated in cisplatin-resistant bladder cancer cells. The combination with ADI-PEG20 increases the susceptibility and induces apoptosis in cisplatin-resistant cancer cells. | [140] |
| Cisplatin | Hepatocellular carcinoma | Sk-Hep1, Huh7, Tong, HCC36, Hep3B, Malhavu, PLC5, Huh6, HepG2, SNU398 and SNU182 | The combination of cisplatin with ADI-PEG20 suppresses ASS1 expression in HCC cell lines | [141] |
| Cisplatin | Melanomas | A375, Sk-Mel2, A2058, Mel1220 | The combination of cisplatin with ADI-PEG20 enhances the cell death via apoptosis in melanoma cells | [142] |
| Cisplatin | Small-cell lung cancer, Ovarian cancer, Ovarian adenocarcinoma, Glioblastoma, Melanoma | SCLC, S, H465, SR2, A2780, A2008, A172, A2058 | The combination of cisplatin with ADI-PEG20 induces synergistical lethality | [143] |
| Docetaxel | Sarcoma, pancreatic cancer, and melanoma | SK-LMS-1, SK-UT-1, HTB-93, HT-1080, SK-MEL-2, AS-Pc-1, MiaPaCa-2, MNNG, RDES, and RD HPAC, SYO-1 and FUJI, LUPI, RH28 | The combination of docetaxel with ADI-PEG20 overcomes the gemcitabine resistance | [144] |
| 5-Flurouracie | Hepatocellular carcinoma (HCC) | BJ1, A2058, Mel1220, SNU398, SNU387, HepG2, Huh-1 | The combination of ADI-PEG20 with 5-FU improves the anti-tumor effect in ASS1-negative HCC cells | [145] |
| Gemcitabine | Pancreatic cancer | MIA-PaCa2, PANC-1, L3.3 | The combination of gemcitabine synergistically enhances ADI-PEG20 anti-tumor effect | [146] |
| Oxaliplatin | Colorectal cancer | HCT116, SW480, RKO, HT29 | The combination ADI-PEG20 with Oxaliplatin shows the synergistic growth inhibition in the ASS1-negative cell lines CRCs | [147] |
| Paclitaxel | Prostate cancer | CSR22Rv1, PC3, LNCaP | The combination of paclitaxel with ADI-PEG20 retards CWR22Rv1 tumor growth in vivo | [75] |
| Temozolomide | Glioblastoma | LN229 and U87 | The combination of ADI-PEG20 with Temozolomide suppresses the tumor growth irrespective of ASS1 status | [148] |
| TNF-related apoptosis-inducing ligan (TRAL) | Malignant pleural mesothelioma (MPM) | H211, H290, H2052, H2373, GARD REN, BJ-1 | The combination of TNF-related apoptosis-inducing ligan (TRAL) enhances ADI-PEG20 mediated apoptosis in MPM cells | [149] |
| TNF-related apoptosis-inducing ligan (TRAL) | Melanoma | A375, A2058 | The combination of TRAIL with ADI-PEG20 accelerates the cell death in melanoma cell lines | [150] |
| HAT inhibitor(s) | Melanoma | A2058, K-Mel-2, RCC4 | The combination of HAT inhibitors enhances ADI-PEG20 cell killing effect. | [151] |
| HDAC inhibitor(s) | Pancreatic cancer | Panc1, MiaPaca2, Panc02.03, HS766t, HPAF-II, Suit2, Su8686, Panc03.27, Panc10.05 | The combination of HDAC inhibitors with ADI-PEG20 induces the degradation of DNA repair enzyme, C-terminal-binding protein interacting protein (CtIP), resulting in DNA damage and apoptosis. | [82] |
| BET bromodomain-targeting c-Myc inhibitor | Melanoma | A2058 | The combination of ADI-PEG20 with JQ1, a BET bromodomain-targeting c-Myc inhibitor, significantly enhances the killing effect in ADI-resistant cells | [152] |
| Polyamide inhibitor | Mesothelioma | MSTO, Ju77, H28, H226 | The combination of polyamide inhibitor with ADI-PEG20 causes synthetically lethal effect in MPM cells | [87] |
| PHGDH or GLS inhibitor | Leiomyosarcoma, Melanoma | SKLMS1, SKUT1, SKMEL2 | The combination of ADI-PEG20 with PHGDH or GLS inhibitor significantly increases cell death | [22] |
| N-acetylcysteine | Breast | MDA-MD-231 | Combination of N-acetylcysteine with ADI-PEG 20 induces the immunogenic cell death. | [106] |
| PI3K/AKT inhibitor | Melanoma, Breast cancer | A2058, SK-MEL-2, MDA-MB-231, and A375 | The combination of PI3K/AKT inhibitor enhances ADI-PEG20–mediated cell apoptosis. | [24] |
| Start Date | NCT Number | Type of Cancer | Treatment | Phase | Status | Ref |
|---|---|---|---|---|---|---|
| Trials for pegylayed arginine deimnase | ||||||
| Jun-2020 | NCT04587830 | Glioblastoma Multiforme (GBM) | ADI-PEG20|Temozolomide | Phase 1 | Recruiting | |
| Apr-2019 | NCT03922880 | Uveal Melanoma | ADI-PEG20|Nivolumab|Ipilimumab | Phase 1 | Active, not recruiting | |
| Jun-2018 | NCT03498222 | Carcinoma, Non-Small-Cell Lung | ADI-PEG20|Atezolizumab|Pemetrexed|Carboplatin | Phase 1 | Withdrawn | |
| May-2018 | NCT03449901 | Soft Tissue Sarcoma|Osteosarcoma|Ewing’s Sarcoma| Small Cell Lung Cancer | ADI-PEG20|Gemcitabine|Docetaxel | Phase 2 | Active, not recruiting | |
| Aug-2017 | NCT02709512 | Mesothelioma | ADI-PEG20 |Pemetrexed and Cisplatin | Phase 2|Phase 3 | Recruiting | |
| Jul-2017 | NCT03254732 | Advanced Solid Cancers | ADI-PEG20|Pembrolizumab | Phase 1 | Active, not recruiting | |
| Jan-2017 | NCT02875093 | Acute Myeloid Leukemia | ADI-PEG20|Cytarabine | Phase 1 | Terminated | [158] |
| Jan-2015 | NCT01910012 | Acute Myeloid Leukemia | ADI-PEG20 | Phase 2 | Completed | [159] |
| Nov-2014 | NCT02101580 | Advanced Pancreatic Cancer | ADI-PEG20 Plus Nab-Paclitaxel and Gemcitabine | Phase 1 | Completed | [160] |
| Nov-2014 | NCT02101593 | Hepatocellular Carcinoma | ADI-PEG20|Sorafenib | Phase 1 | Completed | |
| Nov-2014 | NCT02102022 | Advanced Gastrointestinal (GI) Malignancies| Hepatocellular Carcinoma|Gastric Cancer|Colorectal Cancer | ADI-PEG20|modified FOLFOX6 | Phase 1|Phase 2 | Terminated | [161] |
| Oct-2014 | NCT02006030 | Unresectable Hepatocellular Carcinoma | ADI-PEG20|Transarterial chemoembolization | Phase 2 | Completed | |
| Apr-2014 | NCT02029690 | Pleural Mesothelioma Malignant Advanced|Peritoneal Mesothelioma Malignant Advanced|Non-squamous Non-small Cell Lung Carcinoma|Uveal Melanoma|Hepatocellular Carcinoma|Glioma|Sarcomatoid Carcinoma | ADI-PEG20 With Pemetrexed and Cisplatin | Phase 1 | Terminated | [162,163] |
| Apr-2014 | NCT01948843 | HER2 Negative Metastatic Breast Cancer | ADI-PEG20|Doxorubicin | Phase 1 | Completed | |
| Dec-2013 | NCT01910025 | Non-Hodgkin’s Lymphoma | ADI-PEG20 | Phase 2 | Completed | |
| Sep-2012 | NCT01665183 | Cutaneous Melanoma, Uveal Melanoma, Ovarian Carcinoma or Other Advanced Solid Tumors | ADI-PEG20|Cisplatin | Phase 1 | Completed | [164] |
| Dec-2011 | NCT01528384 | Arginosuccinate Synthetase Deficient | ADI-PEG20 | Phase 1 | Completed | |
| Sep-2011 | NCT01497925 | Solid Tumors|Prostate Cancer|Non-Small Cell Lung Cancer | ADI-PEG20|Docetaxel | Phase 1 | Completed | [165] |
| Jul-2011 | NCT01287585 | Hepatocellular Carcinoma | ADI-PEG20 | Phase 3 | Completed | [156] |
| Jan-2011 | NCT01266018 | Small Cell Lung Cancer | ADI-PEG20 | Phase 2 | Terminated | |
| Jan-2011 | NCT01279967 | Malignant Pleural Mesothelioma | ADI-PEG20 | Phase 2 | Unknown status | [166] |
| Jul-2007 | NCT00520299 | Metastatic Melanoma|Skin Cancer|Neoplasm | ADI-PEG20 | Phase 1|Phase 2 | Completed | [127] |
| Jun-2004 | NCT00450372 | Melanoma (Skin) | ADI-PEG20 | Phase 2 | Completed | [167] |
| Sep-2002 | NCT00056992 | Carcinoma, Hepatocellular | ADI-PEG20 | Phase 2 | Completed | |
| Sep-2001 | NCT00029900 | Melanoma| Neoplasm Metastasis | ADI-PEG20 | Phase 1 | Completed | |
| Trials for pegylated recombinant human arginase and arginase-1 peptide vaccine | ||||||
| Dec-2018 | NCT03689192 | Non Small Cell Lung Cancer|Urothelial Carcinoma|Malignant Melanoma|Ovarian Cancer|Colorectal Cancer|Breast Cancer|Squamous Cell Carcinoma of the Head and Neck|Metastatic Cancer | Arginase-1 Peptide Vaccine (ARG1-18,19,20) | Phase 1 | Recruiting | |
| Aug-2018 | NCT03455140 | Cancer|Pediatric Solid Tumor|Pediatric AML|Pediatric ALL | Pegylated Recombinant Human Arginase (BCT-100) | Phase 1|Phase 2 | Recruiting | |
| Sep-2016 | NCT02899286 | Relapsed or Refractory Acute Myeloid Leukemia | Pegylated Recombinant Human Arginase (BCT-100) | Phase 2 | Unknown status | |
| Aug-2016 | NCT02732184 | Acute Myeloid Leukemia|Myelodysplastic Syndrome | Co-ArgI-PEG modified human arginase I | Phase 2 | Completed | |
| Nov-2014 | NCT02285101 | Melanoma|Prostate Adenocarcinoma | Pegylated recombinant human arginase (PEG-BCT-100) | Phase 1 | Completed | [168] |
| Apr-2014 | NCT02089763 | Hepatocellular Carcinoma | Pegylated recombinant human arginase | Phase 2 | Terminated | |
| Apr-2014 | NCT02089633 | Hepatocellular Carcinoma | Pegylated recombinant human arginase|Oxaliplain|Capecitabine | Phase 2 | Completed | |
| Apr-2012 | NCT01551628 | Leukemia|Lymphoma | Recombinant human arginase 1 Peg5000 | Phase 1 | Terminated | |
| Mar-2010 | NCT01092091 | Neoplasm| Hepatocellular Carcinoma | Pegylated Recombinant Human Arginase I (BCT-100-002) | Phase 1|Phase 2 | Completed | [125,169] |
| May-2008 | NCT00988195 | Neoplasm| Hepatocellular Carcinoma | Pegylated Recombinant Human Arginase I|Doxorubicin | Phase 1 | Completed | |
| Trials for arginase inhibitor (INCB1158) | ||||||
| Sep-2019 | NCT03837509 | Relapsed or Refractory Multiple Myeloma | INCB001158|Daratumumab SC | Phase 1|Phase 2 | Recruiting | |
| Jul-2019 | NCT03910530 | Advanced Solid Tumors|Metastatic Solid Tumors | Retifanlimab|INCB001158| Retifanlimab + INCB001158 | Phase 1 | Active, not recruiting | |
| Mar-2018 | NCT03361228 | Solid Tumors | INCB001158|Epacadostat|Pembrolizumab | Phase 1|Phase 2 | Terminated | |
| Nov-2017 | NCT03314935 | Biliary Tract Cancer|Colorectal Cancer|Endometrial Cancer|Gastroesophageal Cancer|Ovarian Cancer|Solid Tumors | INCB001158|Oxaliplatin|Leucovorin|5-Fluorouracil|Gemcitabine|Cisplatin|Paclitaxel | Phase 1|Phase 2 | Active, not recruiting | |
| Sep-2016 | NCT02903914 | Metastatic Cancer|Solid Tumors|Colorectal Cancer|Gastric Cancer|Head and Neck Cancer|Lung Cancer|Renal Cell Carcinoma|Bladder Cancer|Urothelial Cancer|Mesothelioma | INCB001158|Pembrolizumab | Phase 1|Phase 2 | Active, not recruiting | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-L.; Hsu, S.-C.; Ann, D.K.; Yen, Y.; Kung, H.-J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. https://doi.org/10.3390/cancers13143541
Chen C-L, Hsu S-C, Ann DK, Yen Y, Kung H-J. Arginine Signaling and Cancer Metabolism. Cancers. 2021; 13(14):3541. https://doi.org/10.3390/cancers13143541
Chicago/Turabian StyleChen, Chia-Lin, Sheng-Chieh Hsu, David K. Ann, Yun Yen, and Hsing-Jien Kung. 2021. "Arginine Signaling and Cancer Metabolism" Cancers 13, no. 14: 3541. https://doi.org/10.3390/cancers13143541
APA StyleChen, C.-L., Hsu, S.-C., Ann, D. K., Yen, Y., & Kung, H.-J. (2021). Arginine Signaling and Cancer Metabolism. Cancers, 13(14), 3541. https://doi.org/10.3390/cancers13143541






