Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
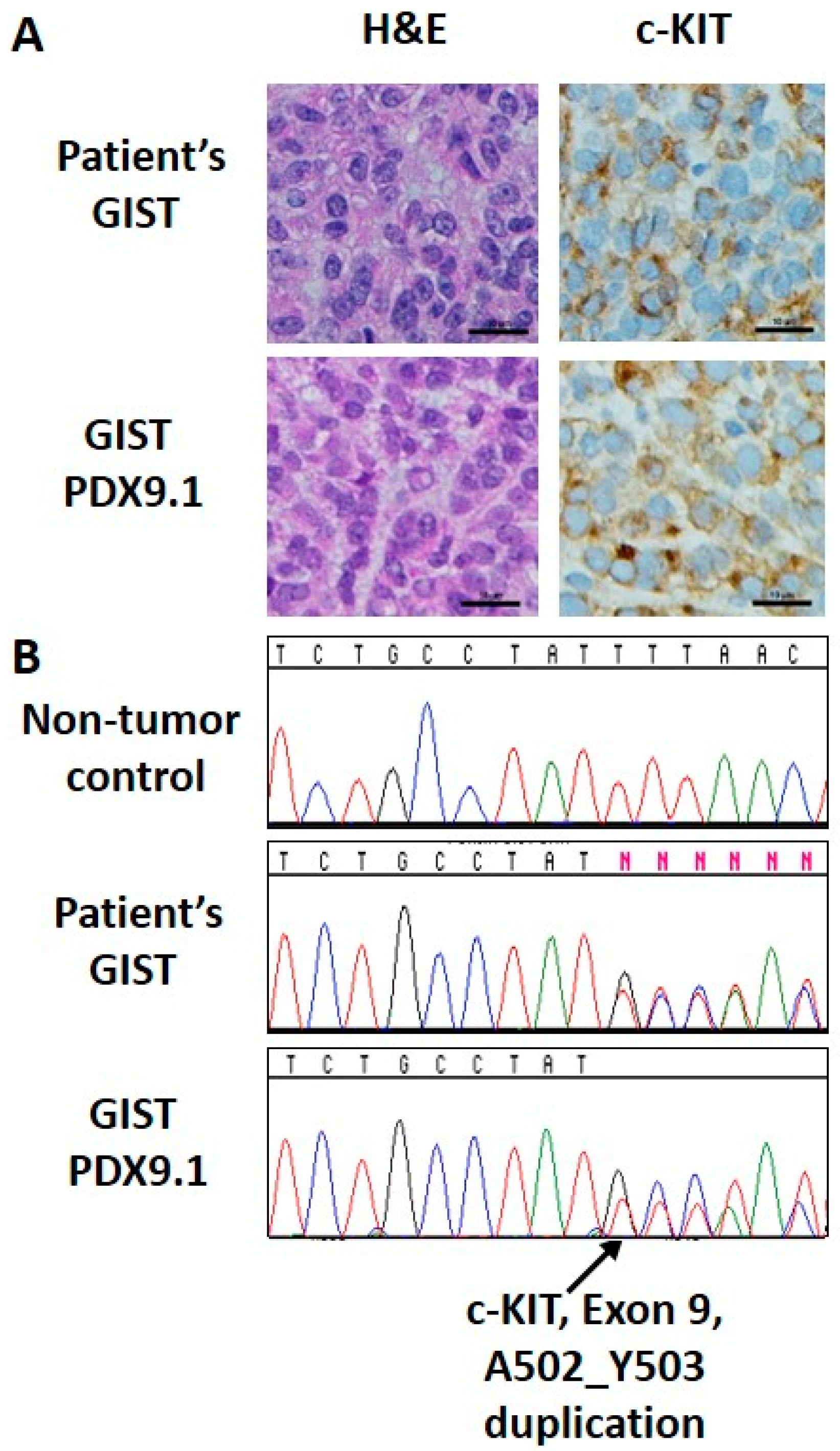
2.1. Cell Lines, Patient Derived Xenograft Model, Compounds, and Antibodies
2.2. Cell Proliferation/Viability Assay
2.3. PDCD4 Overexpression
2.4. BrdU Incorporation Assay
2.5. Preparation of Whole Cell Extract from Cells and Immunoblot Assays
2.6. Protein Extraction and LC-MS/MS Analysis
2.7. Data Processing and Analysis
2.8. Establishment of GIST Patient Derived Xenografts
2.9. GIST Xenografts and Drug Administration
2.10. Tumor Growth Modeling
3. Results
3.1. IM in Combination with MK-4440 Has Enhanced Viability on In Vitro GIST Cell Growth
3.2. Combination Treatment Increases PDCD4 Expression
3.3. Combination Treatment Causes Cell Cycle Arrest and Increased Cell Death
3.4. Combination Treatment Reduces Tumor Growth In Vivo in IM-Sensitive GIST Only
3.5. MK-4440 in Combination with Ripretinib Increases PDCD4 Expression in GIST Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer 2011, 11, 865–878. [Google Scholar] [CrossRef]
- Belinsky, M.G.; Rink, L.; Flieder, D.B.; Jahromi, M.S.; Schiffman, J.D.; Godwin, A.K.; von Mehren, M. Overexpression of insulin-like growth factor 1 receptor and frequent mutational inactivation of SDHA in wild-type SDHB-negative gastrointestinal stromal tumors. Genes Chromosom. Cancer 2013, 52, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.K.; Miettinen, M.; Walker, R.L.; Wang, Y.; Zhu, Y.J.; Waterfall, J.; Noyes, N.; Retnakumar, P.; Yang, Z.; Smith, W.I.; et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci. Transl. Med. 2014, 6, 268ra177. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Vehtari, A.; Riihimäki, J.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Cirilli, C.; Braconi, C.; et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012, 13, 265–274. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, M.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Jakob, J.; Hohenberger, P. Neoadjuvant Therapy to downstage the extent of resection of gastrointestinal stromal tumors. Visc. Med. 2018, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Kang, Y.-K.; Nishida, T.; von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Prim. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Long-term efficacy of imatinib for treatment of metastatic GIST. Cancer Chemother. Pharm. 2013, 72, 277–286. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.-K.; Blay, J.-Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; Von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef]
- Dhillon, S. Avapritinib: First approval. Drugs 2020, 80, 433–439. [Google Scholar] [CrossRef]
- Dhillon, S. Ripretinib: First approval. Drugs 2020, 80, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Kaufman, M.D.; Lu, W.-P.; Gupta, A.; Leary, C.B.; Wise, S.C.; Rutkoski, T.J.; Ahn, Y.M.; Al-Ani, G.; Bulfer, S.L.; et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738–751.e739. [Google Scholar] [CrossRef] [PubMed]
- Debiec-Rychter, M.; Sciot, R.; Le Cesne, A.; Schlemmer, M.; Hohenberger, P.; van Oosterom, A.T.; Blay, J.-Y.; Leyvraz, S.; Stul, M.; Casali, P.G.; et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur. J. Cancer 2006, 42, 1093–1103. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Demetri, G.D.; Blanke, C.D.; Von Mehren, M.; Joensuu, H.; McGreevey, L.S.; Chen, C.-J.; Abbeele, A.D.V.D.; Druker, B.; et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 2003, 21, 4342–4349. [Google Scholar] [CrossRef] [PubMed]
- Parab, T.M.; Derogatis, M.J.; Boaz, A.M.; Grasso, S.A.; Issack, P.S.; Duarte, D.A.; Urayeneza, O.; Vahdat, S.; Qiao, J.-H.; Hinika, G.S. Gastrointestinal stromal tumors: A comprehensive review. J. Gastrointest. Oncol. 2019, 10, 144–154. [Google Scholar] [CrossRef]
- Liegl, B.; Kepten, I.; Le, C.; Zhu, M.; Demetri, G.D.; Heinrich, M.; Fletcher, C.D.M.; Corless, C.L.; Fletcher, J.A. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J. Pathol. 2008, 216, 64–74. [Google Scholar] [CrossRef]
- Nishida, T.; Takahashi, T.; Nishitani, A.; Doi, T.; Shirao, K.; Komatsu, Y.; Nakajima, K.; Hirota, S.; Japanese Study Group on GIST. Sunitinib-resistant gastrointestinal stromal tumors harbor cis-mutations in the activation loop of the KIT gene. Int. J. Clin. Oncol. 2009, 14, 143–149. [Google Scholar] [CrossRef]
- Zook, P.; Pathak, H.B.; Belinsky, M.G.; Gersz, L.; Devarajan, K.; Zhou, Y.; Godwin, A.K.; Von Mehren, M.; Rink, L. Combination of Imatinib Mesylate and AKT Inhibitor Provides Synergistic Effects in Preclinical Study of Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2017, 23, 171–180. [Google Scholar] [CrossRef]
- Tarn, C.; Skorobogatko, Y.V.; Taguchi, T.; Eisenberg, B.; von Mehren, M.; Godwin, A.K. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Cancer Res. 2006, 66, 5477–5486. [Google Scholar] [CrossRef]
- Bauer, S.; Duensing, A.; Demetri, G.D.; Fletcher, J.A. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene 2007, 26, 7560–7568. [Google Scholar] [CrossRef]
- Li, J.; Dang, Y.; Gao, J.; Li, Y.; Zou, J.; Shen, L. PI3K/AKT/mTOR pathway is activated after imatinib secondary resistance in gastrointestinal stromal tumors (GISTs). Med. Oncol. 2015, 32, 111. [Google Scholar] [CrossRef]
- Taguchi, T.; Sonobe, H.; Toyonaga, S.-I.; Yamasaki, I.; Shuin, T.; Takano, A.; Araki, K.; Akimaru, K.; Yuri, K. Conventional and Molecular Cytogenetic Characterization of a New Human Cell Line, GIST-T1, Established from Gastrointestinal Stromal Tumor. Lab. Investig. 2002, 82, 663–665. [Google Scholar] [CrossRef]
- Ye, S.; Sharipova, D.; Kozinova, M.; Klug, L.; D’Souza, J.; Belinsky, M.G.; Johnson, K.J.; Einarson, M.B.; Devarajan, K.; Zhou, Y.; et al. Identification of Wee1 as a target in combination with avapritinib for gastrointestinal stromal tumor treatment. JCI Insight 2021, 6, e143474. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dammer, E.B.; Owino, S.A.; Giddens, M.M.; Madaras, N.S.; Duong, D.M.; Seyfried, N.T.; Hall, R.A. Quantitative Proteomics Reveal an Altered Pattern of Protein Expression in Brain Tissue from Mice Lacking GPR37 and GPR37L1. J. Proteome Res. 2020, 19, 744–755. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, L.S.; Henderson, N.C.; Rathouz, P.J. Fast Pure R Implementation of GEE: Application of the Matrix Package. R Journal 2013, 5, 181–187. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharm. Rev. 1995, 47, 331–385. [Google Scholar] [PubMed]
- Schmid, T.; Jansen, A.P.; Baker, A.R.; Hegamyer, G.; Hagan, J.P.; Colburn, N.H. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008, 68, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, S.; Hamajima, H.; Xia, J.; Zhang, H.; Mizuta, T.; Anzai, K.; Ozaki, I. Control of a tumor suppressor PDCD4: Degradation mechanisms of the protein in hepatocellular carcinoma cells. Cell. Signal. 2014, 26, 603–610. [Google Scholar] [CrossRef]
- Quattrone, A.; Wozniak, A.; Dewaele, B.; Floris, G.; Vanspauwen, V.; Van Looy, T.; Schöffski, P.; Rutkowski, P.; Sciot, R.; Debiec-Rychter, M. Frequent mono-allelic loss associated with deficient PTEN expression in imatinib-resistant gastrointestinal stromal tumors. Mod. Pathol. 2014, 27, 1510–1520. [Google Scholar] [CrossRef][Green Version]
- Floris, G.; Wozniak, A.; Sciot, R.; Li, H.; Friedman, L.; Van Looy, T.; Wellens, J.; Vermaelen, P.; Deroose, C.; Fletcher, J.A.; et al. A potent combination of the novel PI3K Inhibitor, GDC-0941, with imatinib in gastrointestinal stromal tumor xenografts: Long-lasting responses after treatment withdrawal. Clin. Cancer Res. 2013, 19, 620–630. [Google Scholar] [CrossRef]
- Van Looy, T.; Gebreyohannes, Y.K.; Wozniak, A.; Cornillie, J.; Wellens, J.; Li, H.; Vanleeuw, U.; Floris, G.; Debiec-Rychter, M.; Sciot, R.; et al. Characterization and assessment of the sensitivity and resistance of a newly established human gastrointestinal stromal tumour xenograft model to treatment with tyrosine kinase inhibitors. Clin. Sarcoma. Res. 2014, 4, 10. [Google Scholar] [CrossRef]
- García-Valverde, A.; Rosell, J.; Serna, G.; Valverde, C.; Carles, J.; Nuciforo, P.; Fletcher, J.A.; Arribas, J.; Politz, O.; Serrano, C. Preclinical Activity of PI3K Inhibitor Copanlisib in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2020, 19, 1289–1297. [Google Scholar] [CrossRef]
- Bosbach, B.; Rossi, F.; Yozgat, Y.; Loo, J.; Zhang, J.Q.; Berrozpe, G.; Warpinski, K.; Ehlers, I.; Veach, D.; Kwok, A.; et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA 2017, 114, E8448–E8457. [Google Scholar] [CrossRef]
- Kostaras, E.; Kaserer, T.; Lazaro, G.; Heuss, S.F.; Hussain, A.; Casado, P.; Hayes, A.; Yandim, C.; Palaskas, N.; Yu, Y.; et al. A systematic molecular and pharmacologic evaluation of AKT inhibitors reveals new insight into their biological activity. Br. J. Cancer 2020, 123, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, S.; Manirujjaman, M.; Hamajima, H.; Ozaki, I. Control Mechanisms of the Tumor Suppressor PDCD4: Expression and Functions. Int. J. Mol. Sci. 2019, 20, 2304. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, X.; Zhao, M.; Qu, Z.; Huang, S.; Dong, M.; Gao, F. An essential role of PDCD4 in progression and malignant proliferation of gastrointestinal stromal tumors. Med. Oncol. 2011, 29, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Akar, U.; Steiner, M.; Zorrilla-Calancha, I.; Tirado-Gomez, M.; Colburn, N.; Danilenko, M.; Kornblau, S.; Berestein, G.L. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid–induced terminal granulocytic differentiation of human myeloid leukemia Cells. Mol. Cancer Res. 2007, 5, 95–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, N.; Liu, S.S.; Chan, K.K.L.; Ngan, H.Y. Tumour suppressive function and modulation of programmed cell death 4 (PDCD4) in ovarian cancer. PLoS ONE 2012, 7, e30311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wan, L.; Liu, Y.; Sun, Y.; Liu, Y.; Shi, Y.; Zhang, L.; Zhou, H.; Wang, J.; et al. Downregulation of PDCD4 induced by progesterone is mediated by the PI3K/AKT signaling pathway in human endometrial cancer cells. Oncol. Rep. 2019, 42, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Pradayrol, L.; Sonenberg, N. A cell cycle-dependent internal ribosome entry site. Mol. Cell 2000, 5, 607–616. [Google Scholar] [CrossRef]
- Ye, Z.; Zeng, Z.; Shen, Y.; Yang, Q.; Chen, D.; Chen, Z.; Shen, S. ODC1 promotes proliferation and mobility via the AKT/GSK3beta/beta-catenin pathway and modulation of acidotic microenvironment in human hepatocellular carcinoma. Onco. Targets Ther. 2019, 12, 4081–4092. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Bikinieva, F.; Nurgatina, I.; Dunaev, P.; Valeeva, E.; Aukhadieva, A.; Sabirov, A.; Galembikova, A. Inhibition of AKT-Signaling Sensitizes Soft Tissue Sarcomas (STS) and Gastrointestinal Stromal Tumors (GIST) to Doxorubicin via Targeting of Homology-Mediated DNA Repair. Int. J. Mol. Sci. 2020, 21, 8842. [Google Scholar] [CrossRef] [PubMed]






| GIST-T1 | GIST-T1/829 | |||||
|---|---|---|---|---|---|---|
| Significantly Downregulated upon Treatment | ||||||
| ABCF2 ACACA AGFG1 ARMC9 ATAD2 BMP2K CASP7 CBX1 EIF1 EMC8 | FADS2 FBN1 HMGCS1 HN1 HSBP1 IRF2BP2 KIF11 KPNA2 LSM14B MAGT1 | MKI67 NAV1 ODC1 PFN2 PRKCI PRRC2A RRM2 SEC62 SLC4A1AP SPRY4 | SQLE SQSTM1 TACC3 TAX1BP3 TCAF1 TGFB1I1 TPX2 TYMS ZFYVE16 ZPR1 | ASAH1 ODC1 PTGS2 RAC2 RPL35A SPAG5 TCAF1 | ||
| Significantly Upregulated upon Treatment | ||||||
| ATP5D BANF1 DERL1 GSTM2 | H1FX HIST1H1C HIST1H4A | MRPL22 NDUFA4 NDUFS4 | PBXIP1 PDCD4 TMEM109 | ATP5D HMGCR ITM2C | NDUFAB1 PDCD4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozinova, M.; Joshi, S.; Ye, S.; Belinsky, M.G.; Sharipova, D.; Farma, J.M.; Reddy, S.S.; Litwin, S.; Devarajan, K.; Campos, A.R.; et al. Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor. Cancers 2021, 13, 3699. https://doi.org/10.3390/cancers13153699
Kozinova M, Joshi S, Ye S, Belinsky MG, Sharipova D, Farma JM, Reddy SS, Litwin S, Devarajan K, Campos AR, et al. Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor. Cancers. 2021; 13(15):3699. https://doi.org/10.3390/cancers13153699
Chicago/Turabian StyleKozinova, Marya, Shalina Joshi, Shuai Ye, Martin G. Belinsky, Dinara Sharipova, Jeffrey M. Farma, Sanjay S. Reddy, Samuel Litwin, Karthik Devarajan, Alex Rosa Campos, and et al. 2021. "Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor" Cancers 13, no. 15: 3699. https://doi.org/10.3390/cancers13153699
APA StyleKozinova, M., Joshi, S., Ye, S., Belinsky, M. G., Sharipova, D., Farma, J. M., Reddy, S. S., Litwin, S., Devarajan, K., Campos, A. R., Yu, Y., Schwartz, B., von Mehren, M., & Rink, L. (2021). Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor. Cancers, 13(15), 3699. https://doi.org/10.3390/cancers13153699








