Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Collective
2.2. Patient Treatment
2.3. Immunohistochemistry
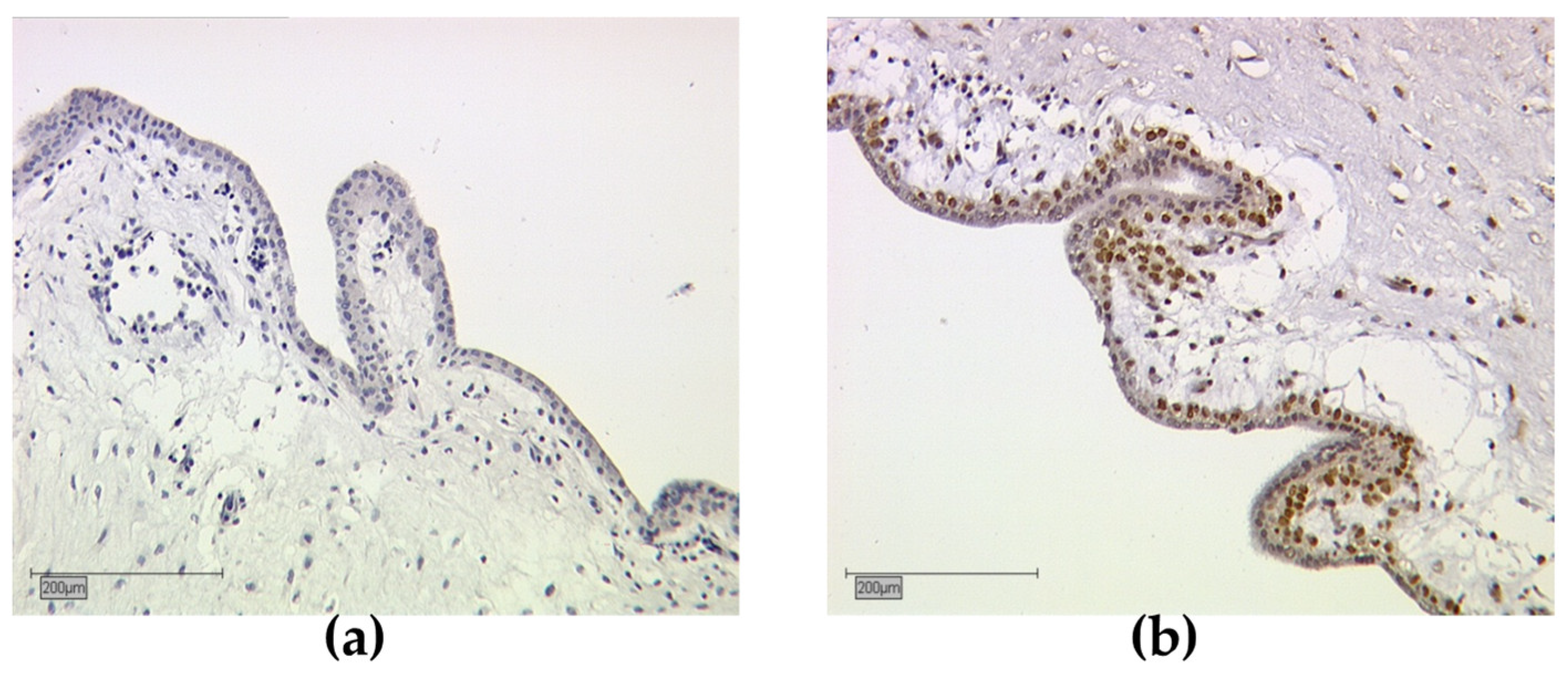
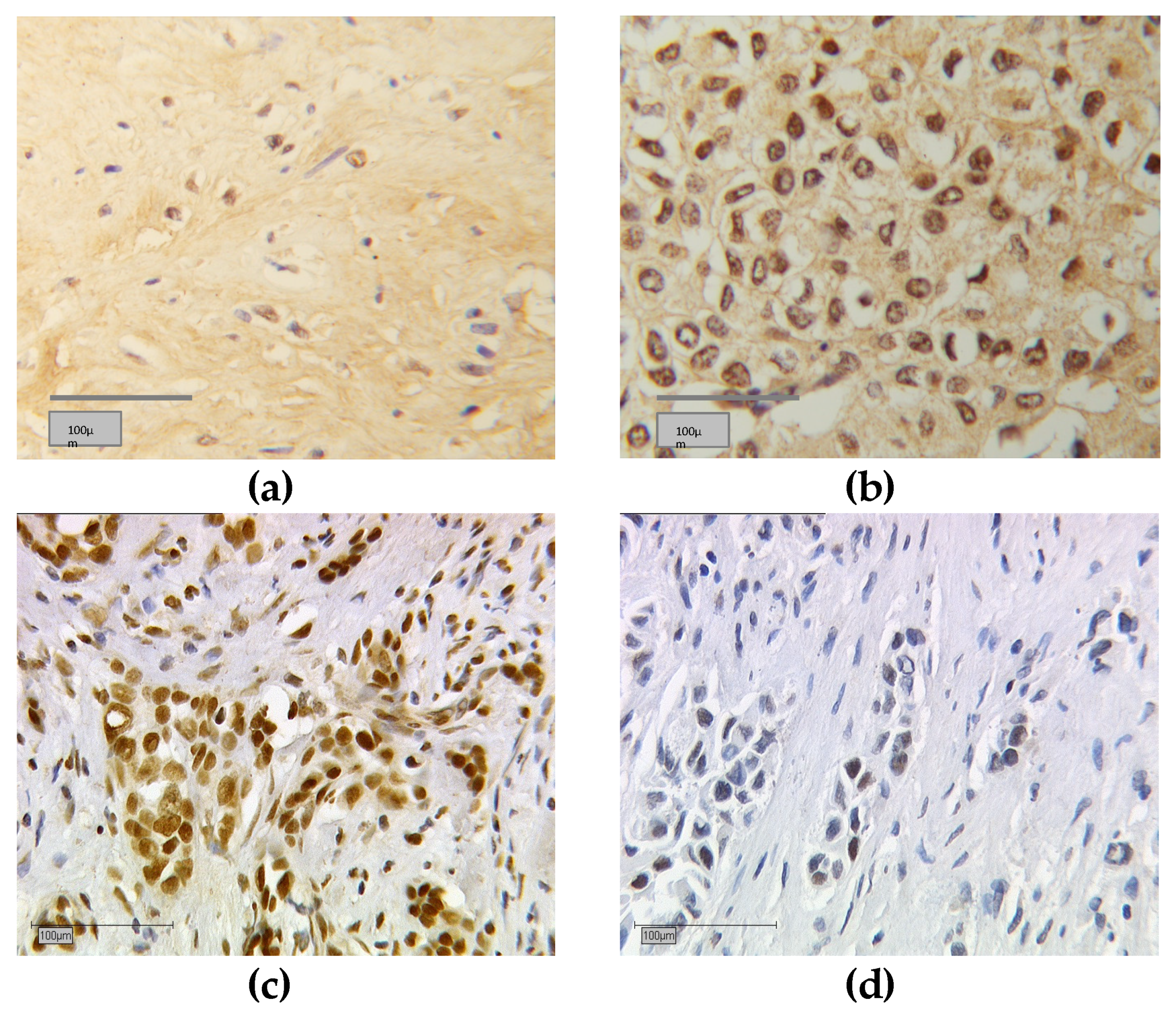
2.4. Staining Evaluation (Immunoreactive Score)
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. RXRα Expression Correlates with Clinicopathological Data
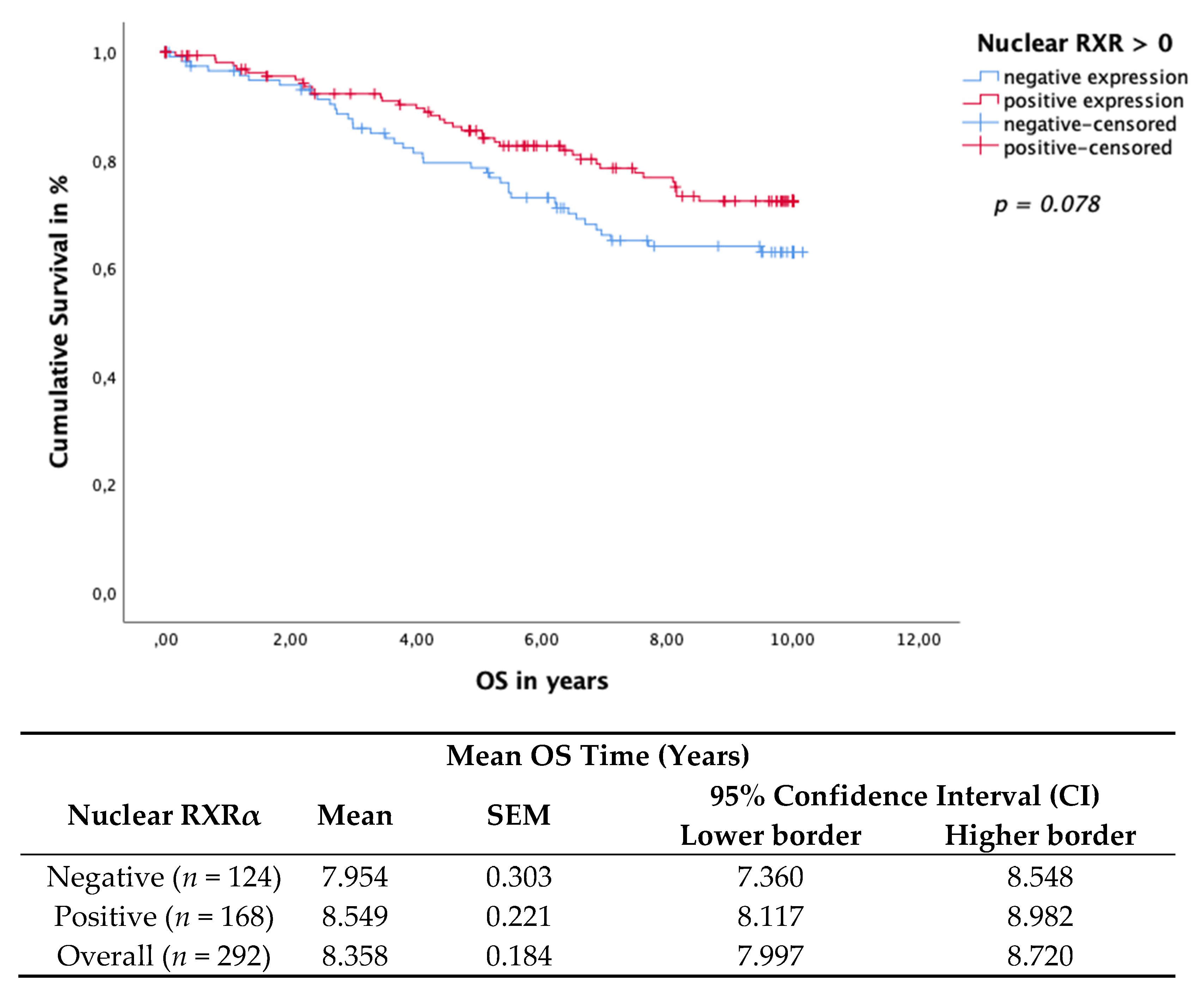
3.3. Nuclear RXRα Expression Correlates with Clinicopathological Parameters Linked with Good Prognosis
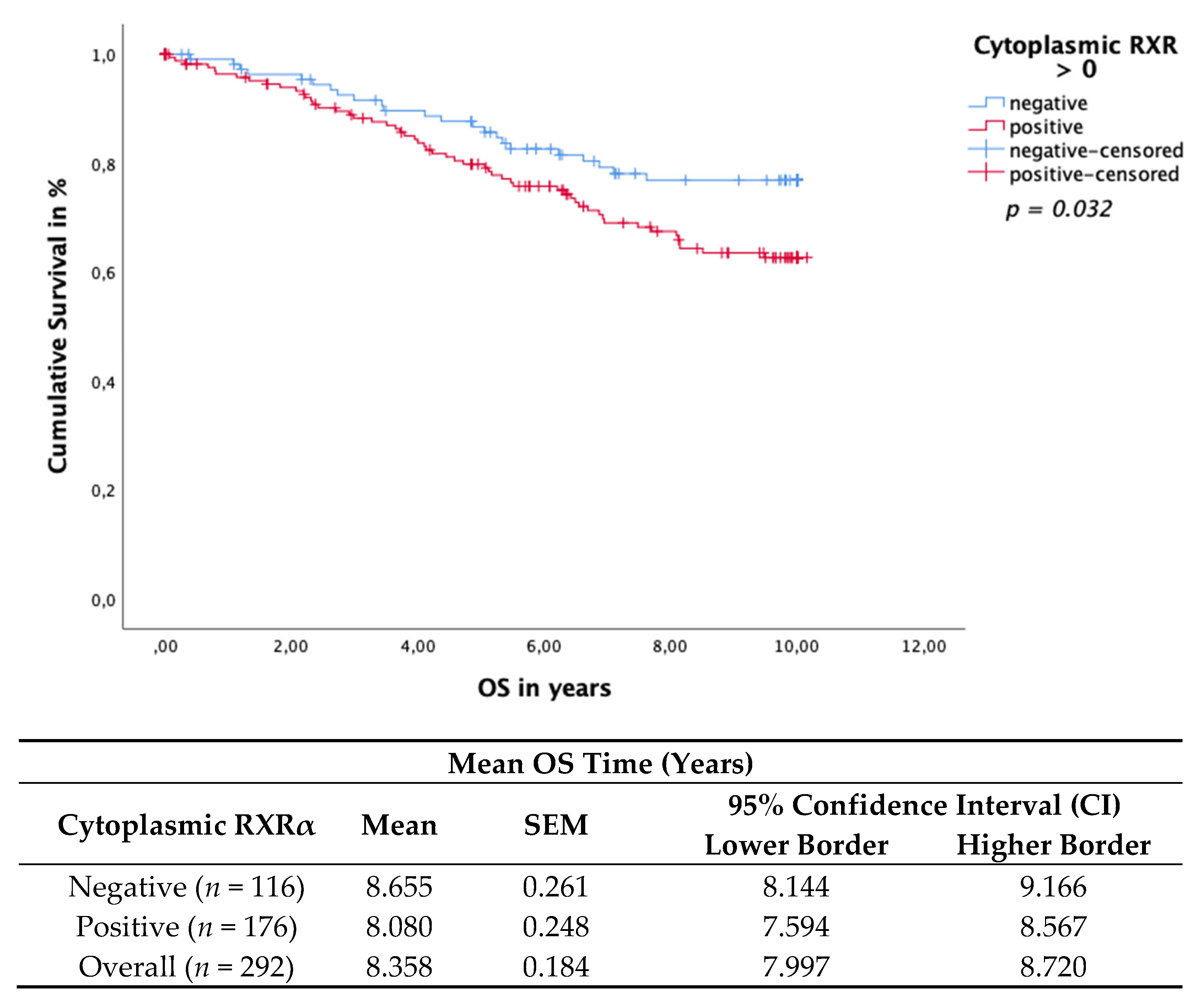
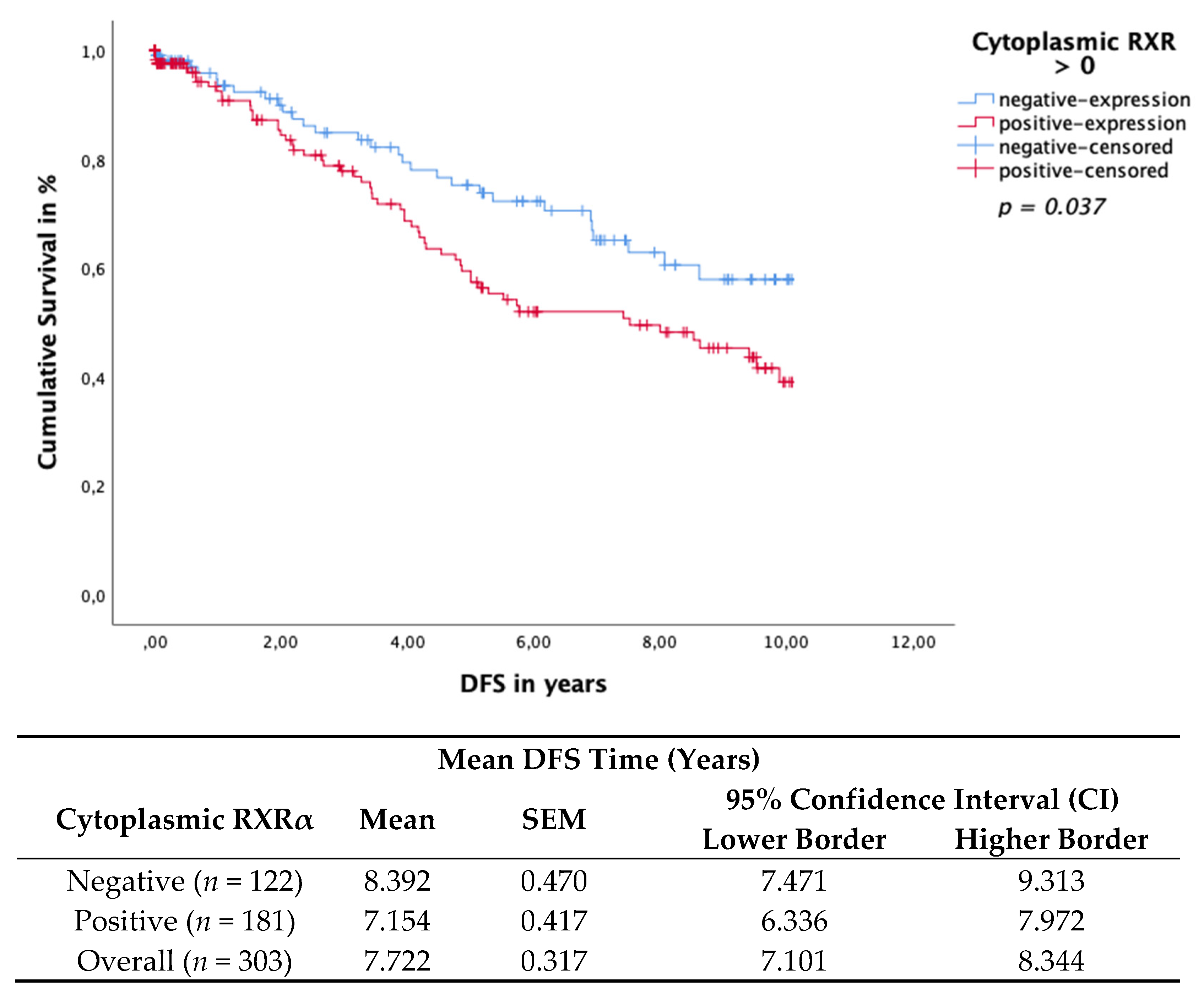
3.4. Cytoplasmic RXRα Is an Independent Prognostic Factor for DFS
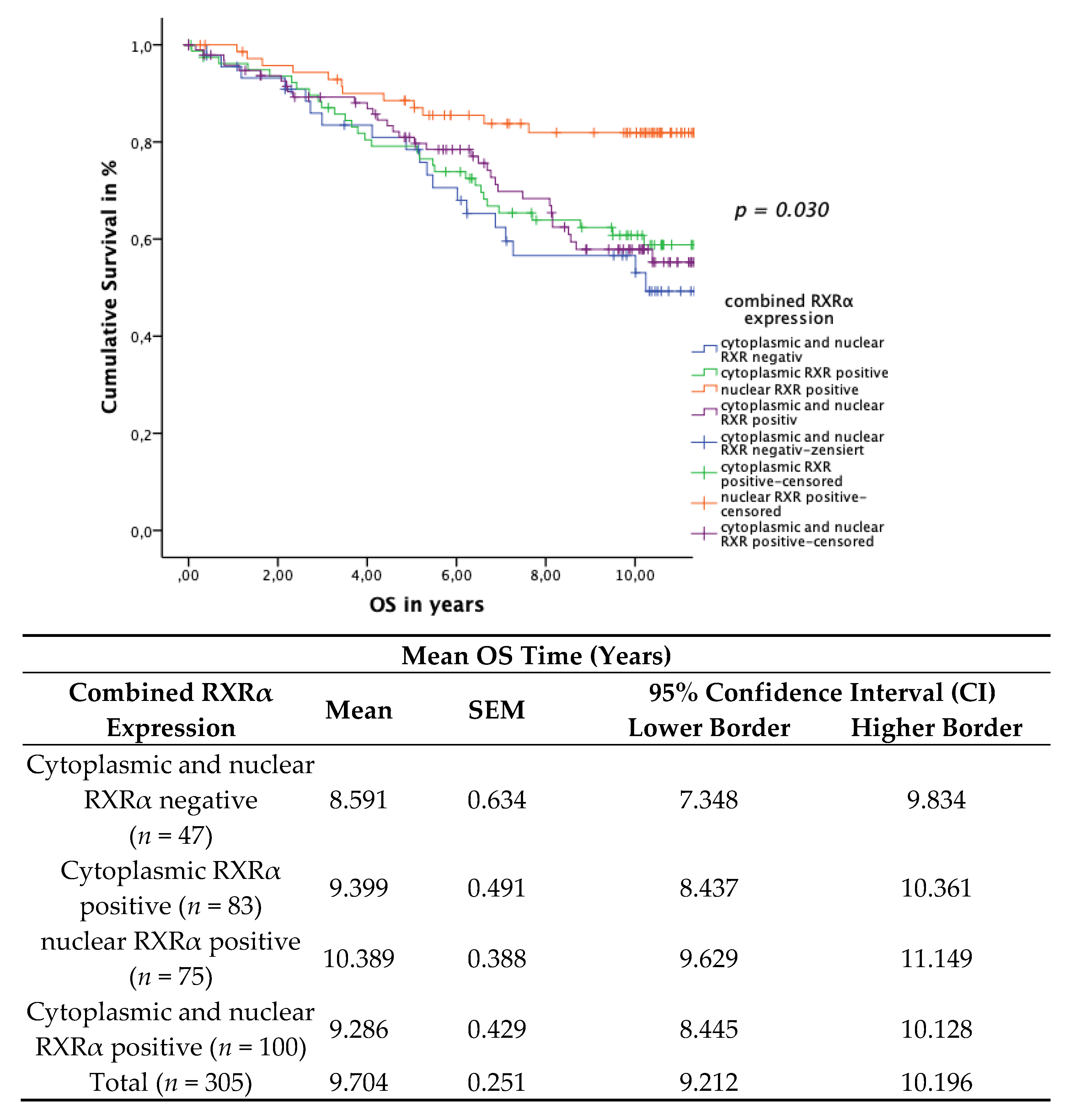
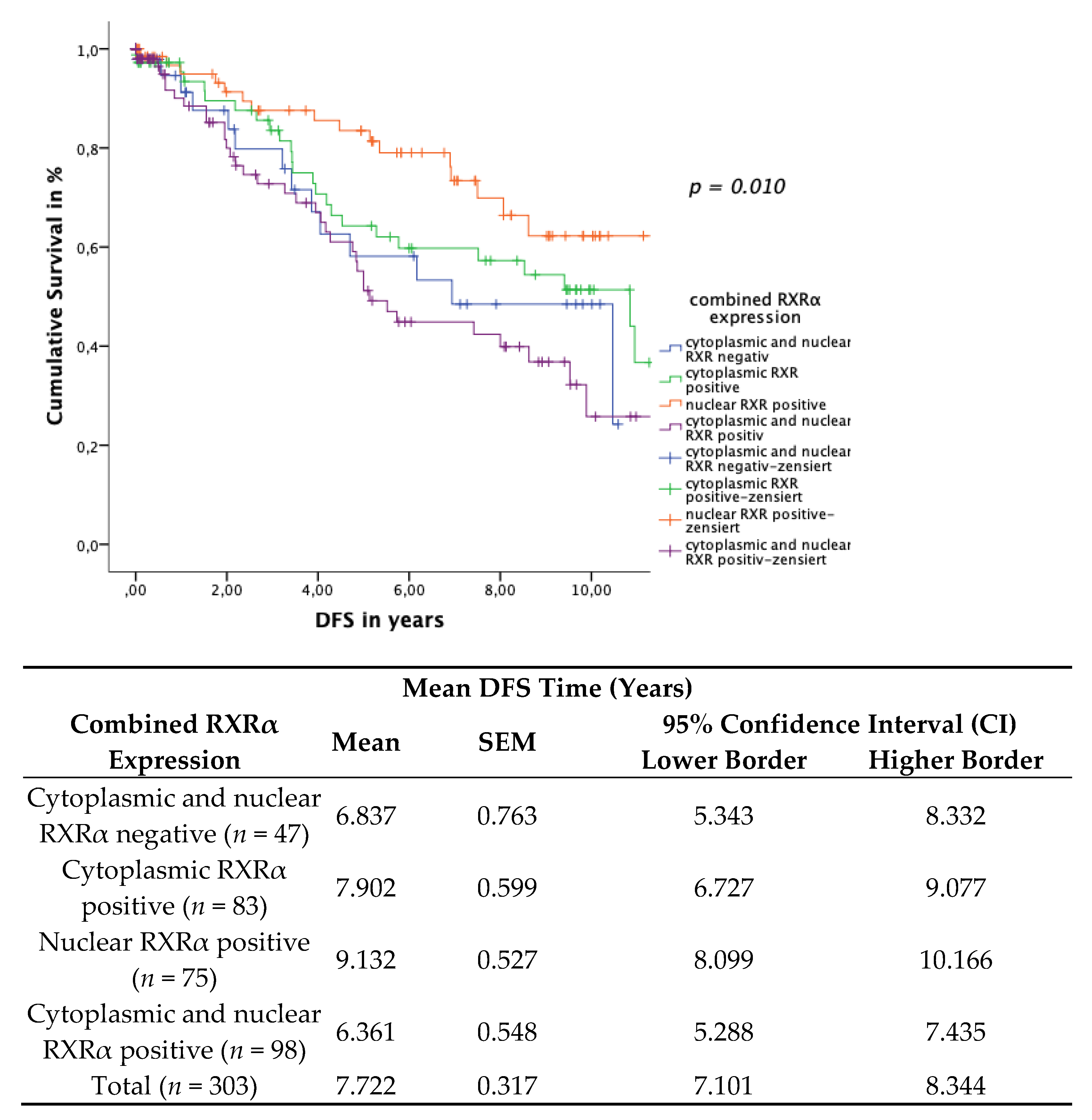
3.5. Subcellular Localisation of RXRα
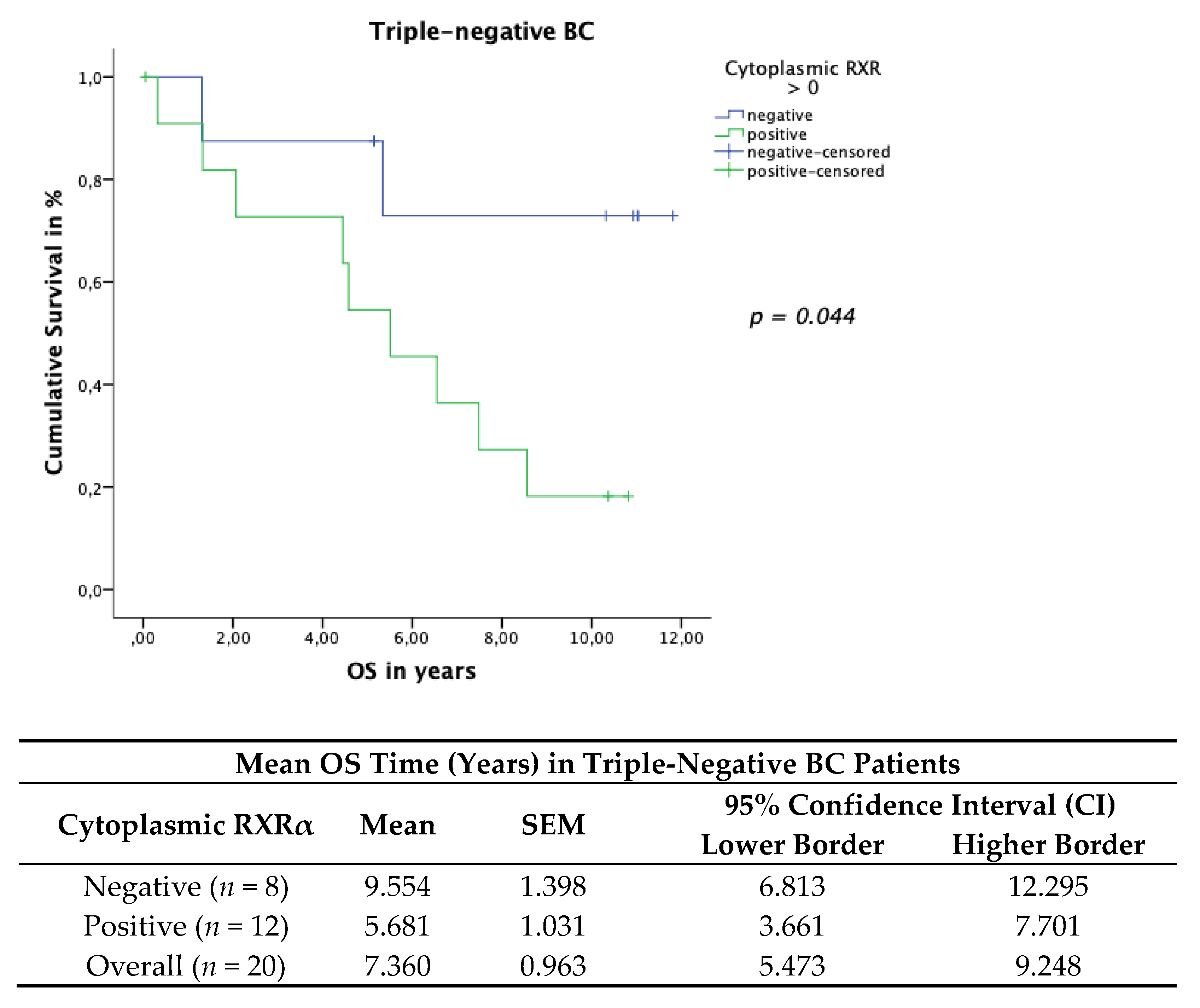
3.6. Cytoplasmic RXRα Is a Negative Prognosticator for Her-2neu-Negative and Triple-Negative Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| DFS | Disease-free survival |
| NR | Nuclear receptor |
| OS | Overall survival |
| IRS | Immmunoreactive score |
| ER | Estrogen receptor |
| PBS | Phosphate-buffered saline |
| PR | Progesterone receptor |
| RXR | Retinoid X receptor |
| RXRα | Retinoid X receptor alpha |
| RXRɣ | Retinoid X receptor gamma |
| TC | Total collective |
| VDR | Vitamin D receptor |
| THR | Thyroid hormone receptor |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- WHO. Breast Cancer; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the Prevention of Breast Cancer: Current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauley, J.A.; Norton, L.; Lippman, M.E.; Eckert, S.; Krueger, K.A.; Purdie, D.W.; Farrerons, J.; Karasik, A.; Mellstrom, D.; Ng, K.W.; et al. Continued Breast Cancer Risk Reduction in Postmenopausal Women Treated with Raloxifene: 4-Year Results from the MORE Trial. Breast Cancer Res. Treat. 2001, 65, 125–134. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Alés-Martínez, J.E.; Cheung, A.M.; Chlebowski, R.T.; Wactawski-Wende, J.; McTiernan, A.; Robbins, J.; Johnson, K.C.; Martin, L.W.; et al. Exemestane for Breast-Cancer Prevention in Postmenopausal Women. N. Engl. J. Med. 2011, 364, 2381–2391. [Google Scholar] [CrossRef] [Green Version]
- Giordano, S.H.; Elias, A.D.; Gradishar, W.J. Gradishar. Nccn Guidelines Updates: Breast Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF). Interdiszipliäre S3-Leitlinie Für Die Früherkennung, Diagnostik, Therapie Und Nachsorge Des Mammakarzinoms. Available online: https://www.awmf.org/uploads/tx_szleitlinien/032-045OLl_S3_Mammakarzinom_2017-12.pdf (accessed on 19 July 2021). (In German).
- Shaikh, T.; Tam, T.Y.; Li, T.; Hayes, S.B.; Goldstein, L.; Bleicher, R.; Boraas, M.; Sigurdson, E.; Ryan, P.D.; Anderson, P. Multifocal and Multicentric Breast Cancer is Associated with Increased Local Recurrence Regardless of Surgery Type. Breast J. 2015, 21, 121–126. [Google Scholar] [CrossRef]
- Zhang, X.; Hofmann, S.; Rack, B.; Harbeck, N.; Jeschke, U.; Sixou, S. Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients. Int. J. Mol. Sci. 2017, 18, 1318. [Google Scholar] [CrossRef] [Green Version]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The Association between Vitamin D Receptor Expression and Prolonged Overall Survival in Breast Cancer. J. Histochem. Cytochem. 2011, 60, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Lang, Z.; Wu, Y.; Li, C.; Li, X.; Wang, X.; Qu, G. Multifocal and Multicentric Breast Carcinoma: A Significantly More Aggressive Tumor Than Unifocal Breast Cancer. Anticancer Res. 2017, 37, 4593–4598. [Google Scholar] [PubMed] [Green Version]
- Reinert, T.; De Paula, B.; Shafaee, M.N.; Souza, P.H.; Ellis, M.J.; Bines, J. Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer. Chin. Clin. Oncol. 2018, 7, 25. [Google Scholar] [CrossRef]
- Welsh, J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol. Cell. Endocrinol. 2017, 453, 88–95. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wu, C.-Y.; Petrossian, K.; Huang, T.-T.; Tseng, L.-M.; Chen, S. Treatment for the endocrine resistant breast cancer: Current options and future perspectives. J. Steroid Biochem. Mol. Biol. 2017, 172, 166–175. [Google Scholar] [CrossRef]
- Muscat, G.E.O.; Eriksson, N.A.; Byth, K.; Loi, S.; Graham, D.; Jindal, S.; Davis, M.J.; Clyne, C.; Funder, J.W.; Simpson, E.R.; et al. Research Resource: Nuclear Receptors as Transcriptome: Discriminant and Prognostic Value in Breast Cancer. Mol. Endocrinol. 2013, 27, 350–365. [Google Scholar] [CrossRef]
- Escriva, H.; Bertrand, S.; Laudet, V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004, 40, 11–26. [Google Scholar]
- Dawson, M.I.; Xia, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 21–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.; Kittler, R.; White, K.P. Genomic Antagonism between Retinoic Acid and Estrogen Signaling in Breast Cancer. Cell 2009, 137, 1259–1271. [Google Scholar] [CrossRef] [Green Version]
- Ditsch, N.; Heublein, S.; Jeschke, U.; Sattler, C.; Kuhn, C.; Hester, A.; Czogalla, B.; Trillsch, F.; Mahner, S.; Engel, J.; et al. Cytoplasmic versus nuclear THR alpha expression determines survival of ovarian cancer patients. J. Cancer Res. Clin. Oncol. 2020, 146, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Zehni, A.Z.; Jacob, S.-N.; Mumm, J.-N.; Heidegger, H.H.; Ditsch, N.; Mahner, S.; Jeschke, U.; Vilsmaier, T. Hormone Receptor Expression in Multicentric/Multifocal versus Unifocal Breast Cancer: Especially the VDR Determines the Outcome Related to Focality. Int. J. Mol. Sci. 2019, 20, 5740. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Kuhn, C.; Mayr, D.; Ditsch, N.; Kailuweit, M.; Wolf, V.; Harbeck, N.; Mahner, S.; Jeschke, U.; Cavailles, V.; et al. Cytoplasmic and Nuclear Forms of Thyroid Hormone Receptor Beta1 Are Inversely Associated with Survival in Primary Breast Cancer. Int. J. Mol. Sci. 2020, 21, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czogalla, B.; Deuster, E.; Liao, Y.; Mayr, D.; Schmoeckel, E.; Sattler, C.; Kolben, T.; Hester, A.; Fürst, S.; Burges, A.; et al. Cytoplasmic VDR expression as an independent risk factor for ovarian cancer. Histochem. Cell Biol. 2020, 154, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.B.; Graham, J.D.; Clarke, C. Emerging functional roles of nuclear receptors in breast cancer. J. Mol. Endocrinol. 2017, 58, R169–R190. [Google Scholar] [CrossRef]
- Röszer, T.; Menéndez-Gutiérrez, M.P.; Cedenilla, M.; Ricote, M. Retinoid X receptors in macrophage biology. Trends Endocrinol. Metab. 2013, 24, 460–468. [Google Scholar] [CrossRef]
- Leal, A.S.M.; Zydeck, K.; Carapellucci, S.; Reich, L.A.; Zhang, D.; Moerland, J.A.; Sporn, M.B.; Liby, K.T. Retinoid X receptor agonist LG100268 modulates the immune microenvironment in preclinical breast cancer models. NPJ Breast Cancer 2019, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nunez, V.; Alameda, D.; Rico, D.; Mota, R.; Gonzalo, P.; Cedenilla, M.; Fischer, T.; Bosca, L.; Glass, C.; Arroyo, K.A.G.; et al. Retinoid X Receptor Alpha Controls Innate Inflammatory Responses through the up-Regulation of Chemokine Expression. Proc. Natl. Acad. Sci. USA 2010, 107, 10626–10631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Roman, J. Peroxisome Proliferator-Activated Receptor Gamma: A Novel Target for Cancer Therapeutics? Anticancer Drugs 2007, 18, 237–244. [Google Scholar] [CrossRef]
- Ditsch, N.; Vrekoussis, T.; Lenhard, M.; Ruhl, I.; Gallwas, J.; Weissenbacher, T.; Friese, K.; Mayr, D.; Makrigiannakis, A.; Jeschke, U. Retinoid X Receptor Alpha (Rxralpha) and Peroxisome Proliferator-Activated Receptor Gamma (Ppargamma) Expression in Breast Cancer: An Immunohistochemical Study. In Vivo 2012, 26, 87–92. [Google Scholar]
- Tang, X.-H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Forman, B.M.; Umesono, K.; Chen, J.; Evans, R. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 1995, 81, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Koeffler, H.P. Peroxisome Proliferator-Activated Receptor Gamma and Cancers. Clin. Cancer Res. 2003, 9, 1–9. [Google Scholar]
- Nuclear Receptors Nomenclature Committee. A Unified Nomenclature System for the Nuclear Receptor Superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, R.; Williams, R.; Xu, M.; Noy, N. Nuclear Import of the Retinoid X Receptor, the Vitamin D Receptor, and Their Mutual Heterodimer. J. Biol. Chem. 2005, 280, 40152–40160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-K.; Su, Y.; Chen, L.; Chen, F.; Liu, J.; Zhou, H. Regulation of the nongenomic actions of retinoid X receptor-α by targeting the coregulator-binding sites. Acta Pharmacol. Sin. 2015, 36, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knabl, J.; Vattai, A.; Hüttenbrenner, R.; Hutter, S.; Karsten, M.; Jeschke, U. RXRα is upregulated in first trimester endometrial glands of spontaneous abortions unlike LXR and PPARγ. Eur. J. Histochem. 2016, 60, 2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laudet, V.; Gronemeyer, H. The Nuclear Receptor FactsBook; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Kersten, S.; Dong, D.; Lee, W.-Y.; Reczek, P.R.; Noy, N. Auto-silencing by the retinoid X receptor 1 1Edited by M. Yaniv. J. Mol. Biol. 1998, 284, 21–32. [Google Scholar] [CrossRef]
- The Human Protein Atlas. 2021. Available online: http://www.proteinatlas.org (accessed on 19 July 2021).
- Raffo, P.; Emionite, L.; Colucci, L.; Belmondo, F.; Moro, M.G.; Bollag, W.; Toma, S. Retinoid Receptors: Pathways of Proliferation Inhibition and Apoptosis Induction in Breast Cancer Cell Lines. Anticancer Res. 2000, 20, 1535–1543. [Google Scholar]
- Friedrich, M.; Axt-Fliedner, R.; Villena-Heinsen, C.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of Vitamin D-Receptor (Vdr) and Retinoid X-Receptor Alpha in Breast Cancer. Histochem. J. 2002, 34, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.; Williamson, E.A.; Zang, C.; Fritz, J.; Heber, D.; Fenner, M.; Possinger, K.; Koeffler, H.P. Novel Therapeutic Approach: Ligands for Ppargamma and Retinoid Receptors Induce Apoptosis in Bcl-2-Positive Human Breast Cancer Cells. Breast Cancer Res. Treat 2002, 74, 155–165. [Google Scholar] [CrossRef]
- Crowe, D.L.; Chandraratna, R.A. A Retinoid X Receptor (Rxr)-Selective Retinoid Reveals That Rxr-Alpha Is Potentially a Therapeutic Target in Breast Cancer Cell Lines, and That It Potentiates Antiproliferative and Apoptotic Responses to Peroxisome Proliferator-Activated Receptor Ligands. Breast Cancer Res. 2004, 6, R546–R555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, N.; Wang, Y.; Williams, C.R.; Risingsong, R.; Gilmer, T.; Willson, T.M.; Sporn, M.B. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999, 59, 5671–5673. [Google Scholar]
- Zanardi, S.; Serrano, D.; Argusti, A.; Barile, M.; Puntoni, M.; DeCensi, A. Clinical trials with retinoids for breast cancer chemoprevention. Endocr. Relat. Cancer 2006, 13, 51–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross-Innes, C.S.; Stark, R.; Holmes, K.A.; Schmidt, D.; Spyrou, C.; Russell, R.; Massie, C.E.; Vowler, S.L.; Eldridge, M.; Carroll, J.S. Cooperative Interaction between Retinoic Acid Receptor-Alpha and Estrogen Receptor in Breast Cancer. Genes Dev. 2010, 24, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heublein, S.; Mayr, D.; Meindl, A.; Kircher, A.; Jeschke, U.; Ditsch, N. Vitamin D Receptor, Retinoid X Receptor and Peroxisome Proliferator-Activated Receptor Gamma Are Overexpressed in Brca1 Mutated Breast Cancer and Predict Prognosis. J. Exp. Clin. Cancer Res. 2017, 36, 57. [Google Scholar] [CrossRef] [Green Version]
- Bonofiglio, D.; Cione, E.; Vizza, D.; Perri, M.; Pingitore, A.; Qi, H.; Catalano, S.; Rovito, D.; Genchi, G.; Ando, S. Bid as a Potential Target of Apoptotic Effects Exerted by Low Doses of Ppargamma and Rxr Ligands in Breast Cancer Cells. Cell Cycle 2011, 10, 2344–2354. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, R.; Kannan-Thulasiraman, P.; Kagechika, H.; Dawson, M.I.; Noy, N. Inhibition of Mammary Carcinoma Cell Growth by Rxr Is Mediated by the Receptor’s Oligomeric Switch. J. Mol. Biol. 2010, 397, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Zehni, A.Z.; Batz, F.; Vattai, A.; Kaltofen, T.; Schrader, S.; Jacob, S.N.; Mumm, J.N.; Heidegger, H.H.; Ditsch, N.; Mahner, S.; et al. The Prognostic Impact of Retinoid X Receptor and Thyroid Hormone Receptor Alpha in Unifocal vs. Multifocal/Multicentric Breast Cancer. Int. J. Mol. Sci. 2021, 22, 957. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Archiv. 2018, 472, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Edge, S.B.; Giuliano, A. New and Important Changes in the TNM Staging System for Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 457–467. [Google Scholar] [CrossRef]
- Elston, C.; Ellis, I. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for Uniform Definition of an Immunoreactive Score (Irs) for Immunohistochemical Estrogen Receptor Detection (Er-Ica) in Breast Cancer Tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Weissenbacher, T.; Hirte, E.; Kuhn, C.; Janni, W.; Mayr, D.; Karsten, U.; Rack, B.; Friese, K.; Jeschke, U.; Heublein, S.; et al. Multicentric and Multifocal Versus Unifocal Breast Cancer: Differences in the Expression of E-Cadherin Suggest Differences in Tumor Biology. BMC Cancer 2013, 13, 361. [Google Scholar] [CrossRef] [Green Version]
- Weissenbacher, T.M.; Zschage, M.; Janni, W.; Jeschke, U.; Dimpfl, T.; Mayr, D.; Rack, B.; Schindlbeck, C.; Friese, K.; Dian, D. Multicentric and Multifocal Versus Unifocal Breast Cancer: Is the Tumor-Node-Metastasis Classification Justified? Breast Cancer Res. Treat 2010, 122, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Ditsch, N.; Toth, B.; Himsl, I.; Lenhard, M.; Ochsenkühn, R.; Friese, K.; Mayr, D.; Jeschke, U. Thyroid hormone receptor (TR)alpha and TRbeta expression in breast cancer. Histol. Histopathol. 2013, 28, 227–237. [Google Scholar] [PubMed]
- Heublein, S.; Mayr, R.; Meindl, A.; Angele, M.; Gallwas, J.; Jeschke, U.; Ditsch, N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PLoS ONE 2015, 10, e0127072. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, N.; Mayr, D.; Lenhard, M.; Strauss, C.; Vodermaier, A.; Gallwas, J.; Stoeckl, D.; Graeser, M.; Weissenbacher, T.; Friese, K.; et al. Correlation of Thyroid Hormone, Retinoid X, Peroxisome Proliferator-Activated, Vitamin D and Oestrogen/Progesterone Receptors in Breast Carcinoma. Oncol. Lett. 2012, 4, 665–671. [Google Scholar] [CrossRef]
- Ghose, R.; Zimmerman, T.L.; Thevananther, S.; Karpen, S.J. Endotoxin Leads to Rapid Subcellular Re-Localization of Hepatic Rxralpha: A Novel Mechanism for Reduced Hepatic Gene Expression in Inflammation. Nucl. Recept. 2004, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mey, J.; Schrage, K.; Wessels, I.; Vollpracht-Crijns, I. Effects of Inflammatory Cytokines Il-1beta, Il-6, and Tnfalpha on the Intracellular Localization of Retinoid Receptors in Schwann Cells. Glia 2007, 55, 152–164. [Google Scholar] [CrossRef]
- Tanaka, T.; Dancheck, B.L.; Trifiletti, L.C.; Birnkrant, R.E.; Taylor, B.J.; Garfield, S.H.; Thorgeirsson, U.; De Luca, L.M. Altered Localization of Retinoid X Receptor Alpha Coincides with Loss of Retinoid Responsiveness in Human Breast Cancer Mda-Mb-231 Cells. Mol. Cell. Biol. 2004, 24, 3972–3982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Liu, W.; Su, Y.; Wei, Z.; Liu, J.; Kolluri, S.K.; Wu, H.; Cao, Y.; Chen, J.Y.; Wu, T.; et al. Nsaid Sulindac and Its Analog Bind Rxralpha and Inhibit Rxralpha-Dependent Akt Signaling. Cancer Cell 2010, 17, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Salehin, D.; Haugk, C.; Thill, M.; Cordes, T.; William, M.; Hemmerlein, B.; Friedrich, M. Vitamin D receptor expression in patients with vulvar cancer. Anticancer. Res. 2012, 32, 283–289. [Google Scholar] [PubMed]
- Matusiak, D.; Murillo, G.; Carroll, R.E.; Mehta, R.G.; Benya, R.V. Expression of Vitamin D Receptor and 25-Hydroxyvitamin D3-1[1]-Hydroxylase in Normal and Malignant Human Colon. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2370–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, P.E.; Halsall, J.A.; Popovici, S.; Papadogeorgakis, E.; Osborne, J.E.; Powley, I.R.; Dasari, D.; Saldanha, G.; Pringle, J.H. Compromised vitamin D receptor signalling in malignant melanoma is associated with tumour progression and mitogen-activated protein kinase activity. Melanoma Res. 2018, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Al-Izzi, S.; Alsaleem, M.; Kurozumi, S.; Toss, M.S.; Arshad, M.; Goh, F.Q.; Alshankyty, I.M.; Aleskandarany, M.; Ali, S.; et al. Retinoid X receptor gamma (RXRG) is an independent prognostic biomarker in ER-positive invasive breast cancer. Br. J. Cancer 2019, 121, 776–785. [Google Scholar] [CrossRef]
- Hennigs, A.; Riedel, F.; Gondos, A.; Sinn, P.; Schirmacher, P.; Marmé, F.; Jäger, D.; Kauczor, H.U.; Stieber, A.; Lindel, K.; et al. Prognosis of Breast Cancer Molecular Subtypes in Routine Clinical Care: A Large Prospective Cohort Study. BMC Cancer 2016, 16, 734. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Lee, J.Y.; Choi, J.E.; Lee, S.Y.; Park, J.Y.; Kim, D.S. Epigenetic inactivation of retinoid X receptor genes in non-small cell lung cancer and the relationship with clinicopathologic features. Cancer Genet. Cytogenet. 2010, 197, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Bapat, S.A. Expression Proteomics Predicts Loss of Rxr-Gamma During Progression of Epithelial Ovarian Cancer. PLoS ONE 2013, 8, e70398. [Google Scholar] [CrossRef]
- Liby, K.; Rendi, M.; Suh, N.; Royce, D.B.; Risingsong, R.C.; Williams, R.; Lamph, W.; Labrie, F.; Krajewski, S.; Xu, X.; et al. The Combination of the Rexinoid, Lg100268, and a Selective Estrogen Receptor Modulator, Either Arzoxifene or Acolbifene, Synergizes in the Prevention and Treatment of Mammary Tumors in an Estrogen Receptor-Negative Model of Breast Cancer. Clin. Cancer Res. 2006, 12, 5902–5909. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Kim, H.T.; Rodriquez, J.L.; Hilsenbeck, S.G.; Mohsin, S.K.; Xu, X.C.; Lamph, W.W.; Kuhn, J.G.; Green, J.E.; Brown, P.H. Suppression of Mammary Tumorigenesis in Transgenic Mice by the Rxr-Selective Retinoid, Lgd1069. Cancer Epidemiol. Biomark. Prev. 2002, 11, 467–474. [Google Scholar]
- Wu, K.; Dupré, E.; Kim, H.; Tin-U, C.K.; Bissonnette, R.P.; Lamph, W.W.; Brown, P.H. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res. Treat. 2005, 96, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Kim, H.-T.; Wu, K.; De Nardo, D.; Hilsenbeck, S.G.; Xu, X.-C.; Lamph, W.W.; Bissonnette, R.; Dannenberg, A.J.; Brown, P.H. The Retinoid X Receptor-Selective Retinoid, LGD1069, Down-regulates Cyclooxygenase-2 Expression in Human Breast Cells through Transcription Factor Crosstalk: Implications for Molecular-Based Chemoprevention. Cancer Res. 2005, 65, 3462–3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gniadecki, R.; Assaf, C.; Bagot, M.; Dummer, R.; Duvic, M.; Knobler, R.; Ranki, A.; Schwandt, P.; Whittaker, S. The optimal use of bexarotene in cutaneous T-cell lymphoma. Br. J. Dermatol. 2007, 157, 433–440. [Google Scholar] [CrossRef]
- Esteva, F.J.; Glaspy, J.; Baidas, S.; Laufman, L.; Hutchins, L.; Dickler, M.; Tripathy, D.; Cohen, R.; De Michele, A.; Yocum, R.C.; et al. Multicenter Phase II Study of Oral Bexarotene for Patients with Metastatic Breast Cancer. J. Clin. Oncol. 2003, 21, 999–1006. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | n (%) |
|---|---|
| Age (years) | Median 59.09 ± 13.1 Standard Deviation |
| Tumor foci | Unifocal 173 (54.2) Multifocal 146 (45.7) |
| Histology | No special type (NST) 188 (61.4) Non-NST 118 (38.5) |
| Estrogen Receptor | Negative 45 (18.3) Positive 201 (81.7) |
| Progesterone Receptor | Negative 92 (37.4) Positive 154 (62.6) |
| Tumor grade | G1 or G2 165 (52.2) G3 151 (47.7) |
| pT | pT1 197 (64.3) pT2–pT4 109 (35.6) |
| pN | pN0 166 (54.2) pN1–pN3 140 (45.7) |
| pM | pM0 239 (78.1) pM1 67 (21.8) |
| Nuclear RXRα | Negative 124 (42.5) Positive 168 (57.5) |
| Cytoplasmic RXRα | Negative 122 (40.0) Positive 183 (60.0) |
| Variables | Cytoplasmic RXRα | Nuclear RXRα | ||
|---|---|---|---|---|
| p | Correlation Coefficient | p | Correlation Coefficient | |
| pT | 0.497 | −0.039 | 0.012 * | −0.143 |
| pN | 0.630 | 0.028 | 0.063 | −0.107 |
| Histology | 0.610 | 0.029 | 0.558 | −0.034 |
| Grading | 0.029 * | 0.125 | 0.227 | −0.069 |
| Variable | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| RXRn > 0 Histology | −0.310 −0.003 | 0.733 (0.487–1.103) 0.997 (0.970–1.024) | 0.136 0.821 |
| Grading | 0.004 | 1.004 (1.000–1.009) | 0.056 |
| pT | 0.551 | 1.736 (1.500–2.008) | 0.001 |
| pN | 0.015 | 1.015 (1.006–1.024) | 0.001 |
| Focality | −0.240 | 0.787 (0.599–1.033) | 0.085 |
| Estrogen Receptor | 0.084 | 1.088 (0.643–1.841) | 0.754 |
| Progesterone Receptor | −0.082 | 0.921 (0.545–1.557) | 0.759 |
| Variable | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| RXRc > 0 Histology | 0.472 −0.003 | 1.603 (0.964–2.665) 0.997 (0.973–1.023 | 0.069 0.837 |
| Grading | 0.004 | 1.004 (0.999–1.009) | 0.132 |
| pT | 0.572 | 1.772 (1.514–2.074) | 0.001 |
| pN | 0.013 | 1.013 (1.003–1.023) | 0.010 |
| Focality | −0.172 | 0.842 (0.623–1.139) | 0.265 |
| Estrogen Receptor | 0.253 | 1.288 (0.725–2.291) | 0.253 |
| Progesterone Receptor | −0.254 | 0.776 (0.437–1.377) | 0.386 |
| Variable | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| RXRc > 0 | 0.528 | 1.696 (1.077–2.671) | 0.023 |
| Histology | −0.059 | 0.942 (0.873–1.017) | 0.127 |
| Grading | −0.001 | 0.999 (0.995–1.004) | 0.811 |
| pT | 0.353 | 1.423 (1.190–1.701) | 0.001 |
| pN | 0.000 | 1.000 (0.986–1.014) | 0.996 |
| Focality | 0.040 | 1.041 (0.782–1.386) | 0.783 |
| Estrogen Receptor | −0.173 | 0.841 (0.488–1.451) | 0.534 |
| Progesterone Receptor | 0.172 | 1.188 (0.689–2.047) | 0.535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zati zehni, A.; Batz, F.; Cavaillès, V.; Sixou, S.; Kaltofen, T.; Keckstein, S.; Heidegger, H.H.; Ditsch, N.; Mahner, S.; Jeschke, U.; et al. Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer. Cancers 2021, 13, 3756. https://doi.org/10.3390/cancers13153756
Zati zehni A, Batz F, Cavaillès V, Sixou S, Kaltofen T, Keckstein S, Heidegger HH, Ditsch N, Mahner S, Jeschke U, et al. Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer. Cancers. 2021; 13(15):3756. https://doi.org/10.3390/cancers13153756
Chicago/Turabian StyleZati zehni, Alaleh, Falk Batz, Vincent Cavaillès, Sophie Sixou, Till Kaltofen, Simon Keckstein, Helene Hildegard Heidegger, Nina Ditsch, Sven Mahner, Udo Jeschke, and et al. 2021. "Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer" Cancers 13, no. 15: 3756. https://doi.org/10.3390/cancers13153756
APA StyleZati zehni, A., Batz, F., Cavaillès, V., Sixou, S., Kaltofen, T., Keckstein, S., Heidegger, H. H., Ditsch, N., Mahner, S., Jeschke, U., & Vilsmaier, T. (2021). Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer. Cancers, 13(15), 3756. https://doi.org/10.3390/cancers13153756







