Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Generalities on UT Cells
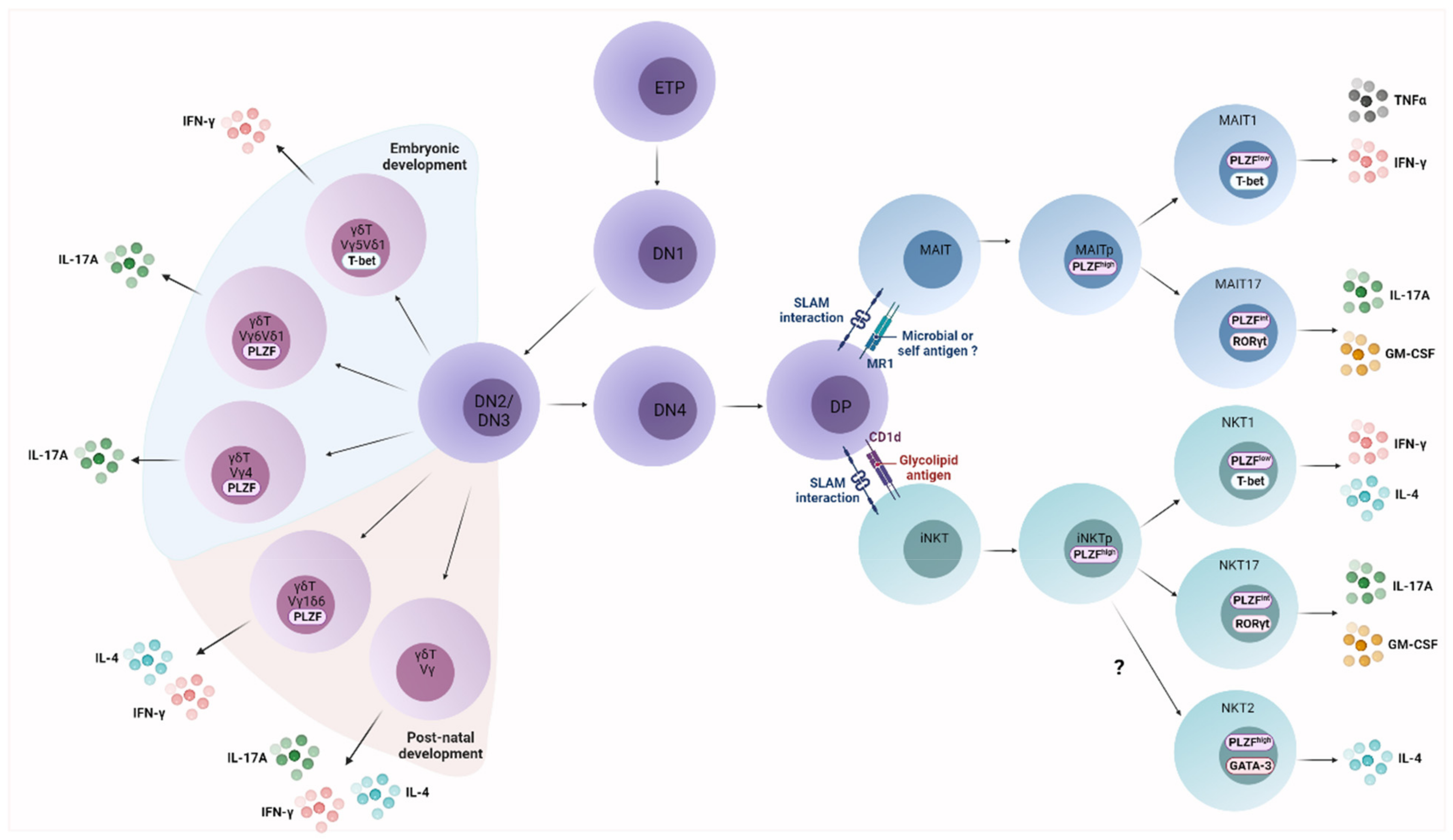
2.1. UT Cell Ontogeny
2.2. UT Cell Effector Differentiation
2.3. Activation Mechanisms of UT Cells
2.3.1. TCR-Dependent Signals
2.3.2. TCR-Independent Signals
2.4. Functional Diversity of UT Cells
2.4.1. Cytotoxicity
2.4.2. Release of Immunoregulatory Factors
2.5. UT Cell Populations: Redundant Functions for Specific Roles?
3. Influence of the Tumor Microenvironment on UT Cell Functions
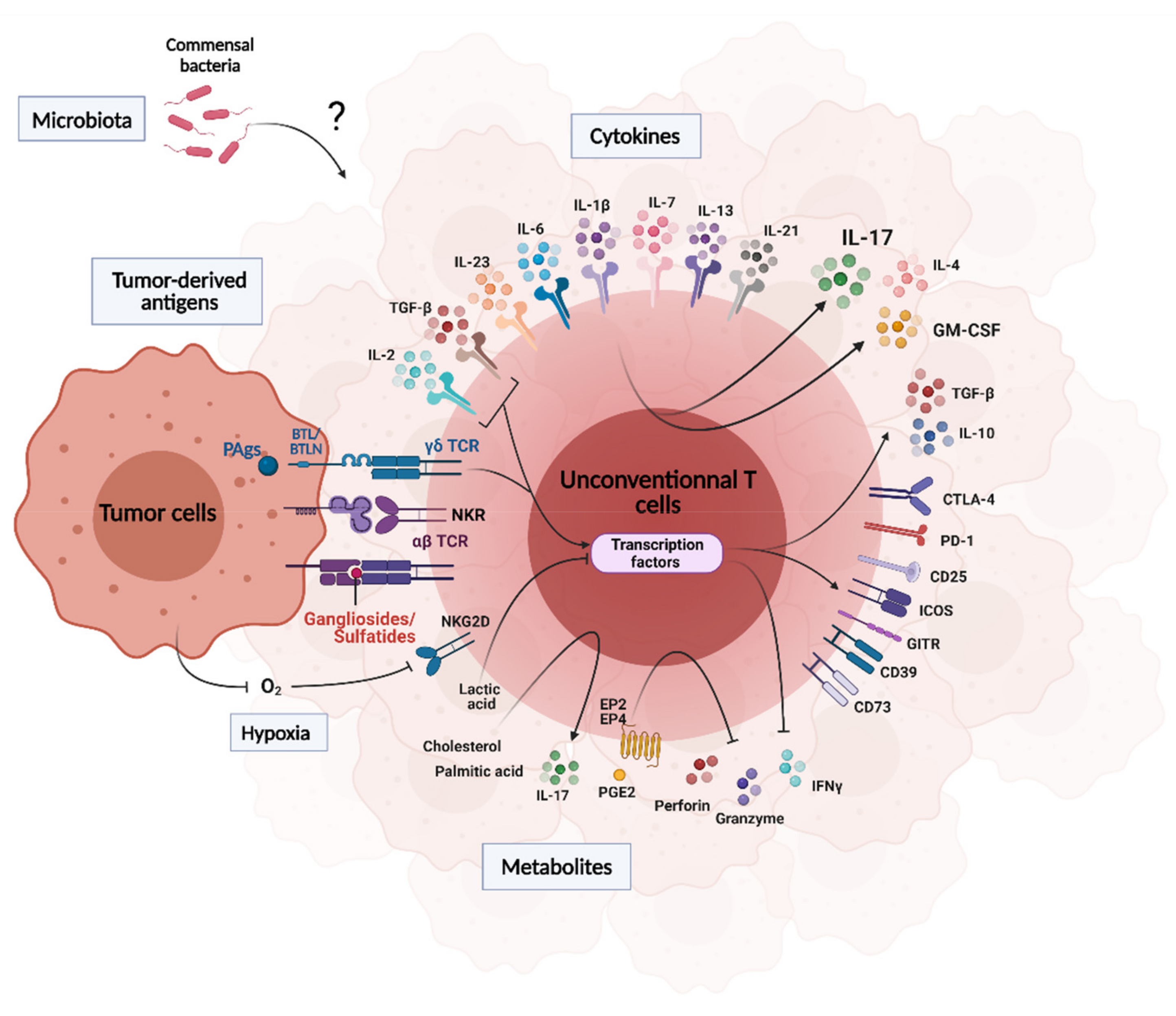
3.1. Tumor-Derived Antigens
3.2. Cytokines, Metabolites, pH and Hypoxia
3.3. Microbiota
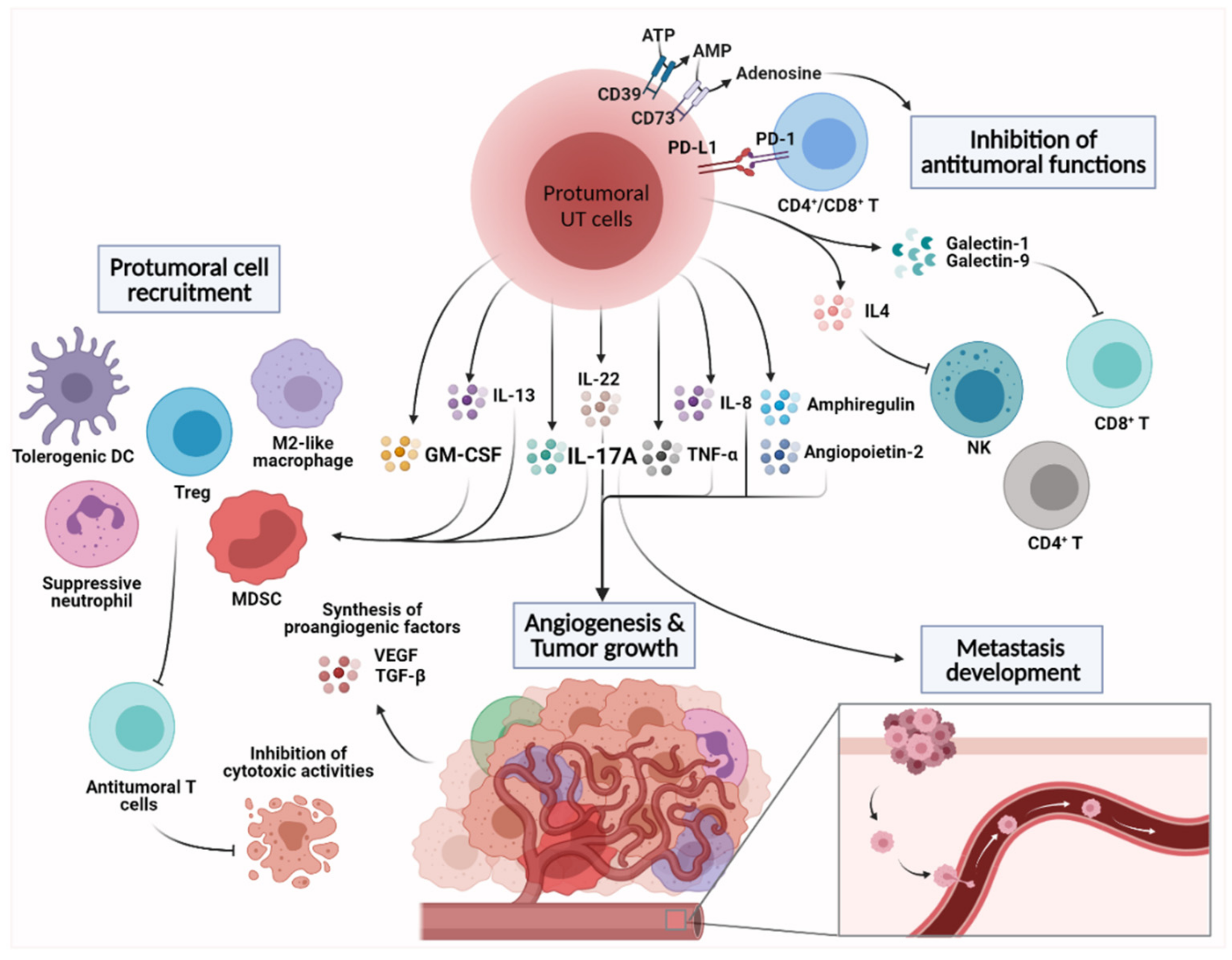
4. Emerging Protumoral Functions of Intratumoral Unconventional T Cells
4.1. Angiogenesis and Tumor Cell Proliferation
4.2. Shaping the Immunosuppressive TME
4.3. Inhibition of Antitumoral Functions
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leone, R.D.; Powell, J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells across Human Cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Robinette, M.L.; Colonna, M. Immune Modules Shared by Innate Lymphoid Cells and T Cells. J. Allergy Clin. Immunol. 2016, 138, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellicci, D.G.; Koay, H.-F.; Berzins, S.P. Thymic Development of Unconventional T Cells: How NKT Cells, MAIT Cells and Γδ T Cells Emerge. Nat. Rev. Immunol. 2020, 20, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, F.; Treiner, E.; Park, S.H.; Garcia, C.; Lemonnier, F.; de la Salle, H.; Bendelac, A.; Bonneville, M.; Lantz, O. An Invariant T Cell Receptor Alpha Chain Defines a Novel TAP-Independent Major Histocompatibility Complex Class Ib-Restricted Alpha/Beta T Cell Subpopulation in Mammals. J. Exp. Med. 1999, 189, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.-F.; McCluskey, J.; Gherardin, N.A. The Biology and Functional Importance of MAIT Cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- Gapin, L.; Matsuda, J.L.; Surh, C.D.; Kronenberg, M. NKT Cells Derive from Double-Positive Thymocytes That Are Positively Selected by CD1d. Nat. Immunol. 2001, 2, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Macho-Fernandez, E.; Brigl, M. The Extended Family of CD1d-Restricted NKT Cells: Sifting through a Mixed Bag of TCRs, Antigens, and Functions. Front. Immunol. 2015, 6, 362. [Google Scholar] [CrossRef] [Green Version]
- Vermijlen, D.; Prinz, I. Ontogeny of Innate T Lymphocytes—Some Innate Lymphocytes Are More Innate than Others. Front. Immunol. 2014, 5, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldrich, A.P.; Rigau, M.; Godfrey, D.I. Immune Recognition of Phosphoantigen-Butyrophilin Molecular Complexes by Γδ T Cells. Immunol. Rev. 2020, 298, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of Evolutionarily Conserved Mucosal-Associated Invariant T Cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, I.; Silva-Santos, B.; Pennington, D.J. Functional Development of Γδ T Cells. Eur. J. Immunol. 2013, 43, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.D.; Boughter, C.T.; Broughton, A.E.; Ramesh, A.; Adams, E.J. Diversity in Recognition and Function of Human Γδ T Cells. Immunol. Rev. 2020, 298, 134–152. [Google Scholar] [CrossRef]
- Corbett, A.J.; Eckle, S.B.G.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-Cell Activation by Transitory Neo-Antigens Derived from Distinct Microbial Pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-Restricted and TCR-Mediated Activation of Valpha14 NKT Cells by Glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef]
- Kinjo, Y.; Illarionov, P.; Vela, J.L.; Pei, B.; Girardi, E.; Li, X.; Li, Y.; Imamura, M.; Kaneko, Y.; Okawara, A.; et al. Invariant Natural Killer T Cells Recognize Glycolipids from Pathogenic Gram-Positive Bacteria. Nat. Immunol. 2011, 12, 966–974. [Google Scholar] [CrossRef]
- Paget, C.; Mallevaey, T.; Speak, A.O.; Torres, D.; Fontaine, J.; Sheehan, K.C.F.; Capron, M.; Ryffel, B.; Faveeuw, C.; Leite de Moraes, M.; et al. Activation of Invariant NKT Cells by Toll-like Receptor 9-Stimulated Dendritic Cells Requires Type I Interferon and Charged Glycosphingolipids. Immunity 2007, 27, 597–609. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/Delta T-Cell Stimulation by Pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.Y.W.; Xu, W.; Reid, R.C.; Corbett, A.J.; Meehan, B.S.; Wang, H.; Chen, Z.; Rossjohn, J.; McCluskey, J.; Liu, L.; et al. Stabilizing Short-Lived Schiff Base Derivatives of 5-Aminouracils That Activate Mucosal-Associated Invariant T Cells. Nat. Commun. 2017, 8, 14599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paget, C.; Deng, S.; Soulard, D.; Priestman, D.A.; Speca, S.; von Gerichten, J.; Speak, A.O.; Saroha, A.; Pewzner-Jung, Y.; Futerman, A.H.; et al. TLR9-Mediated Dendritic Cell Activation Uncovers Mammalian Ganglioside Species with Specific Ceramide Backbones That Activate Invariant Natural Killer T Cells. PLoS Biol. 2019, 17, e3000169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkholz, A.M.; Kronenberg, M. Antigen Specificity of Invariant Natural Killer T-Cells. Biomed. J. 2015, 38, 470–483. [Google Scholar] [CrossRef] [Green Version]
- Livák, F.; Tourigny, M.; Schatz, D.G.; Petrie, H.T. Characterization of TCR Gene Rearrangements During Adult Murine T Cell Development. J. Immunol. 1999, 162, 2575–2580. [Google Scholar]
- Ciofani, M.; Knowles, G.C.; Wiest, D.L.; von Boehmer, H.; Zúñiga-Pflücker, J.C. Stage-Specific and Differential Notch Dependency at the Aβ and Γδ T Lineage Bifurcation. Immunity 2006, 25, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Prinz, I.; Sansoni, A.; Kissenpfennig, A.; Ardouin, L.; Malissen, M.; Malissen, B. Visualization of the Earliest Steps of Γδ T Cell Development in the Adult Thymus. Nat. Immunol. 2006, 7, 995–1003. [Google Scholar] [CrossRef]
- Lantz, O.; Legoux, F. MAIT Cells: Programmed in the Thymus to Mediate Immunity within Tissues. Curr. Opin. Immunol. 2019, 58, 75–82. [Google Scholar] [CrossRef]
- Gapin, L. Development of Invariant Natural Killer T Cells. Curr. Opin. Immunol. 2016, 39, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biol. 2009, 7, e1000054. [Google Scholar] [CrossRef]
- Coles, M.C.; Raulet, D.H. NK1.1+ T Cells in the Liver Arise in the Thymus and Are Selected by Interactions with Class I Molecules on CD4+CD8+ Cells. J. Immunol. 2000, 164, 2412–2418. [Google Scholar] [CrossRef] [Green Version]
- Seach, N.; Guerri, L.; Bourhis, L.L.; Mburu, Y.; Cui, Y.; Bessoles, S.; Soudais, C.; Lantz, O. Double Positive Thymocytes Select Mucosal-Associated Invariant T Cells. J. Immunol. 2013, 191, 6002–6009. [Google Scholar] [CrossRef] [Green Version]
- Koay, H.-F.; Su, S.; Amann-Zalcenstein, D.; Daley, S.R.; Comerford, I.; Miosge, L.; Whyte, C.E.; Konstantinov, I.E.; d’Udekem, Y.; Baldwin, T.; et al. A Divergent Transcriptional Landscape Underpins the Development and Functional Branching of MAIT Cells. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Legoux, F.; Gilet, J.; Procopio, E.; Echasserieau, K.; Bernardeau, K.; Lantz, O. Molecular Mechanisms of Lineage Decisions in Metabolite-Specific T Cells. Nat. Immunol. 2019, 20, 1244–1255. [Google Scholar] [CrossRef]
- Griewank, K.; Borowski, C.; Rietdijk, S.; Wang, N.; Julien, A.; Wei, D.G.; Mamchak, A.A.; Terhorst, C.; Bendelac, A. Homotypic Interactions Mediated by Slamf1 and Slamf6 Receptors Control NKT Cell Lineage Development. Immunity 2007, 27, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovalovsky, D.; Uche, O.U.; Eladad, S.; Hobbs, R.M.; Yi, W.; Alonzo, E.; Chua, K.; Eidson, M.; Kim, H.-J.; Im, J.S.; et al. The BTB–Zinc Finger Transcriptional Regulator PLZF Controls the Development of Invariant Natural Killer T Cell Effector Functions. Nat. Immunol. 2008, 9, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.K.; Constantinides, M.G.; Han, J.; Picard, D.; Martin, E.; Li, B.; Lantz, O.; Bendelac, A. The Transcription Factor PLZF Directs the Effector Program of the NKT Cell Lineage. Immunity 2008, 29, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Seiler, M.P.; Mathew, R.; Liszewski, M.K.; Spooner, C.J.; Barr, K.; Meng, F.; Singh, H.; Bendelac, A. Elevated and Sustained Expression of the Transcription Factors Egr1 and Egr2 Controls NKT Lineage Differentiation in Response to TCR Signaling. Nat. Immunol. 2012, 13, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Koay, H.F.; Enders, A.; Clanchy, R.; Eckle, S.B.G.; Meehan, B.; Chen, Z.; Whittle, B.; Liu, L.; Fairlie, D.P.; et al. Identification of Phenotypically and Functionally Heterogeneous Mouse Mucosal-Associated Invariant T Cells Using MR1 Tetramers. J. Exp. Med. 2015, 212, 1095–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koay, H.-F.; Gherardin, N.A.; Enders, A.; Loh, L.; Mackay, L.K.; Almeida, C.F.; Russ, B.E.; Nold-Petry, C.A.; Nold, M.F.; Bedoui, S.; et al. A Three-Stage Intrathymic Development Pathway for the Mucosal-Associated Invariant T Cell Lineage. Nat. Immunol. 2016, 17, 1300–1311. [Google Scholar] [CrossRef]
- Winter, S.J.; Kunze-Schumacher, H.; Imelmann, E.; Grewers, Z.; Osthues, T.; Krueger, A. MicroRNA MiR-181a/b-1 Controls MAIT Cell Development. Immunol. Cell Biol. 2019, 97, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Dhodapkar, M.V.; Kumar, V. Type II NKT Cells and Their Emerging Role in Health and Disease. J. Immunol. 2017, 198, 1015–1021. [Google Scholar] [CrossRef]
- Harsha Krovi, S.; Zhang, J.; Michaels-Foster, M.J.; Brunetti, T.; Loh, L.; Scott-Browne, J.; Gapin, L. Thymic INKT Single Cell Analyses Unmask the Common Developmental Program of Mouse Innate T Cells. Nat. Commun. 2020, 11, 6238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Adrianto, I.; Wang, J.; Wu, X.; Datta, I.; Mi, Q.-S. Single-Cell RNA-Seq Analysis Uncovers Distinct Functional Human NKT Cell Sub-Populations in Peripheral Blood. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Ben Youssef, G.; Tourret, M.; Salou, M.; Ghazarian, L.; Houdouin, V.; Mondot, S.; Mburu, Y.; Lambert, M.; Azarnoush, S.; Diana, J.-S.; et al. Ontogeny of Human Mucosal-Associated Invariant T Cells and Related T Cell Subsets. J. Exp. Med. 2018, 215, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Jacques, P.; Mortier, C.; Labadia, M.E.; Decruy, T.; Coudenys, J.; Hoyt, K.; Wayne, A.L.; Hughes, R.; Turner, M.; et al. RORγt Inhibition Selectively Targets IL-17 Producing INKT and Γδ-T Cells Enriched in Spondyloarthritis Patients. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranek, T.; Lebrigand, K.; de Amat Herbozo, C.; Gonzalez, L.; Bogard, G.; Dietrich, C.; Magnone, V.; Boisseau, C.; Jouan, Y.; Trottein, F.; et al. High Dimensional Single-Cell Analysis Reveals INKT Cell Developmental Trajectories and Effector Fate Decision. Cell Rep. 2020, 32, 108116. [Google Scholar] [CrossRef] [PubMed]
- Gapin, L.; Godfrey, D.I.; Rossjohn, J. Natural Killer T Cell Obsession with Self-Antigens. Curr. Opin. Immunol. 2013, 25, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Unutmaz, D. Immune Cells for Microbiota Surveillance. Science 2019, 366, 419–420. [Google Scholar] [CrossRef]
- Paget, C.; Chow, M.; Gherardin, N.; Beavis, P.; Uldrich, A.; Duret, H.; Hassane, M.; Souza-Fonseca-Guimaraes, F.; Mogilenko, D.; Staumont-Sallé, D.; et al. CD3bright Signals on Γδ T Cells Identify IL-17A-Producing Vγ6Vδ1+ T Cells. Immunol. Cell Biol. 2015, 93, 198–212. [Google Scholar] [CrossRef]
- Dieudé, M.; Striegl, H.; Tyznik, A.J.; Wang, J.; Behar, S.M.; Piccirillo, C.A.; Levine, J.S.; Zajonc, D.M.; Rauch, J. Cardiolipin Binds to CD1d and Stimulates CD1d-Restricted Γδ T Cells in the Normal Murine Repertoire. J. Immunol. 2011, 186, 4771–4781. [Google Scholar] [CrossRef] [Green Version]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal Structure of Vδ1 T Cell Receptor in Complex with CD1d-Sulfatide Shows MHC-like Recognition of a Self-Lipid by Human Γδ T Cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef] [Green Version]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 Presents Microbial Vitamin B Metabolites to MAIT Cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.-M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key Implication of CD277/Butyrophilin-3 (BTN3A) in Cellular Stress Sensing by a Major Human Γδ T-Cell Subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef] [Green Version]
- Sandstrom, A.; Peigné, C.-M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.-C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human Vγ9Vδ2 T Cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 Is Essential for Phosphoantigen Reactivity by Γδ T Cells. Science 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Russano, A.M.; Agea, E.; Corazzi, L.; Postle, A.D.; De Libero, G.; Porcelli, S.; de Benedictis, F.M.; Spinozzi, F. Recognition of Pollen-Derived Phosphatidyl-Ethanolamine by Human CD1d-Restricted Gamma Delta T Cells. J. Allergy Clin. Immunol. 2006, 117, 1178–1184. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-Lipid Antigen Recognition by the Γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- McEwen-Smith, R.M.; Salio, M.; Cerundolo, V. The Regulatory Role of Invariant NKT Cells in Tumor Immunity. Cancer Immunol. Res. 2015, 3, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Santos, B.; Serre, K.; Norell, H. Γδ T Cells in Cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Seyda, M.; Elkhal, A.; Quante, M.; Falk, C.S.; Tullius, S.G. T Cells Going Innate. Trends Immunol. 2016, 37, 546–556. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, N.D.; Gama, F.M.; de Souza Barros, M.; Ribeiro, T.L.P.; Alves, F.S.; Xabregas, L.A.; Tarragô, A.M.; Malheiro, A.; Costa, A.G. Translating Unconventional T Cells and Their Roles in Leukemia Antitumor Immunity. J. Immunol. Res. 2021, 2021, 6633824. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural Killer Cells and Other Innate Lymphoid Cells in Cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Q.; Peng, H.; Cheng, R.; Sun, Z.; Ye, Z. IFN-γ Enhances HOS and U2OS Cell Lines Susceptibility to Γδ T Cell-Mediated Killing through the Fas/Fas Ligand Pathway. Int. Immunopharmacol. 2011, 11, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Rodin, W.; Sundström, P.; Ahlmanner, F.; Szeponik, L.; Zajt, K.K.; Wettergren, Y.; Bexe Lindskog, E.; Quiding Järbrink, M. Exhaustion in Tumor-Infiltrating Mucosal-Associated Invariant T (MAIT) Cells from Colon Cancer Patients. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-Restricted INKT Cells, the “Swiss-Army Knife” of the Immune System. Curr Opin Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T Cell Effector Functions: A Blend of Innate Programming and Acquired Plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Kinjo, Y.; Kronenberg, M. V Alpha14 i NKT Cells Are Innate Lymphocytes That Participate in the Immune Response to Diverse Microbes. J. Clin. Immunol. 2005, 25, 522–533. [Google Scholar] [CrossRef]
- Toubal, A.; Nel, I.; Lotersztajn, S.; Lehuen, A. Mucosal-Associated Invariant T Cells and Disease. Nat. Rev. Immunol. 2019, 19, 643–657. [Google Scholar] [CrossRef]
- Gold, M.C.; Lewinsohn, D.M. Co-Dependents: MR1-Restricted MAIT Cells and Their Antimicrobial Function. Nat. Rev. Microbiol. 2013, 11, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Joshi, B.; Yang, Z.; Degheidy, H.; Fichtner-Feigl, S.; de Souza, H.; Rieder, F.; Scaldaferri, F.; Schirbel, A.; Scarpa, M.; et al. IL-13Rα2-Bearing, Type II NKT Cells Reactive to Sulfatide Self-Antigen Populate the Mucosa of Ulcerative Colitis. Gut 2014, 63, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Dokouhaki, P.; Schuh, N.W.; Joe, B.; Allen, C.A.D.; Der, S.D.; Tsao, M.-S.; Zhang, L. NKG2D Regulates Production of Soluble TRAIL by Ex Vivo Expanded Human Γδ T Cells. Eur. J. Immunol. 2013, 43, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Nieda, M.; Nicol, A.; Koezuka, Y.; Kikuchi, A.; Lapteva, N.; Tanaka, Y.; Tokunaga, K.; Suzuki, K.; Kayagaki, N.; Yagita, H.; et al. TRAIL Expression by Activated Human CD4(+)V Alpha 24NKT Cells Induces in Vitro and in Vivo Apoptosis of Human Acute Myeloid Leukemia Cells. Blood 2001, 97, 2067–2074. [Google Scholar] [CrossRef] [Green Version]
- Wingender, G.; Krebs, P.; Beutler, B.; Kronenberg, M. Antigen-Specific Cytotoxicity by Invariant NKT Cells in Vivo Is CD95/CD178-Dependent and Is Correlated with Antigenic Potency. J. Immunol. 2010, 185, 2721–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biasi, S.; Gibellini, L.; Lo Tartaro, D.; Puccio, S.; Rabacchi, C.; Mazza, E.M.C.; Brummelman, J.; Williams, B.; Kaihara, K.; Forcato, M.; et al. Circulating Mucosal-Associated Invariant T Cells Identify Patients Responding to Anti-PD-1 Therapy. Nat. Commun. 2021, 12, 1669. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.C.; Aguilera, C.; Maricic, I.; Kumar, V. Type II NKT Cell-Mediated Anergy Induction in Type I NKT Cells Prevents Inflammatory Liver Disease. J. Clin. Investig. 2007, 117, 2302–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imataki, O.; Arai, T.; Yamaoka, G.; Matsuoka, A.; Uemura, M. NKT Cell-Infiltrating Aseptic Meningitis on the Central Nervous System in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Dasatinib. Ann. Hematol. 2014, 93, 1935–1936. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, E.; Terabe, M.; Halder, R.C.; Peng, J.; Takaku, S.; Miyake, S.; Yamamura, T.; Kumar, V.; Berzofsky, J.A. Cross-Regulation between Type I and Type II NKT Cells in Regulating Tumor Immunity: A New Immunoregulatory Axis. J. Immunol. 2007, 179, 5126–5136. [Google Scholar] [CrossRef] [Green Version]
- Paget, C.; Chow, M.T.; Duret, H.; Mattarollo, S.R.; Smyth, M.J. Role of Γδ T Cells in α-Galactosylceramide-Mediated Immunity. J. Immunol. 2012, 188, 3928–3939. [Google Scholar] [CrossRef]
- Jin, N.; Miyahara, N.; Roark, C.L.; French, J.D.; Aydintug, M.K.; Matsuda, J.L.; Gapin, L.; O’Brien, R.L.; Gelfand, E.W.; Born, W.K. Airway Hyperresponsiveness through Synergy of Gammadelta} T Cells and NKT Cells. J. Immunol. 2007, 179, 2961–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, A.; Rausell, A. Primary Immunodeficiencies Suggest Redundancy within the Human Immune System. Sci. Immunol. 2016, 1, eaah5861. [Google Scholar] [CrossRef]
- Cooper, M.D.; Alder, M.N. The Evolution of Adaptive Immune Systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Salou, M.; Legoux, F.; Gilet, J.; Darbois, A.; du Halgouet, A.; Alonso, R.; Richer, W.; Goubet, A.-G.; Daviaud, C.; Menger, L.; et al. A Common Transcriptomic Program Acquired in the Thymus Defines Tissue Residency of MAIT and NKT Subsets. J. Exp. Med. 2018, 216, 133–151. [Google Scholar] [CrossRef]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A Role for Skin Gammadelta T Cells in Wound Repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef]
- Simonian, P.L.; Wehrmann, F.; Roark, C.L.; Born, W.K.; O’Brien, R.L.; Fontenot, A.P. Γδ T Cells Protect against Lung Fibrosis via IL-22. J. Exp. Med. 2010, 207, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-G.; Gong, J.; Yeung, W.S.B.; Haidl, G.; Allam, J.-P. Natural Killer and NKT Cells in the Male Reproductive Tract. J. Reprod. Immunol. 2020, 142, 103178. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, A.; Brigas, H.C.; Sandrock, I.; Ribeiro, M.; Amado, T.; Reinhardt, A.; Demera, A.; Hoenicke, L.; Strowig, T.; Carvalho, T.; et al. Microbiota-Dependent Expansion of Testicular IL-17-Producing Vγ6+ Γδ T Cells upon Puberty Promotes Local Tissue Immune Surveillance. Mucosal Immunol. 2021, 14, 242–252. [Google Scholar] [CrossRef]
- Ribeiro, M.; Brigas, H.C.; Temido-Ferreira, M.; Pousinha, P.A.; Regen, T.; Santa, C.; Coelho, J.E.; Marques-Morgado, I.; Valente, C.A.; Omenetti, S.; et al. Meningeal Γδ T Cell-Derived IL-17 Controls Synaptic Plasticity and Short-Term Memory. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The Determinants of Tumour Immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Griffin, J.L.; Shockcor, J.P. Metabolic Profiles of Cancer Cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The Interplay between Cell Signalling and the Mevalonate Pathway in Cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Metelitsa, L.S. Anti-Tumor Potential of Type-I NKT Cells against CD1d-Positive and CD1d-Negative Tumors in Humans. Clin. Immunol. 2011, 140, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Asgharzadeh, S.; Salo, J.; Engell, K.; Wu, H.; Sposto, R.; Ara, T.; Silverman, A.M.; DeClerck, Y.A.; Seeger, R.C.; et al. Valpha24-Invariant NKT Cells Mediate Antitumor Activity via Killing of Tumor-Associated Macrophages. J. Clin. Investig. 2009, 119, 1524–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, G.; Livingston, P.O. Ganglioside Antigens Expressed by Human Cancer Cells. Semin. Cancer Biol. 1991, 2, 401–409. [Google Scholar] [PubMed]
- Gentilini, M.V.; Pérez, M.E.; Fernández, P.M.; Fainboim, L.; Arana, E. The Tumor Antigen N-Glycolyl-GM3 Is a Human CD1d Ligand Capable of Mediating B Cell and Natural Killer T Cell Interaction. Cancer Immunol. Immunother. 2016, 65, 551–562. [Google Scholar] [CrossRef]
- Wu, D.Y.; Segal, N.H.; Sidobre, S.; Kronenberg, M.; Chapman, P.B. Cross-Presentation of Disialoganglioside GD3 to Natural Killer T Cells. J. Exp. Med. 2003, 198, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-E.; Wu, D.Y.; Prendes, M.; Lu, S.X.; Ragupathi, G.; Schrantz, N.; Chapman, P.B. Fine Specificity of Natural Killer T Cells against GD3 Ganglioside and Identification of GM3 as an Inhibitory Natural Killer T-Cell Ligand. Immunology 2008, 123, 145–155. [Google Scholar] [CrossRef]
- Mallevaey, T.; Clarke, A.J.; Scott-Browne, J.P.; Young, M.H.; Roisman, L.C.; Pellicci, D.G.; Patel, O.; Vivian, J.P.; Matsuda, J.L.; McCluskey, J.; et al. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity 2011, 34, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Tiper, I.V.; Temkin, S.M.; Spiegel, S.; Goldblum, S.E.; Giuntoli, R.L.; Oelke, M.; Schneck, J.P.; Webb, T.J. VEGF Potentiates GD3-Mediated Immunosuppression by Human Ovarian Cancer Cells. Clin. Cancer Res. 2016, 22, 4249–4258. [Google Scholar] [CrossRef] [Green Version]
- Webb, T.J.; Li, X.; Giuntoli, R.L.; Lopez, P.H.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular Identification of GD3 as a Suppressor of the Innate Immune Response in Ovarian Cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT Cells in Patients with Relapsed or Refractory Neuroblastoma: An Interim Analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.N.; Liu, B.; Di Pierro, E.J.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef] [Green Version]
- Heczey, A.; Liu, D.; Tian, G.; Courtney, A.N.; Wei, J.; Marinova, E.; Gao, X.; Guo, L.; Yvon, E.; Hicks, J.; et al. Invariant NKT Cells with Chimeric Antigen Receptor Provide a Novel Platform for Safe and Effective Cancer Immunotherapy. Blood 2014, 124, 2824–2833. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Weissman, A.M. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer 2010, 1, 764–778. [Google Scholar] [CrossRef]
- Govindarajan, S.; Verheugen, E.; Venken, K.; Gaublomme, D.; Maelegheer, M.; Cloots, E.; Gysens, F.; De Geest, B.G.; Cheng, T.-Y.; Moody, D.B.; et al. ER Stress in Antigen-Presenting Cells Promotes NKT Cell Activation through Endogenous Neutral Lipids. EMBO Rep. 2020, 21, e48927. [Google Scholar] [CrossRef]
- Singh, A.K.; Tripathi, P.; Cardell, S.L. Type II NKT Cells: An Elusive Population with Immunoregulatory Properties. Front. Immunol. 2018, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomqvist, M.; Rhost, S.; Teneberg, S.; Löfbom, L.; Osterbye, T.; Brigl, M.; Månsson, J.-E.; Cardell, S.L. Multiple Tissue-Specific Isoforms of Sulfatide Activate CD1d-Restricted Type II NKT Cells. Eur. J. Immunol. 2009, 39, 1726–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Suzuki, T. Role of Sulfatide in Normal and Pathological Cells and Tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef] [Green Version]
- Gober, H.-J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T Cell Receptor Gammadelta Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Scotet, E.; Martinez, L.O.; Grant, E.; Barbaras, R.; Jenö, P.; Guiraud, M.; Monsarrat, B.; Saulquin, X.; Maillet, S.; Estève, J.-P.; et al. Tumor Recognition Following Vgamma9Vdelta2 T Cell Receptor Interactions with a Surface F1-ATPase-Related Structure and Apolipoprotein A-I. Immunity 2005, 22, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Allen, S.; McDonald, E.; Das, I.; Mak, J.Y.W.; Liu, L.; Fairlie, D.P.; Meehan, B.S.; Chen, Z.; Corbett, A.J.; et al. MAIT Cells Promote Tumor Initiation, Growth, and Metastases via Tumor MR1. Cancer Discov. 2020, 10, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Kalinichenko, A.; Calogero, S.; Kumar, P.; Paleja, B.; Schmaler, M.; Narang, V.; Zolezzi, F.; Poidinger, M.; Mori, L.; et al. Functionally Diverse Human T Cells Recognize Non-Microbial Antigens Presented by MR1. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Carmi, Y.; Rinott, G.; Dotan, S.; Elkabets, M.; Rider, P.; Voronov, E.; Apte, R.N. Microenvironment-Derived IL-1 and IL-17 Interact in the Control of Lung Metastasis. J. Immunol. 2011, 186, 3462–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-Infiltrating IL-17-Producing Gammadelta T Cells Support the Progression of Tumor by Promoting Angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.A.A.; Rmali, K.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Aberrant Expression of Interleukin-7 (IL-7) and Its Signalling Complex in Human Breast Cancer. Eur. J. Cancer 2004, 40, 494–502. [Google Scholar] [CrossRef]
- Silva, A.; Laranjeira, A.B.A.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Yunes, J.A.; Seddon, B.; Barata, J.T. IL-7 Contributes to the Progression of Human T-Cell Acute Lymphoblastic Leukemias. Cancer Res. 2011, 71, 4780–4789. [Google Scholar] [CrossRef] [Green Version]
- Schroten, C.; Dits, N.F.; Steyerberg, E.W.; Kranse, R.; van Leenders, A.G.J.L.H.; Bangma, C.H.; Kraaij, R. The Additional Value of TGFβ1 and IL-7 to Predict the Course of Prostate Cancer Progression. Cancer Immunol. Immunother. 2012, 61, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patin, E.C.; Soulard, D.; Fleury, S.; Hassane, M.; Dombrowicz, D.; Faveeuw, C.; Trottein, F.; Paget, C. Type I IFN Receptor Signaling Controls IL7-Dependent Accumulation and Activity of Protumoral IL17A-Producing ΓδT Cells in Breast Cancer. Cancer Res. 2018, 78, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rei, M.; Gonçalves-Sousa, N.; Lança, T.; Thompson, R.G.; Mensurado, S.; Balkwill, F.R.; Kulbe, H.; Pennington, D.J.; Silva-Santos, B. Murine CD27(-) Vγ6(+) Γδ T Cells Producing IL-17A Promote Ovarian Cancer Growth via Mobilization of Protumor Small Peritoneal Macrophages. Proc. Natl. Acad. Sci. USA 2014, 111, E3562–E3570. [Google Scholar] [CrossRef] [Green Version]
- Hassane, M.; Jouan, Y.; Creusat, F.; Soulard, D.; Boisseau, C.; Gonzalez, L.; Patin, E.C.; Heuzé-Vourc’h, N.; Sirard, J.-C.; Faveeuw, C.; et al. Interleukin-7 Protects against Bacterial Respiratory Infection by Promoting IL-17A-Producing Innate T-Cell Response. Mucosal Immunol. 2020, 13, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.-L.; Pang, D.J.; Haque, S.F.Y.; Potocnik, A.J.; Pennington, D.J.; Hayday, A.C. Interleukin 7 (IL-7) Selectively Promotes Mouse and Human IL-17-Producing Γδ Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17549–17554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, K.E.; Kim, H.-O.; Kyparissoudis, K.; Corpuz, T.M.; Pinget, G.V.; Uldrich, A.P.; Brink, R.; Belz, G.T.; Cho, J.-H.; Godfrey, D.I.; et al. IL-17-Producing NKT Cells Depend Exclusively on IL-7 for Homeostasis and Survival. Mucosal Immunol. 2014, 7, 1058–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.-Z.; Jo, J.; Tan, A.T.; Sandalova, E.; Chia, A.; Tan, K.C.; Lee, K.H.; Gehring, A.J.; De Libero, G.; Bertoletti, A. IL-7 Licenses Activation of Human Liver Intrasinusoidal Mucosal-Associated Invariant T Cells. J. Immunol. 2013, 190, 3142–3152. [Google Scholar] [CrossRef]
- Casetti, R.; Agrati, C.; Wallace, M.; Sacchi, A.; Martini, F.; Martino, A.; Rinaldi, A.; Malkovsky, M. Cutting Edge: TGF-Beta1 and IL-15 Induce FOXP3+ Gammadelta Regulatory T Cells in the Presence of Antigen Stimulation. J. Immunol. 2009, 183, 3574–3577. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Cui, Q.; Gu, Y.; Sheng, L.; Wu, K.; Shi, J.; Tan, Y.; Fu, H.; Liu, L.; Fu, S.; et al. Decitabine Facilitates the Generation and Immunosuppressive Function of Regulatory ΓδT Cells Derived from Human Peripheral Blood Mononuclear Cells. Leukemia 2013, 27, 1580–1585. [Google Scholar] [CrossRef]
- Monteiro, M.; Almeida, C.F.; Caridade, M.; Ribot, J.C.; Duarte, J.; Agua-Doce, A.; Wollenberg, I.; Silva-Santos, B.; Graca, L. Identification of Regulatory Foxp3+ Invariant NKT Cells Induced by TGF-Beta. J. Immunol. 2010, 185, 2157–2163. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Teixeira, L.; Resende, M.; Devergne, O.; Herbeuval, J.-P.; Hermine, O.; Schneider, E.; Dy, M.; Cordeiro-da-Silva, A.; Leite-de-Moraes, M.C. Rapamycin Combined with TGF-β Converts Human Invariant NKT Cells into Suppressive Foxp3+ Regulatory Cells. J. Immunol. 2012, 188, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Wu, P.; Cheng, P.; Zhang, Z.; Wang, Z.; Yu, X.; Shao, X.; Wu, D.; Ye, J.; Zhang, T.; et al. Tumor-Infiltrating CD39+γδTregs Are Novel Immunosuppressive T Cells in Human Colorectal Cancer. Oncoimmunology 2017, 6, e1277305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Yin, S.; Zhang, J.; Hu, Y.; Huang, B.; Cui, L.; Kang, N.; He, W. A New Effect of IL-4 on Human Γδ T Cells: Promoting Regulatory Vδ1 T Cells via IL-10 Production and Inhibiting Function of Vδ2 T Cells. Cell Mol. Immunol. 2016, 13, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Dong, S.; Xia, S.; He, W.; Jia, H.; Zhang, S.; Wei, J.; O’Brien, R.L.; Born, W.K.; Wu, Z.; et al. Regulatory Role of Vγ1 Γδ T Cells in Tumor Immunity through IL-4 Production. J. Immunol. 2011, 187, 4979–4986. [Google Scholar] [CrossRef]
- Terabe, M.; Matsui, S.; Noben-Trauth, N.; Chen, H.; Watson, C.; Donaldson, D.D.; Carbone, D.P.; Paul, W.E.; Berzofsky, J.A. NKT Cell-Mediated Repression of Tumor Immunosurveillance by IL-13 and the IL-4R-STAT6 Pathway. Nat. Immunol. 2000, 1, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. Interleukin-21: A Double-Edged Sword with Therapeutic Potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Chabab, G.; Bonnefoy, N.; Lafont, V. IL-21 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 73–82. [Google Scholar] [CrossRef]
- Chabab, G.; Barjon, C.; Abdellaoui, N.; Salvador-Prince, L.; Dejou, C.; Michaud, H.-A.; Boissière-Michot, F.; Lopez-Crapez, E.; Jacot, W.; Pourquier, D.; et al. Identification of a Regulatory Vδ1 Gamma Delta T Cell Subpopulation Expressing CD73 in Human Breast Cancer. J. Leukoc. Biol. 2020, 107, 1057–1067. [Google Scholar] [CrossRef]
- Barjon, C.; Michaud, H.-A.; Fages, A.; Dejou, C.; Zampieri, A.; They, L.; Gennetier, A.; Sanchez, F.; Gros, L.; Eliaou, J.-F.; et al. IL-21 Promotes the Development of a CD73-Positive Vγ9Vδ2 T Cell Regulatory Population. Oncoimmunology 2017, 7, e1379642. [Google Scholar] [CrossRef] [Green Version]
- Coquet, J.M.; Skak, K.; Davis, I.D.; Smyth, M.J.; Godfrey, D.I. IL-21 Modulates Activation of NKT Cells in Patients with Stage IV Malignant Melanoma. Clin. Transl. Immunol. 2013, 2, e6. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with PH Regulation in Tumours as a Therapeutic Strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, W.R.; Hay, M.P. Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Delgoffe, G.M. Metabolic Barriers to Cancer Immunotherapy. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhu, S.; Bai, L. Lactic Acid in Tumor Microenvironments Causes Dysfunction of NKT Cells by Interfering with MTOR Signaling. Sci. China Life Sci. 2016, 59, 1290–1296. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; He, K.; Tian, C.; Sun, H.; Zhu, C.; Bai, S.; Liu, J.; Wu, Q.; Xie, D.; Yue, T.; et al. Impaired Lipid Biosynthesis Hinders Anti-Tumor Efficacy of Intratumoral INKT Cells. Nat. Commun. 2020, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, A.; Loftus, R.M.; Pisarska, M.M.; Tobin, L.M.; Bergin, R.; Wood, N.A.W.; Foley, C.; Mat, A.; Tinley, F.C.; Bannan, C.; et al. Obesity Reduces MTORC1 Activity in Mucosal-Associated Invariant T Cells, Driving Defective Metabolic and Functional Responses. J. Immunol. 2019, 202, 3404–3411. [Google Scholar] [CrossRef]
- O’Neill, C.; Cassidy, F.C.; O’Shea, D.; Hogan, A.E. Mucosal Associated Invariant T Cells in Cancer-Friend or Foe? Cancers 2021, 13, 1582. [Google Scholar] [CrossRef]
- Martinet, L.; Fleury-Cappellesso, S.; Gadelorge, M.; Dietrich, G.; Bourin, P.; Fournié, J.-J.; Poupot, R. A Regulatory Cross-Talk between Vgamma9Vdelta2 T Lymphocytes and Mesenchymal Stem Cells. Eur. J. Immunol. 2009, 39, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Jean, C.; Dietrich, G.; Fournié, J.-J.; Poupot, R. PGE2 Inhibits Natural Killer and Gamma Delta T Cell Cytotoxicity Triggered by NKR and TCR through a CAMP-Mediated PKA Type I-Dependent Signaling. Biochem. Pharmacol. 2010, 80, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Gonnermann, D.; Oberg, H.-H.; Kellner, C.; Peipp, M.; Sebens, S.; Kabelitz, D.; Wesch, D. Resistance of Cyclooxygenase-2 Expressing Pancreatic Ductal Adenocarcinoma Cells against Γδ T Cell Cytotoxicity. Oncoimmunology 2015, 4, e988460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung Cancer Is Associated with Decreased Expression of Perforin, Granzyme B and Interferon (IFN)-γ by Infiltrating Lung Tissue T Cells, Natural Killer (NK) T-like and NK Cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Klatka, J.; Grywalska, E.; Hymos, A.; Guz, M.; Polberg, K.; Roliński, J.; Stepulak, A. Cyclooxygenase-2 Inhibition Enhances Proliferation of NKT Cells Derived from Patients with Laryngeal Cancer. Anticancer Res. 2017, 37, 4059–4066. [Google Scholar] [CrossRef] [Green Version]
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.C.; Fiala, G.J.; Agudelo, L.Z.; Dyck, L.; Kane, H.; et al. Distinct Metabolic Programs Established in the Thymus Control Effector Functions of Γδ T Cell Subsets in Tumor Microenvironments. Nat. Immunol. 2021, 22, 179–192. [Google Scholar] [CrossRef]
- Ko, J.S.; Koh, J.M.; So, J.-S.; Jeon, Y.K.; Kim, H.Y.; Chung, D.H. Palmitate Inhibits Arthritis by Inducing T-Bet and Gata-3 MRNA Degradation in INKT Cells via IRE1α-Dependent Decay. Sci. Rep. 2017, 7, 14940. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, H.-J.; Kim, C.W.; Kim, H.C.; Jung, Y.; Lee, H.-S.; Lee, Y.; Ju, Y.S.; Oh, J.E.; Park, S.-H.; et al. Tumor Hypoxia Represses Γδ T Cell-Mediated Antitumor Immunity against Brain Tumors. Nat. Immunol. 2021, 22, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Wu, X.; Sun, R.; Chen, Y.; Zheng, X.; Bai, L.; Lian, Z.; Wei, H.; Tian, Z. Oral Ampicillin Inhibits Liver Regeneration by Breaking Hepatic Innate Immune Tolerance Normally Maintained by Gut Commensal Bacteria. Hepatology 2015, 62, 253–264. [Google Scholar] [CrossRef]
- Qian, X.; Chen, H.; Wu, X.; Hu, L.; Huang, Q.; Jin, Y. Interleukin-17 Acts as Double-Edged Sword in Anti-Tumor Immunity and Tumorigenesis. Cytokine 2017, 89, 34–44. [Google Scholar] [CrossRef]
- Lefrancois, L.; Goodman, T. In Vivo Modulation of Cytolytic Activity and Thy-1 Expression in TCR-Gamma Delta+ Intraepithelial Lymphocytes. Science 1989, 243, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Bailey, J.M.; Alsina, J.; Nirschl, C.J.; Sharma, R.; Fan, H.; Rattigan, Y.; Roeser, J.C.; Lankapalli, R.H.; Zhang, H.; et al. Oncogenic Kras Activates a Hematopoietic-to-Epithelial IL-17 Signaling Axis in Preinvasive Pancreatic Neoplasia. Cancer Cell 2014, 25, 621–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 Can Promote Tumor Growth through an IL-6-Stat3 Signaling Pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; Le Bourhis, L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J.; et al. Identification of an Innate T Helper Type 17 Response to Intestinal Bacterial Pathogens. Nat. Med. 2011, 17, 837–844. [Google Scholar] [CrossRef]
- Wingender, G.; Stepniak, D.; Krebs, P.; Lin, L.; McBride, S.; Wei, B.; Braun, J.; Mazmanian, S.K.; Kronenberg, M. Intestinal Microbes Affect Phenotypes and Functions of Invariant Natural Killer T Cells in Mice. Gastroenterology 2012, 143, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial Exposure during Early Life Has Persistent Effects on Natural Killer T Cell Function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Sundström, P.; Ahlmanner, F.; Akéus, P.; Sundquist, M.; Alsén, S.; Yrlid, U.; Börjesson, L.; Sjöling, Å.; Gustavsson, B.; Wong, S.B.J.; et al. Human Mucosa-Associated Invariant T Cells Accumulate in Colon Adenocarcinomas but Produce Reduced Amounts of IFN-γ. J. Immunol. 2015, 195, 3472–3481. [Google Scholar] [CrossRef] [Green Version]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Duan, M.; Goswami, S.; Shi, J.-Y.; Wu, L.-J.; Wang, X.-Y.; Ma, J.-Q.; Zhang, Z.; Shi, Y.; Ma, L.-J.; Zhang, S.; et al. Activated and Exhausted MAIT Cells Foster Disease Progression and Indicate Poor Outcome in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 3304–3316. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Oh, S.F.; Olszak, T.; Neves, J.F.; Avci, F.Y.; Erturk-Hasdemir, D.; Lu, X.; Zeissig, S.; Blumberg, R.S.; Kasper, D.L. Sphingolipids from a Symbiotic Microbe Regulate Homeostasis of Host Intestinal Natural Killer T Cells. Cell 2014, 156, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Ridaura, V.K.; Bouladoux, N.; Claesen, J.; Chen, Y.E.; Byrd, A.L.; Constantinides, M.G.; Merrill, E.D.; Tamoutounour, S.; Fischbach, M.A.; Belkaid, Y. Contextual Control of Skin Immunity and Inflammation by Corynebacterium. J. Exp. Med. 2018, 215, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via Γδ T Cells. Cell 2019, 176, 998.e16–1013.e16. [Google Scholar] [CrossRef] [Green Version]
- Selvanantham, T.; Lin, Q.; Guo, C.X.; Surendra, A.; Fieve, S.; Escalante, N.K.; Guttman, D.S.; Streutker, C.J.; Robertson, S.J.; Philpott, D.J.; et al. NKT Cell-Deficient Mice Harbor an Altered Microbiota That Fuels Intestinal Inflammation during Chemically Induced Colitis. J. Immunol. 2016, 197, 4464–4472. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome-Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A Role for G-CSF and GM-CSF in Nonmyeloid Cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef]
- Van Hede, D.; Polese, B.; Humblet, C.; Wilharm, A.; Renoux, V.; Dortu, E.; de Leval, L.; Delvenne, P.; Desmet, C.J.; Bureau, F.; et al. Human Papillomavirus Oncoproteins Induce a Reorganization of Epithelial-Associated Γδ T Cells Promoting Tumor Formation. Proc. Natl. Acad. Sci. USA 2017, 114, E9056–E9065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulig, P.; Burkhard, S.; Mikita-Geoffroy, J.; Croxford, A.L.; Hövelmeyer, N.; Gyülvészi, G.; Gorzelanny, C.; Waisman, A.; Borsig, L.; Becher, B. IL17A-Mediated Endothelial Breach Promotes Metastasis Formation. Cancer Immunol. Res. 2016, 4, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Akther, H.D.; Hackstein, C.-P.; Powell, K.; King, T.; Friedrich, M.; Christoforidou, Z.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; et al. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019, 28, 3077.e5–3091.e5. [Google Scholar] [CrossRef] [Green Version]
- Hinks, T.S.C.; Marchi, E.; Jabeen, M.; Olshansky, M.; Kurioka, A.; Pediongco, T.J.; Meehan, B.S.; Kostenko, L.; Turner, S.J.; Corbett, A.J.; et al. Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality. Cell Rep. 2019, 28, 3249.e5–3262.e5. [Google Scholar] [CrossRef] [Green Version]
- Xie, K. Interleukin-8 and Human Cancer Biology. Cytokine Growth Factor Rev. 2001, 12, 375–391. [Google Scholar] [CrossRef]
- Khosravi, N.; Caetano, M.S.; Cumpian, A.M.; Unver, N.; De la Garza Ramos, C.; Noble, O.; Daliri, S.; Hernandez, B.J.; Gutierrez, B.A.; Evans, S.E.; et al. IL22 Promotes Kras-Mutant Lung Cancer by Induction of a Protumor Immune Response and Protection of Stemness Properties. Cancer Immunol. Res. 2018, 6, 788–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.-F. Tumor-Infiltrating Gammadelta T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 Ligand Galectin-9 Negatively Regulates T Helper Type 1 Immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. ΓδT17 Cells Promote the Accumulation and Expansion of Myeloid-Derived Suppressor Cells in Human Colorectal Cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Zhu, M.; Marley, J.L.; Bi, K.; Wang, K.; Zhai, M.; Hu, H.; Guo, P.; Li, C.; Xu, Y.; et al. The Combined Action of Monocytic Myeloid-Derived Suppressor Cells and Mucosal-Associated Invariant T Cells Promotes the Progression of Cervical Cancer. Int. J. Cancer 2021, 148, 1499–1507. [Google Scholar] [CrossRef]
- Terabe, M.; Matsui, S.; Park, J.-M.; Mamura, M.; Noben-Trauth, N.; Donaldson, D.D.; Chen, W.; Wahl, S.M.; Ledbetter, S.; Pratt, B.; et al. Transforming Growth Factor-Beta Production and Myeloid Cells Are an Effector Mechanism through Which CD1d-Restricted T Cells Block Cytotoxic T Lymphocyte-Mediated Tumor Immunosurveillance: Abrogation Prevents Tumor Recurrence. J. Exp. Med. 2003, 198, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Minoda, Y.; Meredith, T.; Cameron, G.; Philipp, M.-S.; Pellicci, D.G.; Corbett, A.J.; Kurts, C.; Gray, D.H.; Godfrey, D.I.; et al. Chronically Stimulated Human MAIT Cells Are Unexpectedly Potent IL-13 Producers. Immunol. Cell Biol. 2019, 97, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sedimbi, S.; Löfbom, L.; Singh, A.K.; Porcelli, S.A.; Cardell, S.L. Unique Invariant Natural Killer T Cells Promote Intestinal Polyps by Suppressing TH1 Immunity and Promoting Regulatory T Cells. Mucosal Immunol. 2018, 11, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venken, K.; Decruy, T.; Aspeslagh, S.; Van Calenbergh, S.; Lambrecht, B.N.; Elewaut, D. Bacterial CD1d-Restricted Glycolipids Induce IL-10 Production by Human Regulatory T Cells upon Cross-Talk with Invariant NKT Cells. J. Immunol. 2013, 191, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A Potent Suppressor of Antitumor Immune Responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T Cells Mediated by Galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Zambirinis, C.P.; Seifert, L.; Akkad, N.; Mohan, N.; Werba, G.; Barilla, R.; Torres-Hernandez, A.; Hundeyin, M.; Mani, V.R.K.; et al. Γδ T Cells Support Pancreatic Oncogenesis by Restraining Aβ T Cell Activation. Cell 2016, 166, 1485.e15–1499.e15. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Robert, J. Evolutionary Underpinnings of Innate-Like T Cell Interactions with Cancer. Immunol. Investig. 2019, 48, 737–758. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer Immunotherapy with Γδ T Cells: Many Paths Ahead of Us. Cell Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Cogswell, D.T.; Gapin, L.; Tobin, H.M.; McCarter, M.D.; Tobin, R.P. MAIT Cells: Partners or Enemies in Cancer Immunotherapy? Cancers 2021, 13, 1502. [Google Scholar] [CrossRef]
- Aehnlich, P.; Carnaz Simões, A.M.; Skadborg, S.K.; Holmen Olofsson, G.; Thor Straten, P. Expansion with IL-15 Increases Cytotoxicity of Vγ9Vδ2 T Cells and Is Associated with Higher Levels of Cytotoxic Molecules and T-Bet. Front. Immunol. 2020, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Iyoda, T.; Vizcardo, R.; Shimizu, K.; Sato, Y.; Endo, T.A.; Kitahara, G.; Okoshi, M.; Kobayashi, M.; Sakurai, M.; et al. Efficient Regeneration of Human Vα24+ Invariant Natural Killer T Cells and Their Anti-Tumor Activity In Vivo. Stem Cells 2016, 34, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Parrot, T.; Healy, K.; Boulouis, C.; Sobkowiak, M.J.; Leeansyah, E.; Aleman, S.; Bertoletti, A.; Sällberg Chen, M.; Sandberg, J.K. Expansion of Donor-Unrestricted MAIT Cells with Enhanced Cytolytic Function Suitable for TCR Redirection. JCI Insight 2021, 6, 140074. [Google Scholar] [CrossRef] [PubMed]
- Akkapeddi, P.; Fragoso, R.; Hixon, J.A.; Ramalho, A.S.; Oliveira, M.L.; Carvalho, T.; Gloger, A.; Matasci, M.; Corzana, F.; Durum, S.K.; et al. A Fully Human Anti-IL-7Rα Antibody Promotes Antitumor Activity against T-Cell Acute Lymphoblastic Leukemia. Leukemia 2019, 33, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; CANTOS Trial Group. Effect of Interleukin-1β Inhibition with Canakinumab on Incident Lung Cancer in Patients with Atherosclerosis: Exploratory Results from a Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 Therapy Elevates Treg/Th Balance and Increases Tumor Cell PSmad3 That Are Both Targeted by α-TGFβ Antibody to Promote Durable Rejection and Immunity in Squamous Cell Carcinomas. J. Immunother. Cancer 2019, 7, 62. [Google Scholar] [CrossRef]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic Reprogramming of Natural Killer Cells in Obesity Limits Antitumor Responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Wang, T.; Gnanaprakasam, J.N.R.; Chen, X.; Kang, S.; Xu, X.; Sun, H.; Liu, L.; Rodgers, H.; Miller, E.; Cassel, T.A.; et al. Inosine Is an Alternative Carbon Source for CD8+-T-Cell Function under Glucose Restriction. Nat. Metab. 2020, 2, 635–647. [Google Scholar] [CrossRef]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Singh, K.; Ritchie, T.M.; Aguiar, J.A.; Fan, I.Y.; Portillo, A.L.; Rojas, E.A.; Vahedi, F.; El-Sayes, A.; Xing, S.; et al. Metabolic Flexibility Determines Human NK Cell Functional Fate in the Tumor Microenvironment. Cell Metab. 2021, 33, 1205.e5–1220.e5. [Google Scholar] [CrossRef]
- Qiu, J.; Villa, M.; Sanin, D.E.; Buck, M.D.; O’Sullivan, D.; Ching, R.; Matsushita, M.; Grzes, K.M.; Winkler, F.; Chang, C.-H.; et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep. 2019, 27, 2063.e5–2074.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verykokakis, M.; Kee, B.L. Transcriptional and Epigenetic Regulation of Innate-like T Lymphocyte Development. Curr. Opin Immunol. 2018, 51, 39–45. [Google Scholar] [CrossRef]
- Inácio, D.P.; Amado, T.; Silva-Santos, B.; Gomes, A.Q. Control of T Cell Effector Functions by MiRNAs. Cancer Lett. 2018, 427, 63–73. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Subedi, K.; Yi, Q.; Zhou, L.; Mi, Q.-S. MicroRNA-155 Regulates MAIT1 and MAIT17 Cell Differentiation. Front. Cell Dev. Biol. 2021, 9, 670531. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, N.; Papotto, P.H.; Romero, P.V.; Amado, T.; Enguita, F.J.; Amorim, A.; Rodrigues, A.F.; Gordon, K.E.; Coroadinha, A.S.; Boldin, M.; et al. MicroRNA-146a Controls Functional Plasticity in Γδ T Cells by Targeting NOD1. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Rollins, D.; Ruhn, K.A.; Stubblefield, J.J.; Green, C.B.; Kashiwada, M.; Rothman, P.B.; Takahashi, J.S.; Hooper, L.V. TH17 Cell Differentiation Is Regulated by the Circadian Clock. Science 2013, 342, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.-P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
| Lineages | γδT Cells | MAIT Cells | iNKT Cells | vNKT Cells | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Mouse | Human | Mouse | Human | Mouse | Human | Mouse | Human | |
| TCR repertoire | Restricted including germline-encoded TCRs (e.g., Vγ1Vδ6.3, Vγ5Vδ1, Vγ6Vδ1) [5] | Semi-invariant or variant Restricted number of γ and δ chains [5] | Semi-invariant Vα19-Jα33 Restricted number of β chains [6,7] | Semi-invariant Vα7.2-Jα33 Restricted number of β chains [7] | Semi-invariant Vα14-Jα18 Restricted number of β chains [8] | Semi-invariant Vα24-Jα18 Restricted number of β chains [8] | Diverse, including oligoclonal Vα3.2Jα7, Vα1Jα9 Restricted number of β chains including Vβ8.1 and Vβ3.1 segments [9] | Diverse [9] | |
| Restricting elements | Mainly unknown CD1d T10/T22 butyrophilin-like molecules (Skint-1) [10] | Butyrophilin 3A1/2A1 (Vγ9Vδ2) CD1d (Vδ1) CD1c viral glycoproteins [11] | MR1 [12] | CD1d [13] | CD1d [9] | ||||
| TCR ligands | Natural | Unknown Cardiolipin Phycoerythrin [14] | IPP, HMBPP (Vγ9Vδ2) Sulfatide and α-GalCer (Vδ1) EPCR (Vγ4Vδ5) [15] | Microbial-derived vitamin B2 metabolites (5-OP-RU, 5-OE-RU) [16] | α-GalCer Microbial α-derived glycolipids Ganglioside [17,18,19] | Sulfatide, LPC, PG Hydrophobic peptides β-GlcCer, LysoGL1 [20] | Sulfatide, LPC, PG β-GlcCer, LysoGL1 [20] | ||
| Synthetic | - | Zoledronate *, Pamidronate * (Vγ9Vδ2) [21] | 5-OP-RU derivatives [16,22] | KRN7000 ** and derivatives Ganglioside (C24:1 GM3 and GD3) [23,24] | KRN7000 ** and derivatives [25] | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsac, E.; de Amat Herbozo, C.; Gonzalez, L.; Baranek, T.; Mallevaey, T.; Paget, C. Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers 2021, 13, 3578. https://doi.org/10.3390/cancers13143578
Barsac E, de Amat Herbozo C, Gonzalez L, Baranek T, Mallevaey T, Paget C. Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers. 2021; 13(14):3578. https://doi.org/10.3390/cancers13143578
Chicago/Turabian StyleBarsac, Emilie, Carolina de Amat Herbozo, Loïc Gonzalez, Thomas Baranek, Thierry Mallevaey, and Christophe Paget. 2021. "Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors" Cancers 13, no. 14: 3578. https://doi.org/10.3390/cancers13143578
APA StyleBarsac, E., de Amat Herbozo, C., Gonzalez, L., Baranek, T., Mallevaey, T., & Paget, C. (2021). Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers, 13(14), 3578. https://doi.org/10.3390/cancers13143578






