NUPR1: A Critical Regulator of the Antioxidant System
Abstract
:Simple Summary
Abstract
1. Introduction
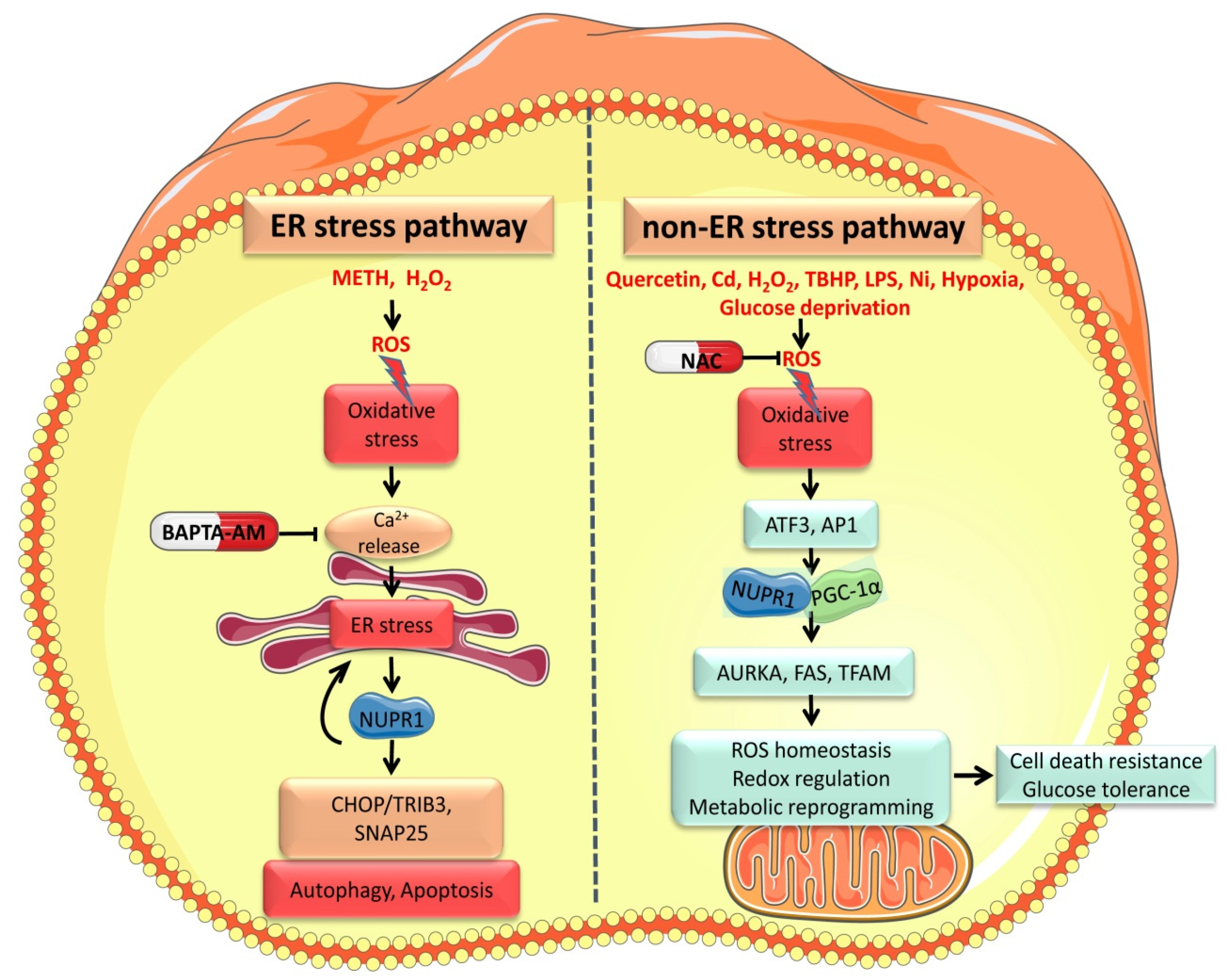
2. Oxidative Stress Activates NUPR1
2.1. Oxidative Stress Regulates NUPR1 Expression via a ER Stress-Mediated Pathway
2.2. Oxidative Stress Mediates NUPR1 Expression through Non-ER Pathways
3. NUPR1 Controls Redox Homeostasis and Protects Mitochondria
3.1. NUPR1 Regulates the Antioxidant System
3.2. NUPR1 Maintains Mitochondrial Function
3.3. NUPR1 Regulates Energy Metabolism
4. NUPR1 Is a Key Factor of Ferroptosis
4.1. NUPR1 Regulates Multiple Transcription Factors Involved in Ferroptosis
4.2. NUPR1 Controls Iron Metabolism
4.3. NUPR1 Regulates Mitochondrial-Related Ferroptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| NUPR1 | Nuclear protein 1 |
| IDP | Intrinsically disordered protein |
| ER | Endoplasmic reticulum |
| ROS | Reactive oxygen species |
| KRAS | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog |
| NAC | N-acetyl cysteine |
| Cd | Cadmium |
| GSH | Glutathione |
| METH | Methamphetamine |
| mTOR | Mechanistic target of rapamycin |
| UPS | Ubiquitin-proteasome system |
| PDAC | Pancreas ductal adenocarcinoma |
| HCC | Hepatocarcinoma |
| TFP | Trifluoperazine |
| PDX | Patient-derived xenograft |
| IKE | Imidazole ketone erastin |
| 5-FU | 5-fluorouracil |
| TMZ | Temozolomide |
| UPR | Unfolded protein response |
| TBHP | Tert-butyl hydroperoxide |
| ATF3 | Activating transcription factor 3 |
| eIF2α | Eukaryotic initiation factor 2α |
| GRP75 | Glucose-regulated protein 75 |
| GRP94 | Glucose-regulated protein 94 |
| H2O2 | Hydrogen peroxide |
| AP-1 | Activator protein 1 |
| Ni | Nickel |
| Ca2+ | Calcium ions |
| Fe2+ | Ferrous iron |
| HO• | Hydroxyl radical |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| AKR1C1 | Aldo-keto reductase family 1 member C1 |
| SNAP25 | Synaptosome Associated Protein 25 |
| MMP | Mitochondrial membrane potential |
| TCA | Tricarboxylic acid cycle |
| AURKA | Aurora kinase A |
| Nox1 | NADPH oxidase 1 |
| DHODH | dihydroorotate dehydrogenase |
| BQR | Brequinar sodium |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| PGC-1α | Coactivator 1-alpha |
| FAS | Fatty acid synthase |
| HFD | High-fat diet |
| LPS | Lipopolysaccharide |
| OXPHOS | Oxidative phosphorylation |
| GST | Glutathione S-transferase |
| SOD | Superoxide dismutase |
| MPO | Myeloperoxidase |
| HO-1 | Heme oxygenase-1 |
| MEF | Mouse embryonic fibroblasts |
| ZnPP | Zinc protoporphyrin IX |
| LCN2 | Lipocalin 2 |
| RUNX2 | Runt-related transcription factor 2 |
| MitoQ | Mitoquinone |
| PUFAs | Polyunsaturated fatty acids |
| TFAM | Mitochondrial transcription factor A |
| LONP1 | Lon protease |
| NFAT | Nuclear factor of activated T cells |
References
- Pommier, R.; Gout, J.; Vincent, D.F.; Cano, C.E.; Kaniewski, B.; Martel, S.; Rodriguez, J.; Fourel, G.; Valcourt, U.; Marie, J.; et al. The human NUPR1/P8 gene is transcriptionally activated by transforming growth factor β via the SMAD signalling pathway. Biochem. J. 2012, 445, 285–293. [Google Scholar] [CrossRef]
- Cano, C.E.; Hamidi, T.; Sandi, M.J.; Iovanna, J. Nupr1: The Swiss-knife of cancer. J. Cell. Physiol. 2011, 226, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Giampietri, C.; Petrungaro, S.; Conti, S.; Facchiano, A.; Filippini, A.; Ziparo, E. Cancer Microenvironment and Endoplasmic Reticulum Stress Response. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avril, T.; Vauléon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Rizzuti, B.; Xia, Y.; Abian, O.; Peng, L.; Velazquez-Campoy, A.; Neira, J.L.; Iovanna, J. Targeting intrinsically disordered proteins involved in cancer. Cell. Mol. Life Sci. 2020, 77, 1695–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idelchik, M.D.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef]
- Qu, J.; Zou, T.; Lin, Z. The Roles of the Ubiquitin–Proteasome System in the Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2021, 22, 1526. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic Reticulum Stress Sensing in the Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.K.; Chae, S.-W.; Kim, H.-R.; Chae, H.J. Endoplasmic Reticulum Stress and Cancer. J. Cancer Prev. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Xu, X.; Huang, E.; Tai, Y.; Zhao, X.; Chen, X.; Chen, C.; Chen, R.; Liu, C.; Lin, Z.; Wang, H.; et al. Nupr1 Modulates Methamphetamine-Induced Dopaminergic Neuronal Apoptosis and Autophagy through CHOP-Trib3-Mediated Endoplasmic Reticulum Stress Signaling Pathway. Front. Mol. Neurosci. 2017, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Huang, E.; Luo, B.; Yang, Y.; Zhang, F.; Liu, C.; Lin, Z.; Xie, W.-B.; Wang, H. Nupr1/Chop signal axis is involved in mitochondrion-related endothelial cell apoptosis induced by methamphetamine. Cell Death Dis. 2016, 7, e2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Huang, E.; Luo, B.; Cai, D.; Zhao, X.; Luo, Q.; Jin, Y.; Chen, L.; Wang, Q.; Liu, C.; et al. Methamphetamine exposure triggers apoptosis and autophagy in neuronal cells by activating the C/EBPβ-related signaling pathway. FASEB J. 2018, 32, 6737–6759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, J.-S. ROS homeostasis and metabolism: A critical liaison for cancer therapy. Exp. Mol. Med. 2016, 48, e269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Sun, J.; Yin, Y.; Sun, Y.; Ma, J.; Zhou, R.; Chang, X.; Li, D.; Yao, Z.; Tian, S.; et al. Transcriptional coregualtor NUPR1 maintains tamoxifen resistance in breast cancer cells. Cell Death Dis. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Emma, M.R.; Iovanna, J.L.; Bachvarov, D.; Puleio, R.; Loria, G.R.; Augello, G.; Candido, S.; Libra, M.; Gulino, A.; Cancila, V.; et al. NUPR1, a new target in liver cancer: Implication in controlling cell growth, migration, invasion and sorafenib resistance. Cell Death Dis. 2016, 7, e2269. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Kapralov, O.; Yang, Q.; Dar, H.H.; Tyurina, Y.; Anthonymuthu, T.S.; Kim, R.; Croix, C.M.S.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Y.; Zhang, Y.; Wen, J.; Cai, N.; Cheng, K.; Liang, H.; Zhang, W. The Molecular Mechanisms of Regulating Oxidative Stress-Induced Ferroptosis and Therapeutic Strategy in Tumors. Oxid. Med. Cell. Longev. 2020, 2020, 8810785. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nat. Cell Biol. 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nat. Cell Biol. 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ping, J.; Wen, Y.; Wu, Y. The Mechanism of Ferroptosis and Applications in Tumor Treatment. Front. Pharmacol. 2020, 11, 1061. [Google Scholar] [CrossRef]
- Murphy, A.; Costa, M. Nuclear protein 1 imparts oncogenic potential and chemotherapeutic resistance in cancer. Cancer Lett. 2020, 494, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, X.; Kuang, F.; Zhang, Q.; Xie, Y.; Kang, R.; Kroemer, G.; Tang, D. NUPR1 is a critical repressor of ferroptosis. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Hamidi, T.; Cano, C.E.; Grasso, D.; Garcia, M.N.; Sandí, M.J.; Calvo, E.L.; Dagorn, J.-C.; Lomberk, G.; Goruppi, S.; Urrutia, R.; et al. NUPR1 works against the metabolic stress-induced autophagy-associated cell death in pancreatic cancer cells. Autophagy 2013, 9, 95–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Zeng, W.; Ouyang, W.; Xu, Q.; Chen, J.; Wang, B.; Zhang, X. Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells. J. Clin. Biochem. Nutr. 2020, 67, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Bolduc, J.-S.; Denizeau, F.; Jumarie, C. Cadmium-Induced Mitochondrial Membrane-Potential Dissipation Does Not Necessarily Require Cytosolic Oxidative Stress: Studies Using Rhodamine-123 Fluorescence Unquenching. Toxicol. Sci. 2004, 77, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. SAGE-Hindawi Access Res. 2011, 2011, 457327. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Chen, Y.; He, Z.; Wang, Q.; Yang, X.; Ren, Z.; Zhang, S. Inhibition of ROS/NUPR1-dependent autophagy antagonises repeated cadmium exposure -induced oral squamous cell carcinoma cell migration and invasion. Toxicol. Lett. 2019, 314, 142–152. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Alam Riaz, T.; Kim, H.-R.; Chae, H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandary, B.; Marahatta, A.; Kim, H.-R.; Chae, H.-J. An Involvement of Oxidative Stress in Endoplasmic Reticulum Stress and Its Associated Diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Morshed, M.; Remex, N.S.; Nitu, S.; Kolluru, G.K.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; et al. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun. Biol. 2020, 3, 682. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Jee, B.A.; Kwon, S.M.; Yoon, Y.-S.; Xu, W.G.; Wang, H.-J.; Wang, X.W.; Thorgeirsson, S.S.; Lee, J.-S.; Woo, H.G.; et al. Identification of a mitochondrial defect gene signature reveals NUPR1 as a key regulator of liver cancer progression. Hepatology 2015, 62, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Iwamoto, Y.; Maru, Y. Oxidative Stress-Responsive Transcription Factor ATF3 Potentially Mediates Diabetic Angiopathy. Mol. Cell. Biol. 2006, 26, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Yammani, R.R.; Loeser, R.F. Brief report: Stress-inducible nuclear protein 1 regulates matrix metalloproteinase 13 expression in human articular chondrocytes. Arth. Rheumatol. 2014, 66, 1266–1271. [Google Scholar] [CrossRef] [Green Version]
- Wenz, C.; Faust, D.; Linz, B.; Turmann, C.; Nikolova, T.; Bertin, J.; Gough, P.; Wipf, P.; Schroder, A.S.; Krautwald, S.; et al. t-BuOOH induces ferroptosis in human and murine cell lines. Arch. Toxicol. 2018, 92, 759–775. [Google Scholar] [CrossRef]
- Vasseur, S.; Hoffmeister, A.; Garcia-Montero, A.; Barthet, M.; Saint-Michel, L.; Berthézène, P.; Fiedler, F.; Closa, D.; Dagorn, J.C.; Iovanna, J.L. Mice with targeted disruption of p8gene show increased sensitivity to lipopolysaccharide and DNA microarray analysis of livers reveals an aberrant gene expression response. BMC Gastroenterol. 2003, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.; Roy, N.; Sun, H.; Jin, C.; Costa, M. Induction of NUPR1 and AP-1 contributes to the carcinogenic potential of nickel. Oncol. Rep. 2021, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, T.; Cano, C.E.; Grasso, D.; Garcia, M.N.; Sandi, M.J.; Calvo, E.L.; Dagorn, J.-C.; Lomberk, G.; Urrutia, R.; Goruppi, S.; et al. Nupr1-Aurora Kinase A Pathway Provides Protection against Metabolic Stress-Mediated Autophagic-Associated Cell Death. Clin. Cancer Res. 2012, 18, 5234–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.A.; So, E.-Y.; Simons, A.L.; Spitz, D.; Ouchi, T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 2012, 3, e249. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Sampaio, H.C.; Drynda, R.; Liu, B.; De Ledesma, A.R.; Malicet, C.; Iovanna, J.; Jones, P.; Muller, D.; Persaud, S. Reduced nuclear protein 1 expression improves insulin sensitivity and protects against diet-induced glucose intolerance through up-regulation of heat shock protein 70. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, E.-J.; Ock, M.-S.; Choi, Y.-H.; Iovanna, J.; Mun, S.; Han, K.; Kim, H.-S.; Cha, H.-J. Human Endogenous Retrovirus (HERV)-K env Gene Knockout Affects Tumorigenic Characteristics of nupr1 Gene in DLD-1 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 3941. [Google Scholar] [CrossRef]
- Pierre, N.; Barbé, C.; Gilson, H.; Deldicque, L.; Raymackers, J.-M.; Francaux, M. Activation of ER stress by hydrogen peroxide in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2014, 450, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.T.; Santofimia-Castaño, P.; Bocchio, M.; Listi, A.; Fraunhoffer, N.; Soubeyran, P.; Chevet, E.; Pin, C.; Iovanna, J. NUPR1 interacts with eIF2α and is required for resolution of the ER stress response in pancreatic tissue. FEBS J. 2021. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Lan, W.; Bintz, J.; Gayet, O.; Carrier, A.; Lomberk, G.; Neira, J.L.; González, A.; Urrutia, R.; Soubeyran, P.; et al. Inactivation of NUPR1 promotes cell death by coupling ER-stress responses with necrosis. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Uppala, R.; McKinney, R.W.; Brant, K.A.; Fabisiak, J.P.; Goetzman, E.S. Nickel inhibits mitochondrial fatty acid oxidation. Biochem. Biophys. Res. Commun. 2015, 463, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Narzt, M.-S.; Nagelreiter, I.M.; Oskolkova, O.; Bochkov, V.N.; Latreille, J.; Fedorova, M.; Ni, Z.; Sialana, F.; Lubec, G.; Filzwieser, M.; et al. A novel role for NUPR1 in the keratinocyte stress response to UV oxidized phospholipids. Redox Biol. 2019, 20, 467–482. [Google Scholar] [CrossRef]
- Mu, Y.; Yan, X.; Li, D.; Zhao, D.; Wang, L.; Wang, X.; Gao, D.; Yang, J.; Zhang, H.; Li, Y.; et al. NUPR1 maintains autolysosomal efflux by activating SNAP25 transcription in cancer cells. Autophagy 2018, 14, 654–670. [Google Scholar] [CrossRef] [Green Version]
- Giniatullin, A.; Darios, F.; Shakirzyanova, A.; Davletov, B. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. J. Neurochem. 2006, 98, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing Inflammation and Oxidative Stress in Cardiovascular Disease. Curr. Top. Med. Chem. 2013, 13, 115–138. [Google Scholar] [CrossRef]
- Meng, N.; Mu, X.; Lv, X.; Wang, L.; Li, N.; Gong, Y. Autophagy represses fascaplysin-induced apoptosis and angiogenesis inhibition via ROS and p8 in vascular endothelia cells. Biomed. Pharmacother. 2019, 114, 108866. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Xia, Y.; Lan, W.; Zhou, Z.; Huang, C.; Peng, L.; Soubeyran, P.; Velazquez-Campoy, A.; Abián, O.; Rizzuti, B.; et al. Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis. J. Clin. Investig. 2019, 129, 2500–2513. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Aguado-Llera, D.; Hamidi, T.; Doménech, R.; Pantoja-Uceda, D.; Gironella, M.; Santoro, J.; Velazquez-Campoy, A.; Neira, J.L.; Iovanna, J.L. Deciphering the Binding between Nupr1 and MSL1 and Their DNA-Repairing Activity. PLoS ONE 2013, 8, e78101. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Peng, D.; Chen, Z.; Soutto, M.; Abouelezz, K.; Corvalan, A.; El-Rifai, W. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci. Rep. 2019, 9, 16970. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Zhong, M.; Yang, M.; Sun, X.; Liu, J.; Kroemer, G.; Lotze, M.; Zeh, H.J.; Kang, R.; et al. Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma. Gastroenterology 2017, 153, 1429–1443.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouysségur, J.; Shiu, R.P.; Pastan, I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell 1977, 11, 941–947. [Google Scholar] [CrossRef]
- Chen, S.-D.; Yang, D.-I.; Lin, T.-K.; Shaw, F.-Z.; Liou, C.-W.; Chuang, Y.-C. Roles of Oxidative Stress, Apoptosis, PGC-1α and Mitochondrial Biogenesis in Cerebral Ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, Y.; Ueha, T.; Kawamoto, T.; Hara, H.; Toda, M.; Harada, R.; Minoda, M.; Kurosaka, M.; Akisue, T. Regulation of Mitochondrial Proliferation by PGC-1α Induces Cellular Apoptosis in Musculoskeletal Malignancies. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.G.; Davies, G.; Kynaston, H.; Mason, M.D.; Fodstad, O. Does the PGC-1/PPARgamma pathway play a role in Com-1/p8 mediated cell growth inhibition in prostate cancer? Int. J. Mol. Med. 2006, 18, 1169–1175. [Google Scholar]
- Hollenbach, M.; Klöting, N.; Sommerer, I.; Lorenz, J.; Heindl, M.; Kern, M.; Mossner, J.; Bluher, M.; Hoffmeister, A. p8 deficiency leads to elevated pancreatic beta cell mass but does not contribute to insulin resistance in mice fed with high-fat diet. PLoS ONE 2018, 13, e0201159. [Google Scholar] [CrossRef]
- Li, Z.; Rasmussen, M.L.; Li, J.; Henríquez-Olguín, C.; Knudsen, J.R.; Madsen, A.B.; Quant, E.S.S.; Kleinert, M.; Jensen, T.E. Periodized low protein-high carbohydrate diet confers potent, but transient, metabolic improvements. Mol. Metab. 2018, 17, 112–121. [Google Scholar] [CrossRef]
- Päth, G.; Opel, A.; Knoll, A.; Seufert, J. Nuclear Protein p8 Is Associated with Glucose-Induced Pancreatic β-Cell Growth. Diabetes 2004, 53, S82–S85. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [PubMed] [Green Version]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, W.; Santofimia-Castaño, P.; Xia, Y.; Zhou, Z.; Huang, C.; Fraunhoffer, N.; Barea, D.; Cervello, M.; Giannitrapani, L.; Montalto, G.; et al. Targeting NUPR1 with the small compound ZZW-115 is an efficient strategy to treat hepatocellular carcinoma. Cancer Lett. 2020, 486, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Santofimia-Castaño, P.; Iovanna, J. Combating pancreatic cancer chemoresistance by triggering multiple cell death pathways. Pancreatol. 2021, 21, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Miura, G. A recipe for execution. Nat. Chem. Biol. 2021, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Liu, X.-Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.-L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef]
- Shostak, K.; Jiang, Z.; Charloteaux, B.; Mayer, A.; Habraken, Y.; Tharun, L.; Klein, S.; Xu, X.; Duong, H.Q.; Vislovukh, A.; et al. The X-linked trichothiodystrophy-causing gene RNF113A links the spliceosome to cell survival upon DNA damage. Nat. Commun. 2020, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. The Antioxidant and Anti-Inflammatory Properties of Rice Bran Phenolic Extracts. Foods 2020, 9, 829. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Kuwabara, T.; Imajoh-Ohmi, S. LPS-induced apoptosis is dependent upon mitochondrial dysfunction. Apoptosis 2004, 9, 467–474. [Google Scholar] [CrossRef]
- Jin, H.-O.; Seo, S.-K.; Woo, S.-H.; Choe, T.-B.; Hong, S.-I.; Kim, J.-I.; Park, I.-C. Nuclear protein 1 induced by ATF4 in response to various stressors acts as a positive regulator on the transcriptional activation of ATF4. IUBMB Life 2009, 61, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Huang, C.; Santofimia-Castaño, P.; Lan, W.; Fraunhoffer, N.; Meilerman, A.; Iovanna, J. Inducing ferroptosis by the NUPR1 inhibitor ZZW115 to kill pancreatic cancer cells. Pancreatology 2020, 20, S122. [Google Scholar] [CrossRef]
- Bauer, M.; Bauer, I. Heme Oxygenase-1: Redox Regulation and Role in the Hepatic Response to Oxidative Stress. Antioxid. Redox Signal. 2002, 4, 749–758. [Google Scholar] [CrossRef]
- Deng, H.-F.; Yue, L.-X.; Wang, N.-N.; Zhou, Y.-Q.; Zhou, W.; Liu, X.; Ni, Y.-H.; Huang, C.-S.; Qiu, L.-Z.; Liu, H.; et al. Mitochondrial Iron Overload-Mediated Inhibition of Nrf2-HO-1/GPX4 Assisted ALI-Induced Nephrotoxicity. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Peng, X.; Fan, Q.; Wei, S.; Yang, S.; Li, X.; Jin, H.; Wu, B.; Huang, M.; et al. Emerging mechanisms and applications of ferroptosis in the treatment of resistant cancers. Biomed. Pharmacother. 2020, 130, 110710. [Google Scholar] [CrossRef]
- Carracedo, A.; Egia, A.; Guzman, M.; Velasco, G. p8 Upregulation sensitizes astrocytes to oxidative stress. FEBS Lett. 2006, 580, 1571–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-C.; Chiang, S.-K.; Chen, S.-E.; Yu, Y.-L.; Chou, R.-H.; Chang, W.-C. Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, S.; Bielow, T.; Sommerer, I.; Iovanna, J.; Malicet, C.; Mössner, J.; Hoffmeister, A. P8 deficiency increases cellular ROS and induces HO-1. Arch. Biochem. Biophys. 2015, 565, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [Green Version]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.E.; Culmsee, C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free. Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Pennington, E.R.; Green, W.D.; A Beck, M.; A Brown, D.; Shaikh, S.R. Mechanisms by Which Dietary Fatty Acids Regulate Mitochondrial Structure-Function in Health and Disease. Adv. Nutr. 2018, 9, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Bermudez, J.; Birsoy, K. A mitochondrial gatekeeper that helps cells escape death by ferroptosis. Nat. Cell Biol. 2021, 593, 514–515. [Google Scholar] [CrossRef]
- Kunkel, G.H.; Chaturvedi, P.; Tyagi, S.C. Mitochondrial pathways to cardiac recovery: TFAM. Hear. Fail. Rev. 2016, 21, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Harris, I.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Sandi, M.J.; Hamidi, T.; Malicet, C.; Cano, C.; Loncle, C.; Pierres, A.; Dagorn, J.C.; Iovanna, J.L. p8 Expression controls pancreatic cancer cell migration, invasion, adhesion, and tumorigenesis. J. Cell. Physiol. 2011, 226, 3442–3451. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wu, S.-M.; Lin, Y.-H.; Chi, H.-C.; Lin, S.-L.; Yeh, C.-T.; Chuang, W.-Y.; Lin, K.-H. Induction of nuclear protein-1 by thyroid hormone enhances platelet-derived growth factor A mediated angiogenesis in liver cancer. Theranostics 2019, 9, 2361–2379. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Hu, J.; Feng, K.; Pan, Y.; Zhang, L.; Feng, Y. Lentivirus-mediated RNAi knockdown of NUPR1 inhibits human nonsmall cell lung cancer growth in vitro and in vivo. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 2114–2121. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.; Ma, J.; Sun, Y.; Zhou, R.; Cui, B.; Zhang, Y.; Yang, J.; Yan, X.; Liu, Z.; et al. Combination of AAV-mediated NUPR1 knockdown and trifluoperazine induces premature senescence in human lung adenocarcinoma A549 cells in nude mice. Oncol. Rep. 2020, 43, 681–688. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, J.; Lin, J.; Lin, R.; Chen, K.; Kong, J.; Shui, X. Long Noncoding RNA FEZF1-AS1 Promotes Osteosarcoma Progression by Regulating the miR-4443/NUPR1 Axis. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, S.; Liu, Y.; Lian, Z.; Dong, B.; Yao, Y.; Xu, Y. Knockdown of NUPR1 inhibits the proliferation of glioblastoma cells via ERK1/2, p38 MAPK and caspase-3. J. Neuro-Oncol. 2017, 132, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Jin, D.-I.; Yoon, S.; Baek, S.-Y.; Kim, B.-S.; Oh, S.-O. Expression and roles of NUPR1 in cholangiocarcinoma cells. Anat. Cell Biol. 2012, 45, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Yi, B.; Li, X.; Chen, J. Knockdown of nuclear protein 1 (NUPR1) gene inhibits proliferation and promotes apoptosis of human multiple myeloma U266 cells. Chin. J. Cell. Mol. Immunol. 2017, 33, 1240–1246. [Google Scholar]
- Zeng, C.; Li, X.; Li, A.; Yi, B.; Peng, X.; Huang, X.; Chen, J. Knockdown of NUPR1 inhibits the growth of U266 and RPMI8226 multiple myeloma cell lines via activating PTEN and caspase activation-dependent apoptosis. Oncol. Rep. 2018, 40, 1487–1494. [Google Scholar] [CrossRef]
- Li, A.; Li, X.; Chen, X.; Zeng, C.; Wang, Z.; Li, Z.; Chen, J. NUPR1 Silencing Induces Autophagy-Mediated Apoptosis in Multiple Myeloma Cells Through the PI3K/AKT/mTOR Pathway. DNA Cell Biol. 2020, 39, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, H.; Li, R.; Jiang, Q.; Luan, W.; Shi, J.; Liu, P. Oncogenic Role of NUPR1 in Ovarian Cancer. Oncotargets Ther. 2020, 13, 12289–12300. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cheng, F.; Zheng, B.; Wang, J.; Zhao, G.; Yao, Z.; Zhang, T. NUPR1 is a novel potential biomarker and confers resistance to sorafenib in clear cell renal cell carcinoma by increasing stemness and targeting the PTEN/AKT/mTOR pathway. Aging 2021, 13, 14015–14038. [Google Scholar] [CrossRef]
- Lan, W.; Santofimia-Castaño, P.; Swayden, M.; Xia, Y.; Zhou, Z.; Audebert, S.; Camoin, L.; Huang, C.; Peng, L.; Jiménez-Alesanco, A.; et al. ZZW-115–dependent inhibition of NUPR1 nuclear translocation sensitizes cancer cells to genotoxic agents. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Santofimia-Castaño, P.; Iovanna, J. NUPR1: A Critical Regulator of the Antioxidant System. Cancers 2021, 13, 3670. https://doi.org/10.3390/cancers13153670
Huang C, Santofimia-Castaño P, Iovanna J. NUPR1: A Critical Regulator of the Antioxidant System. Cancers. 2021; 13(15):3670. https://doi.org/10.3390/cancers13153670
Chicago/Turabian StyleHuang, Can, Patricia Santofimia-Castaño, and Juan Iovanna. 2021. "NUPR1: A Critical Regulator of the Antioxidant System" Cancers 13, no. 15: 3670. https://doi.org/10.3390/cancers13153670





