Epstein-Barr Virus Positive B-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract
Abstract
Simple Summary
Abstract
1. Introduction
2. EBV Biology and the GI Tract
3. EBV-Positive Mucocutaneous Ulcer (EBVMCU)
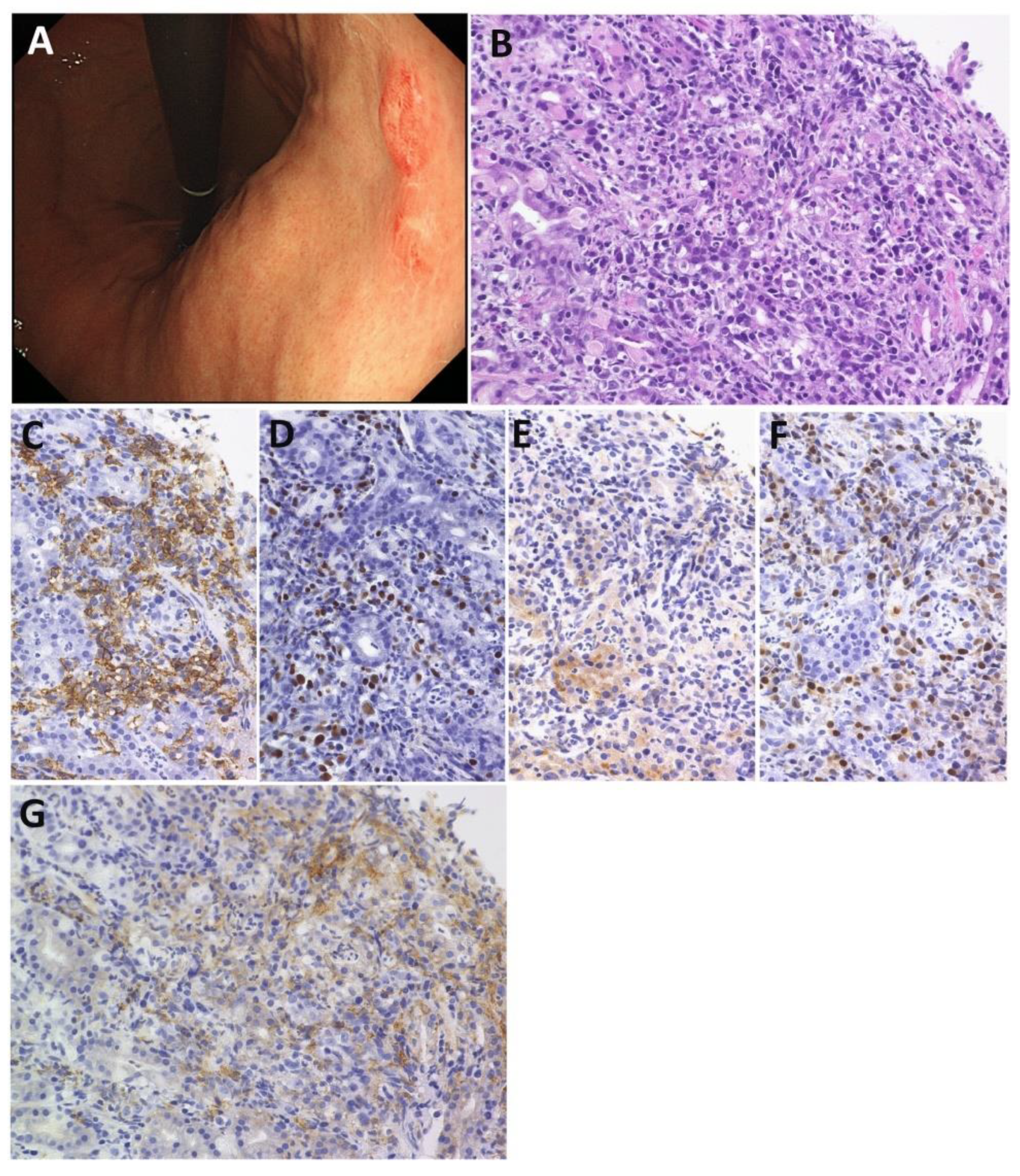
3.1. Clinical Features
3.2. Pathological Features
3.3. PD-L1 Expression
3.4. Treatment and Clinical Course
3.5. giEBVMCU
3.5.1. giEBVMCU in Patients with IBD
3.5.2. giEBVMCU in Patients with Organ Transplant
3.5.3. giEBVMCU in Patients with irColitis
3.5.4. giEBVMCU in the Other Patients
4. EBV-Positive Diffuse Large B-Cell Lymphoma (EBV+ DLBCL)
4.1. Clinical Features
4.2. PD-L1 Expression
4.3. Overall Perspective of Primary giDLBCL as a Control Cohort
4.4. Primary EBV+ giDLBCL
4.4.1. Clinical Features of Primary EBV+ giDLBCL
4.4.2. Pathological Features of Primary EBV+ giDLBCL
4.4.3. PD-L1 Expression of Primary EBV+ giDLBCL
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dojcinov, S.D.; Fend, F.; Quintanilla-Martinez, L. EBV-Positive Lymphoproliferations of B-T-and NK-Cell Derivation in Non-Immunocompromised Hosts. Pathogens 2018, 7, 28. [Google Scholar] [CrossRef]
- Luzuriaga, K.; Sullivan, J.L. Infectious Mononucleosis. N. Engl. J. Med. 2010, 362, 1993–2000. [Google Scholar] [CrossRef]
- Kimura, H.; Cohen, J.I. Chronic Active Epstein-Barr Virus Disease. Front. Immunol. 2017, 8, 1867. [Google Scholar] [CrossRef]
- Tian, S.F.; Westbrook, L.M.; Xiao, S.Y.; Zhang, Y.L.; Huang, Y.; Wang, H.L.L. The Morphologic Features of Primary Epstein-Barr Virus Infection in the Gastrointestinal Tract an Approach to Correct Diagnosis. Am. J. Surg. Pathol. 2019, 43, 1253–1263. [Google Scholar] [CrossRef]
- Xu, W.J.; Jiang, X.Y.; Chen, J.J.; Mao, Q.Q.; Zhao, X.G.; Sun, X.; Zhong, L.; Rong, L. Chronic active Epstein-Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Oyama, T.; Ichimura, K.; Suzuki, R.; Suzumiya, J.; Ohshima, K.; Yatabe, Y.; Yokoi, T.; Kojima, M.; Kamiya, Y.; Taji, H.; et al. Senile EBV plus B-cell Lymphoproliferative disorders—A clinicopathologic study of 22 patients. Am. J. Surg. Pathol. 2003, 27, 16–26. [Google Scholar] [CrossRef]
- Oyama, T.; Yamamoto, K.; Asano, N.; Oshiro, A.; Suzuki, R.; Kagami, Y.; Morishima, Y.; Takeuchi, K.; Izumo, T.; Mori, S.; et al. Age-related EBV-Associated B-Cell Lymphoproliferative disorders constitute a distinct clinicopathologic group: A study of 96 patients. Clin. Cancer Res. 2007, 13, 5124–5132. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Ko, Y.H.; Han, A.; Jun, H.J.; Lee, S.C.; Hwang, I.G.; Park, Y.H.; Ahn, J.S.; Jung, C.W.; et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood 2007, 110, 972–978. [Google Scholar] [CrossRef]
- Morales, D.; Beltran, B.; De Mendoza, F.H.; Riva, L.; Yabar, A.; Quinones, P.; Butera, J.N.; Castillo, J. Epstein-Barr virus as a prognostic factor in de novo nodal diffuse large B-cell lymphoma. Leuk. Lymphoma 2010, 51, 66–72. [Google Scholar] [CrossRef]
- Nicolae, A.; Pittaluga, S.; Abdullah, S.; Steinberg, S.M.; Pham, T.A.; Davies-Hill, T.; Xi, L.Q.; Raffeld, M.; Jaffe, E.S. EBV-positive large B-cell lymphomas in young patients: A nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015, 126, 863–872. [Google Scholar] [CrossRef]
- Ishikawa, E.; Tanaka, T.; Shimada, K.; Kohno, K.; Satou, A.; EladI, A.E.; Sakakibara, A.; Furukawa, K.; Funasaka, K.; Miyahara, R.; et al. A prognostic model, including the EBV status of tumor cells, for primary gastric diffuse large B-cell lymphoma in the rituximab era. Cancer Med. 2018, 7, 3510–3520. [Google Scholar] [CrossRef]
- Ishikawa, E.; Kato, S.; Shimada, K.; Tanaka, T.; Suzuki, Y.; Satou, A.; Kohno, K.; Sakakibara, A.; Yamamura, T.; Nakamura, M.; et al. Clinicopathological analysis of primary intestinal diffuse large B-cell lymphoma: Prognostic evaluation of CD5, PD-L1, and Epstein-Barr virus on tumor cells. Cancer Med. 2018, 7, 6051–6063. [Google Scholar] [CrossRef]
- Maeshima, A.M.; Taniguchi, H.; Ito, Y.; Hatta, S.; Suzuki, T.; Yuda, S.; Makita, S.; Fukuhara, S.; Munakata, W.; Suzuki, T.; et al. Clinicopathological characteristics of diffuse large B-cell lymphoma involving small and large intestines: An analysis of 126 patients. Int. J. Hematol. 2019, 110, 340–346. [Google Scholar] [CrossRef]
- Ahn, J.S.; Yang, D.H.; Choi, Y.D.; Jung, S.H.; Yhim, H.Y.; Kwak, J.Y.; Park, H.S.; Shin, M.G.; Kim, Y.K.; Kim, H.J.; et al. Clinical outcome of elderly patients with Epstein-Barr virus positive diffuse large B-cell lymphoma treated with a combination of rituximab and CHOP chemotherapy. Am. J. Hematol. 2013, 88, 774–779. [Google Scholar] [CrossRef]
- Sato, A.; Nakamura, N.; Kojima, M.; Ohmachi, K.; Carreras, J.; Kikuti, Y.Y.; Numata, H.; Ohgiya, D.; Tazume, K.; Amaki, J.; et al. Clinical outcome of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly in the rituximab era. Cancer Sci. 2014, 105, 1170–1175. [Google Scholar] [CrossRef]
- Hong, J.Y.; Yoon, D.H.; Suh, C.; Huh, J.; Do, I.G.; Sohn, I.; Jo, J.; Jung, S.H.; Hong, M.E.; Yoon, H.; et al. EBV-positive diffuse large B-cell lymphoma in young adults: Is this a distinct disease entity? Ann. Oncol. 2015, 26, 548–555. [Google Scholar] [CrossRef]
- Ok, C.Y.; Ye, Q.; Li, L.; Manyam, G.C.; Deng, L.J.; Goswami, R.R.; Wang, X.X.; Montes-Moreno, S.; Visco, C.; Tzankov, A.; et al. Age cutoff for Epstein-Barr virus-positive diffuse large B-cell lymphoma—is it necessary? Oncotarget 2015, 6, 13935–13946. [Google Scholar] [CrossRef]
- Dojcinov, S.D.; Venkataraman, G.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. EBV Positive Mucocutaneous Ulcer-A Study of 26 Cases Associated with Various Sources of Immunosuppression. Am. J. Surg. Pathol. 2010, 34, 405–417. [Google Scholar] [CrossRef]
- Matnani, R.; Peker, D. Azathioprine induced Epstein Barr virus-positive mucocutaneous ulcer arising in perianal fistula and abscess associated with Crohn’s disease. J. Crohns Colitis 2014, 8, 1747–1748. [Google Scholar] [CrossRef]
- Moran, N.R.; Webster, B.; Lee, K.M.; Trotman, J.; Kwan, Y.L.; Napoli, J.; Leong, R.W. Epstein Barr virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in Crohn’s disease. World J. Gastroenterol. 2015, 21, 6072–6076. [Google Scholar] [CrossRef]
- Juan, A.; Lobaton, T.; Tapia, G.; Manosa, M.; Cabre, E.; Domenech, E. Epstein-Barr virus-positive mucocutaneous ulcer in Crohn’s disease. A condition to consider in immunosuppressed IBD patients. Dig. Liver Dis. 2017, 49, 934–937. [Google Scholar] [CrossRef]
- Goetgebuer, R.L.; van der Woude, C.J.; de Ridder, L.; Doukas, M.; de Vries, A.C. Clinical and endoscopic complications of Epstein-Barr virus in inflammatory bowel disease: An illustrative case series. Int. J. Colorectal Dis. 2019, 34, 923–926. [Google Scholar] [CrossRef]
- Prieto-Torres, L.; Erana, I.; Gil-Redondo, R.; de la Riva, I.G.; Manso, R.; Pajares, R.; Cordoba, R.; Machan, S.; Ara, M.; Requena, L.; et al. The Spectrum of EBV-Positive Mucocutaneous Ulcer A Study of 9 Cases. Am. J. Surg. Pathol. 2019, 43, 201–210. [Google Scholar] [CrossRef]
- Pugh, M.R.; Leopold, G.D.; Morgan, M.; Christian, A.D.; Hewett, R.; Durai, D.; Wagstaff, J.; Harris, D.; Dojcinov, S.D. Epstein-Barr Virus-Positive Mucocutaneous Ulcers Complicate Colitis Caused by Immune Checkpoint Regulator Therapy and Associate with Colon Perforation. Clin. Gastroenterol. Hepatol. 2020, 18, 1785. [Google Scholar] [CrossRef]
- Narimatsu, H.; Ota, Y.; Kami, M.; Takeuchi, K.; Suzuki, R.; Matsuo, K.; Matsumura, T.; Yuji, K.; Kishi, Y.; Hamaki, T.; et al. Clinicopathological features of pyothorax-associated lymphoma; a retrospective survey involving 98 patients. Ann. Oncol. 2007, 18, 122–128. [Google Scholar] [CrossRef]
- Song, J.Y.; Pittaluga, S.; Dunleavy, K.; Grant, N.; White, T.; Jiang, L.Y.; Davies-Hill, T.; Raffeld, M.; Wilson, W.H.; Jaffe, E.S. Lymphomatoid Granulomatosis-A Single Institute Experience Pathologic Findings and Clinical Correlations. Am. J. Surg. Pathol. 2015, 39, 141–156. [Google Scholar] [CrossRef]
- Beaty, M.W.; Toro, J.; Sorbara, L.; Stern, J.B.; Pittaluga, S.; Raffeld, M.; Wilson, W.H.; Jaffe, E.S. Cutaneous lymphomatoid granulomatosis—Correlation of clinical and biologic features. Am. J. Surg. Pathol. 2001, 25, 1111–1120. [Google Scholar] [CrossRef]
- Dong, H.D.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.F.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Chen, B.J.; Chapuy, B.; Jing, O.Y.; Sun, H.H.; Roemer, M.G.M.; Xu, M.L.; Yu, H.B.; Fletcher, C.D.M.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-cell Lymphomas and Virus-Associated Malignancies. Clin. Cancer Res. 2013, 19, 3462–3473. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Balar, A.V.; Weber, J.S. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol. Immunother. 2017, 66, 551–564. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients with Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, A.; Kohno, K.; Ishikawa, E.; Suzuki, Y.; Shimada, S.; Eladl, A.E.; Elsayed, A.A.; Daroontum, T.; Satou, A.; Takahara, T.; et al. Age-related EBV-associated B-cell lymphoproliferative disorders and other EBV plus lymphoproliferative diseases: New insights into immune escape and immunodeficiency through staining with anti-PD-L1 antibody clone SP142. Pathol. Int. 2020, 70, 481–492. [Google Scholar] [CrossRef]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015, 126, 2193–2201. [Google Scholar] [CrossRef]
- Ishikawa, E.; Nakamura, M.; Shimada, K.; Tanaka, T.; Satou, A.; Kohno, K.; Sakakibara, A.; Furukawa, K.; Yamamura, T.; Miyahara, R.; et al. Prognostic impact of PD-L1 expression in primary gastric and intestinal diffuse large B-cell lymphoma. J. Gastroenterol. 2020, 55, 39–50. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A.; Gross, A. Mechanisms of disease—Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004, 350, 1328–1337. [Google Scholar] [CrossRef]
- Taylor, G.S.; Long, H.M.; Brooks, J.M.; Rickinson, A.B.; Hislop, A.D. The immunology of Epstein-Barr Virus Induced Disease. Annu. Rev. Immunol. 2015, 33, 787. [Google Scholar] [CrossRef]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Satou, A.; Asano, N.; Nakazawa, A.; Osumi, T.; Tsurusawa, M.; Ishiguro, A.; Elsayed, A.A.; Nakamura, N.; Ohshima, K.; Kinoshita, T.; et al. Epstein-Barr Virus (EBV)-positive Sporadic Burkitt Lymphoma an Age-related Lymphoproliferative Disorder? Am. J. Surg. Pathol. 2015, 39, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Shen, Y.J.; Morgan, D.R.; Thorne, L.B.; Kenney, S.C.; Dominguez, R.L.; Gulley, M.L. Epstein-Barr Virus Infection Is Common in Inflamed Gastrointestinal Mucosa. Dig. Dis. Sci. 2012, 57, 1887–1898. [Google Scholar] [CrossRef][Green Version]
- Yanai, H.; Shimizu, N.; Nagasaki, S.; Mitani, N.; Okita, K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am. J. Gastroenterol. 1999, 94, 1582–1586. [Google Scholar] [CrossRef]
- Shukla, S.K.; Prasad, K.N.; Tripathi, A.; Singh, A.; Saxena, A.; Chand Ghoshal, U.; Krishnani, N.; Husain, N. Epstein-Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases. Braz. J. Infect. Dis. 2011, 15, 583–590. [Google Scholar] [CrossRef]
- Yanai, H.; Murakami, T.; Yoshiyama, H.; Takeuchi, H.; Nishikawa, J.; Nakamura, H.; Okita, K.; Miura, O.; Shimizu, N.; Takada, K. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J. Clin. Gastroenterol. 1999, 29, 39–43. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar]
- Saito, R.; Abe, H.; Kunita, A.; Yamashita, H.; Seto, Y.; Fukayama, M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: The prognostic implications. Mod. Pathol. 2017, 30, 427–439. [Google Scholar] [CrossRef]
- Fang, W.L.; Chen, M.H.; Huang, K.H.; Lin, C.H.; Chao, Y.; Lo, S.S.; Li, A.F.Y.; Wu, C.W.; Shyr, Y.M. The Clinicopathological Features and Genetic Alterations in Epstein-Barr Virus-Associated Gastric Cancer Patients after Curative Surgery. Cancers 2020, 12, 1517. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.W.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449. [Google Scholar] [CrossRef]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef]
- Sankaran-Walters, S.; Ransibrahmanakul, K.; Grishina, I.; Hung, J.; Martinez, E.; Prindiville, T.; Dandekar, S. Epstein-Barr virus replication linked to B cell proliferation in inflamed areas of colonic mucosa of patients with inflammatory bowel disease. J. Clin. Virol. 2011, 50, 31–36. [Google Scholar] [CrossRef]
- Lopes, S.; Andrade, P.; Conde, S.; Liberal, R.; Dias, C.C.; Fernandes, S.; Pinheiro, J.; Simoes, J.S.; Carneiro, F.; Magro, F.; et al. Looking into Enteric Virome in Patients with IBD: Defining Guilty or Innocence? Inflamm. Bowel Dis. 2017, 23, 278–284. [Google Scholar] [CrossRef]
- Dayharsh, G.A.; Loftus, E.V.; Sandborn, W.J.; Tremaine, W.J.; Zinsmeister, A.R.; Witzig, T.E.; Macon, W.R.; Burgart, L.J. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology 2002, 122, 72–77. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lemann, M.; Cosnes, J.; Hebuterne, X.; Cortot, A.; Bouhnik, Y.; Gendre, J.P.; et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA J. Am. Med. Assoc. 2017, 318, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Rezk, S.A.; Weiss, L.M. Epstein-Barr virus-associated lymphoproliferative disorders. Hum. Pathol. 2007, 38, 1293–1304. [Google Scholar] [CrossRef]
- Ghia, P.; Prato, G.; Stella, S.; Scielzo, C.; Geuna, M.; Caligaris-Cappio, F. Age-dependent accumulation of monoclonal CD4(+)CD8(+)double positive T lymphocytes in the peripheral blood of the elderly. Br. J. Haematol. 2007, 139, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Gion, Y.; Sakamoto, M.; Tachibana, T.; Nishikori, A.; Nishimura, M.F.; Yoshino, T.; Sato, Y. Clinicopathological analysis of 34 Japanese patients with EBV-positive mucocutaneous ulcer. Mod. Pathol. 2020, 33, 2437–2448. [Google Scholar] [CrossRef]
- Hart, M.; Thakral, B.; Yohe, S.; Balfour, H.H.; Singh, C.; Spears, M.; McKenna, R.W. EBV-positive Mucocutaneous Ulcer in Organ Transplant Recipients a Localized Indolent Posttransplant Lymphoproliferative Disorder. Am. J. Surg. Pathol. 2014, 38, 1522–1529. [Google Scholar] [CrossRef]
- Satou, A.; Banno, S.; Hanamura, I.; Takahashi, E.; Takahara, T.; Nobata, H.; Katsuno, T.; Takami, A.; Ito, Y.; Ueda, R.; et al. EBV-positive mucocutaneous ulcer arising in rheumatoid arthritis patients treated with methotrexate: Single center series of nine cases. Pathol. Int. 2019, 69, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Daroontum, T.; Kohno, K.; Eladl, A.E.; Satou, A.; Sakakibara, A.; Matsukage, S.; Yakushiji, N.; Ya-In, C.; Nakamura, S.; Asano, N.; et al. Comparison of Epstein-Barr virus-positive mucocutaneous ulcer associated with treated lymphoma or methotrexate in Japan. Histopathology 2018, 72, 1115–1127. [Google Scholar] [CrossRef]
- Daroontum, T.; Kohno, K.; Inaguma, Y.; Okamoto, A.; Okamoto, M.; Kimura, Y.; Nagahama, M.; Sakakibara, A.; Satou, A.; Nakamura, S. Epstein-Barr virus (EBV)-positive diffuse large B-cell lymphoma arising in patient with a history of EBV-positive mucocutaneous ulcer and EBV-positive nodal polymorphous B-lymphoproliferative disorder. Pathol. Int. 2019, 69, 37–41. [Google Scholar] [CrossRef]
- Sinit, R.B.; Horan, K.L.; Dorer, R.K.; Aboulafia, D.M. Epstein-Barr Virus-Positive Mucocutaneous Ulcer: Case Report and Review of the First 100 Published Cases. Clin. Lymphoma Myeloma Leuk. 2019, 19, E81–E92. [Google Scholar] [CrossRef]
- Morita, N.; Okuse, C.; Suetani, K.; Nakano, H.; Hiraishi, T.; Ishigooka, S.; Mori, S.; Shimamura, T.; Asakura, T.; Koike, J.; et al. A rare case of Epstein-Barr virus-positive mucocutaneous ulcer that developed into an intestinal obstruction: A case report. BMC Gastroenterol. 2020, 20, 9. [Google Scholar] [CrossRef]
- Osman, M.; Al Salihi, M.; Abu Sitta, E.; Al Hadidi, S. A rare case of Epstein-Barr virus mucocutaneous ulcer of the colon. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Zanelli, M.; Zizzo, M.; Foroni, M.; De Marco, L.; Martino, G.; Ascani, S. EBV-positive mucocutaneous ulcer within colonic diverticulitis mimicking diffuse large B cell lymphoma. Ann. Hematol. 2019, 98, 1795–1797. [Google Scholar] [CrossRef]
- Di Napoli, A.; Giubettini, M.; Duranti, E.; Ferrari, A.; Guglielmi, C.; Uccini, S.; Ruco, L. Iatrogenic EBV-positive lymphoproliferative disorder with features of EBV plus mucocutaneous ulcer: Evidence for concomitant TCR gamma/IGH rearrangements in the Hodgkin-like neoplastic cells. Virchows Archiv 2011, 458, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Karube, K.; Takatori, M.; Kohno, K.; Tomoyose, T.; Ohshiro, K.; Nakazato, I. Co-occurrence of EBV-positive classic Hodgkin lymphoma and B-cell lymphomas of different clonal origins: A case report and literature review. Pathol. Int. 2020, 70, 893–898. [Google Scholar] [CrossRef]
- Nomura, M.; Sumiya, R.; Ono, H.; Nagai, T.; Kumazawa, K.; Shimizu, A.; Endo, D.; Aoyanagi, N. Cessation of methotrexate and a small intestinal resection provide a good clinical course for a patient with a jejunum perforation induced by a methotrexate-associated lymphoproliferative disorder: A case report. World J. Surg. Oncol. 2021, 19, s12957. [Google Scholar] [CrossRef] [PubMed]
- Isnard, P.; Bruneau, J.; Sberro-Soussan, R.; Wendum, D.; Legendre, C.; Molina, T.; Chatenoud, L.; Hermine, O.; Rossignol, J. Dissociation of humoral and cellular immune responses in kidney transplant recipients with EBV mucocutaneous ulcer. Transpl. Infect. Dis. 2021, 23, e13552. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, S.; Jhaveri, D.; Caimi, P.; Cameron, R.; Lemonovich, T.; Meyerson, H.; Hostoffer, R.; Tcheurekdjian, H. A rare presentation of EBV+ mucocutaneous ulcer that led to a diagnosis of hypogammaglobulinemia. J. Allergy Clin. Immunol. Pract. 2014, 2, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chapman, J.R.; Vega, F. A case of EBV-associated blastic lymphoplasmacytic proliferation in an oesophageal ulcer with a self-limiting course: Overlapping lesion between EBV mucocutaneous ulcer and polymorphic lymphoplasmacytic disorder. Histopathology 2019, 74, 964–966. [Google Scholar] [CrossRef]
- Zanelli, M.; Mengoli, M.C.; Valli, R.; Froio, E.; Bisagni, A.; Zizzo, M.; De Marco, L.; Ascani, S. Primary classic Hodgkin lymphoma of the ileum and Epstein-Barr virus mucocutaneous ulcer of the colon: Two entities compared. Virchows Archiv 2019, 474, 117–123. [Google Scholar] [CrossRef]
- Ma, S.D.; Xu, X.Q.; Jones, R.; Delecluse, H.J.; Zumwalde, N.A.; Sharma, A.; Gumperz, J.E.; Kenney, S.C. PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model. PLoS Pathog. 2016, 12, e1005642. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.Y.; Hwang, J.E.; Keam, B.; Kim, S.B.; Shim, J.J.; Jang, H.J.; Park, S.; Sohn, B.H.; Cha, M.; Ajani, J.A.; et al. Genomic landscape associated with potential response to anti-CTLA-4 treatment in cancers. Nat. Commun. 2017, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res. 2017, 77, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Tzankov, A.; Pileri, S.A.; Went, P.; Dirnhofer, S. Epstein-Barr virus positive diffuse large B-cell lymphoma in elderly patients is rare in Western populations. Hum. Pathol. 2010, 41, 352–357. [Google Scholar] [CrossRef]
- Dojcinov, S.D.; Venkataraman, G.; Pittaluga, S.; Wlodarska, I.; Schrager, J.A.; Raffeld, M.; Hills, R.K.; Jaffe, E.S. Age-related EBV-associated lymphoproliferative disorders in the Western population: A spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011, 117, 4726–4735. [Google Scholar] [CrossRef]
- Asano, N.; Yamamoto, K.; Tamaru, J.I.; Oyama, T.; Ishida, F.; Ohshima, K.; Yoshino, T.; Nakamura, N.; Mori, S.; Yoshie, O.; et al. Age-related Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders: Comparison with EBV-positive classic Hodgkin lymphoma in elderly patients. Blood 2009, 113, 2629–2636. [Google Scholar] [CrossRef]
- Olsson, J.; Wikby, A.; Johansson, B.; Lofgren, S.; Nilsson, B.O.; Ferguson, F.G. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: The Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 2000, 121, 187–201. [Google Scholar] [CrossRef]
- Vescovini, R.; Telera, A.; Fagnoni, F.F.; Biasini, C.; Medici, M.C.; Valcavi, P.; di Pede, P.; Lucchini, G.; Zanlari, L.; Passeri, G.; et al. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8(+) T cells. Exp. Gerontol. 2004, 39, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, D.; Velez, G.; Orfao, A.; Herrera, M.V.; Solano, J.; Olaya, M.; Uribe, A.M.; Saavedra, C.; Duarte, M.; Rodriguez, M.; et al. Epstein-Barr virus-specific CD8(+) T lymphocytes from diffuse large B cell lymphoma patients are functionally impaired. Clin. Exp. Immunol. 2015, 182, 173–183. [Google Scholar] [CrossRef]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Kuhnt, E.; Trumper, L.; Osterborg, A.; Trneny, M.; Shepherd, L.; Gill, D.S.; Walewski, J.; Pettengell, R.; Jaeger, U.; et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011, 12, 1013–1022. [Google Scholar] [CrossRef]
- Witte, H.M.; Merz, H.; Biersack, H.; Bernard, V.; Riecke, A.; Gebauer, J.; Lehnert, H.; von Bubnoff, N.; Feller, A.C.; Gebauer, N. Impact of treatment variability and clinicopathological characteristics on survival in patients with Epstein-Barr-Virus positive diffuse large B cell lymphoma. Br. J. Haematol. 2020, 189, 257–268. [Google Scholar] [CrossRef]
- Georgiou, K.; Chen, L.Y.; Berglund, M.; Ren, W.C.; de Miranda, N.; Lisboa, S.; Fangazio, M.; Zhu, S.D.; Hou, Y.; Wu, K.; et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood 2016, 127, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- McCord, R.; Bolen, C.R.; Koeppen, H.; Kadel, E.E.; Oestergaard, M.Z.; Nielsen, T.; Sehn, L.H.; Venstrom, J.M. PD-L1 and tumor-associated macrophages in de novo DLBCL. Blood Adv. 2019, 3, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Xu, X.L.; Rao, H.L.; Chen, J.; Lai, R.C.; Huang, H.Q.; Jiang, W.Q.; Lin, T.Y.; Xia, Z.J.; Cai, Q.Q. Expression and clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse large B cell lymphoma: A retrospective study. Chin. J. Cancer 2017, 36, s40880. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3 ‘-UTR disruption in multiple cancers. Nature 2016, 534, 402. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Kim, S.; Kim, P.J.; Go, H.; Nam, S.J.; Paik, J.H.; Kim, Y.A.; Kim, T.M.; Heo, D.S.; Kim, C.W.; et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 2016, 68, 1079–1089. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Satou, A.; Ishikawa, E.; Kohno, K.; Kato, S.; Suzuki, Y.; Takahashi, E.; Ohashi, A.; Asano, N.; Tsuzuki, T.; et al. Clinicopathological analysis of neoplastic PD-L1-positive EBV(+)diffuse large B cell lymphoma, not otherwise specified, in a Japanese cohort. Virchows Archiv 2021, 478, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Matsumoto, T.; Iida, M.; Yao, T.; Tsuneyoshi, M. Primary gastrointestinal lymphoma in Japan—A clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003, 97, 2462–2473. [Google Scholar] [CrossRef]
- Papaxoinis, G.; Papageorgiou, S.; Rontogianni, D.; Kaloutsi, V.; Fountzilas, G.; Pavlidis, N.; Dimopoulos, M.; Tsatalas, C.; Xiros, N.; Economopoulos, T. Primary gastrointestinal non-Hodgkin’s lymphoma: A clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk. Lymphoma 2006, 47, 2140–2146. [Google Scholar] [CrossRef]
- Ding, W.S.; Zhao, S.; Wang, J.C.; Yang, Q.P.; Sun, H.; Yan, J.Q.; Gao, L.M.; Yao, W.Q.; Zhang, W.Y.; Liu, W.P. Gastrointestinal Lymphoma in Southwest China: Subtype Distribution of 1,010 Cases Using the WHO (2008) Classification in a Single Institution. Acta Haematol. 2016, 135, 21–28. [Google Scholar] [CrossRef]
- Chuang, S.S.; Ye, H.; Yang, S.F.; Huang, W.T.; Chen, H.K.; Hsieh, P.P.; Hwang, W.S.; Chang, K.Y.; Lu, C.L.; Du, M.Q. Perforation predicts poor prognosis in patients with primary intestinal diffuse large B-cell lymphoma. Histopathology 2008, 53, 432–440. [Google Scholar] [CrossRef]
- Tanaka, T.; Shimada, K.; Yamamoto, K.; Hirooka, Y.; Niwa, Y.; Sugiura, I.; Kitamura, K.; Kosugi, H.; Kinoshita, T.; Goto, H.; et al. Retrospective analysis of primary gastric diffuse large B cell lymphoma in the rituximab era: A multicenter study of 95 patients in Japan. Ann. Hematol. 2012, 91, 383–390. [Google Scholar] [CrossRef]
- Kuo, S.H.; Yeh, K.H.; Chen, L.T.; Lin, C.W.; Hsu, P.N.; Hsu, C.; Wu, M.S.; Tzeng, Y.S.; Tsai, H.J.; Wang, H.P.; et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: A distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014, 4, e220. [Google Scholar] [CrossRef]
- Yoshino, T.; Nakamura, S.; Matsuno, Y.; Ochiai, A.; Yokoi, T.; Kitadai, Y.; Suzumiya, J.; Tobinai, K.; Kobayashi, Y.; Oda, I.; et al. Epstein-Barr virus involvement is a predictive factor for the resistance to chemoradiotherapy of gastric diffuse large B-cell lymphoma. Cancer Sci. 2006, 97, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ye, H.; Bacon, C.M.; Goatly, A.; Liu, H.X.; Kerr, L.; Banham, A.H.; Streubel, B.; Yao, T.; Tsuneyoshi, M.; et al. Translocations involving the immunoglobulin heavy chain gene locus predict better survival in gastric diffuse large B-cell lymphoma. Clin. Cancer Res. 2008, 14, 3002–3010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.J.; Kang, H.J.; Kim, J.S.; Oh, S.Y.; Choi, C.W.; Lee, S.I.; Won, J.H.; Kim, M.K.; Kwon, J.H.; Mun, Y.C.; et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: Surgical resection followed by chemotherapy versus chemotherapy alone. Blood 2011, 117, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, S.; Ishikawa, E.; Nakamura, M.; Shimada, K.; Yamamura, T.; Furukawa, K.; Tanaka, T.; Mabuchi, S.; Tsuyuki, Y.; Kohno, K.; et al. Reappraisal of Primary Epstein-Barr Virus (EBV)-positive Diffuse Large B-Cell Lymphoma of the Gastrointestinal Tract Comparative Analysis Among Immunosuppressed and Nonimmunosuppressed Stage I and II–IV Patients. Am. J. Surg. Pathol. 2020, 44, 1173–1183. [Google Scholar] [CrossRef]
- Montes-Moreno, S.; Odqvist, L.; Diaz-Perez, J.A.; Lopez, A.B.; de Villambrosia, S.G.; Mazorra, F.; Castillo, M.E.; Lopez, M.; Pajares, R.; Garcia, J.F.; et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Mod. Pathol. 2012, 25, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Li, L.; Xu-Monette, Z.Y.; Visco, C.; Tzankov, A.; Manyam, G.C.; Montes-Moreno, S.; Dybaer, K.; Chiu, A.; Orazi, A.; et al. Prevalence and Clinical Implications of Epstein-Barr Virus Infection in De Novo Diffuse Large B-Cell Lymphoma in Western Countries. Clin. Cancer Res. 2014, 20, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sakakibara, A.; Shimada, K.; Shimada, S.; Ishikawa, E.; Nakamura, S.; Kato, S.; Takahara, T.; Asano, N.; Satou, A.; et al. Immune evasion-related extranodal large B-cell lymphoma: A report of six patients with neoplastic PD-L1-positive extranodal diffuse large B-cell lymphoma. Pathol. Int. 2019, 69, 13–20. [Google Scholar] [CrossRef]
- Tsuyuki, Y.; Ishikawa, E.; Kohno, K.; Shimada, K.; Ohka, F.; Suzuki, Y.; Mabuchi, S.; Satou, A.; Takahara, T.; Kato, S.; et al. Expression of programmed cell death ligand-1 by immune cells in the microenvironment is a favorable prognostic factor for primary diffuse large B-cell lymphoma of the central nervous system. Neuropathology 2021, 41, 99–108. [Google Scholar] [CrossRef] [PubMed]

| Age/Sex | Site | Endoscopic Finding | No. Lesions | Clinical Setting | Source of IS | Treatment | Outcome (Length of CR) | Length of FU (mo) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 57 F | Rectum | Nonspecific erythema without ulcers | NA | IBD | (Only mesalazine) | FU | SD | 6 | [30] |
| 26 M | Rectum | Large and deep ulcer | Single | IBD (CD, 11y) | AZA+IFX | RIS+Sur | CR | 12 | [28] |
| 63 M | Anus | Large superficial perianal wound | Single | IBD (CD, 30y) | AZA | RIS | CR (at 6 wks) | NA | [26] |
| 53 F | Colon, rectum | Multiple ulceration | Multiple | IBD (CD, 6y) | MTX+IFX | RIS | SD, progression to HL | 18 | [27] |
| 34 M | Colon, rectum | Small and large ulcers | Multiple | IBD (UC) | 6-MP | RIS+R | CR | 12 | [29] |
| 78 M | Rectum | Anorectal ulcer | Single | IBD (UC) | CYA | RIS | CR | 23 | [25] |
| 61 M | Esophagus | Well-circumscribed mucosal ulcer | NA | Renal transplantation | PSL+MMF | RIS | CR (at 4 wks) | 16.5 | [57] |
| 29 M | Colon | Ulcerative necrotic lesion | Single | Renal transplantation | PSL+MMF+CYA | RIS+R | CR (at 5 mos) | 11 | [68] |
| 27 M | Colon, rectum | Superficial lesions | Multiple | Renal transplantation | PSL+MMF+CYA | RIS+R | PD→CR * | 13 | [68] |
| 70 M | Rectum | Well-circumscribed mucosal ulcer | NA | Renal transplantation | PSL+MMF | RIS+R+Velcade | CR (at 12 wks), but DOC | 17 | [57] |
| 32 M | Terminal ileum | Well-circumscribed mucosal ulcer | NA | Lung transplantation | PSL+MMF+Tac | RIS+R | CR (at 4 wks), but DOC | 60 | [57] |
| 69 M | Colon | Well-defined punched-out ulcers | Multiple | Melanoma (Ipi), irColitis | PSL+IFX | Sur (perforation) | CR | >60 | [31] |
| 66 M | Colon | Well-defined punched-out ulcers | Multiple | Melanoma (Ipi+Nivo), irColitis | PSL | Sur (perforation) | CR | 25 | [31] |
| 70 M | Small bowel, colon, rectum | Well-defined punched-out ulcers | Multiple | Melanoma (Ipi), irColitis | PSL | Sur (perforation) | CR | >50 | [31] |
| 77 M | Colon, rectum | Well-defined punched-out ulcers | Multiple | Melanoma (Ipi), irColitis | PSL | Sur (perforation) | PD (died of perforation) | <1 | [31] |
| 75 F | Esophagus | Esophageal ulcer | Single | RA | AZA | RIS | CR | 17 | [25] |
| 81 F | Jejunum | Ulcerative lesion | Single | RA | PSL+MTX | Sur (perforation) | CR | 24 | [67] |
| 69 F | Colon | Colonic mass | Single | RA | MTX | NA | NA | NA | [25] |
| 51 F | Stomach | Small shallow ulcer | Multiple | ATLL | mLSG15 | FU | CR (at 4 wks), but DOC | 4 | [58] |
| 35 F | Ileum, colon, rectum | NA | Multiple | ATLL | mLSG15, CHASE, M, HSCT, Tac | RIS | CR (at 12 wks) | 19 | [58] |
| 38 M | Colon, rectum | Multiple ulcers and elevated lesions | Multiple | ED, cHL | ABVD | FU | NA | NA | [66] |
| 81 F | Colon | NA | NA | AITP | PSL+AZA | Sur (perforation) | CR, but DOC | 1 | [65] |
| 70 M | Rectum | Tumoral lesion | NA | HIV | HIV | FU | CR | 9 | [30] |
| 64 F | Colon | Small shallow ulcer | Single | HSCT(ET, sMDS) | CYA | RIS | CR | 6 | [25] |
| 61 F | Esophagus | Esophageal ulcer | Multiple | Hypogammaglobulinemia | PI | R+IVIG+B | PD | <6 | [69] |
| 83 F | Colon | Sharply circumscribed mucosal ulcer | Single | RP | PSL+MTX | Sur (diverticulitis) | CR | 4 | [71] |
| 84 F | Colon | NA | Multiple | Age | Sur (diverticulitis) | CR | NA | [64] | |
| 84 F | Esophagus | NA | Single | Age | FU | CR | 6 | [70] | |
| 64 F | Ileocecum | Partially necrotic ulcer | Single | Age | Sur | CR, but DOC | 6 | [63] | |
| 81 M | Colon | Tumor with circumferential ulcer | NA | Age | Sur (obstruction) | CR | 20 | [62] |
| Male | 16 | (55%) | Source of IS | ||
| Median age, years (range) | 65 | (26–84) | AZA or 6-MP | 3 | (10%) |
| Site | AZA+IFX | 1 | (3%) | ||
| Esophagus | 4 | (13%) | CYA | 2 | (7%) |
| Stomach | 1 | (3%) | MTX | 2 | (7%) |
| Small intestine | 2 | (7%) | MTX+IFX | 1 | (3%) |
| Ileocecum | 1 | (3%) | PSL | 3 | (10%) |
| Colon | 9 | (30%) | PSL+AZA, IFX, MMF, or MTX | 5 | (17%) |
| Rectum | 5 | (17%) | PSL+MMF+CYA or Tac | 3 | (10%) |
| Colon, rectum | 6 | (20%) | CTx | 3 | (10%) |
| Small intestine, colon, rectum | 1 | (3%) | Age | 4 | (13%) |
| Anus | 1 | (3%) | Others | 2 | (7%) |
| The number of lesions | None | 1 | (3%) | ||
| Single lesion | 11 | (55%) | Treatment | ||
| Multiple lesions | 9 | (45%) | RIS | 8 | (27%) |
| NA | 10 | RIS+R | 4 | (13%) | |
| Clinical setting | RIS+CTx | 2 | (7%) | ||
| IBD | 6 | (20%) | Surgery | 10 | (33%) |
| Organ transplant | 5 | (17%) | Follow up | 5 | (17%) |
| irColitis | 4 | (13%) | NA | 1 | (3%) |
| RA | 3 | (10%) | Outcome | ||
| Treated-lymphoma | 3 | (10%) | CR | 22 | (73%) |
| Old age | 4 | (13%) | SD | 2 | (7%) |
| Others | 5 | (17%) | PD | 4 | (13%) |
| NA | 2 | (7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, E.; Satou, A.; Nakamura, M.; Nakamura, S.; Fujishiro, M. Epstein-Barr Virus Positive B-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract. Cancers 2021, 13, 3815. https://doi.org/10.3390/cancers13153815
Ishikawa E, Satou A, Nakamura M, Nakamura S, Fujishiro M. Epstein-Barr Virus Positive B-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract. Cancers. 2021; 13(15):3815. https://doi.org/10.3390/cancers13153815
Chicago/Turabian StyleIshikawa, Eri, Akira Satou, Masanao Nakamura, Shigeo Nakamura, and Mitsuhiro Fujishiro. 2021. "Epstein-Barr Virus Positive B-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract" Cancers 13, no. 15: 3815. https://doi.org/10.3390/cancers13153815
APA StyleIshikawa, E., Satou, A., Nakamura, M., Nakamura, S., & Fujishiro, M. (2021). Epstein-Barr Virus Positive B-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract. Cancers, 13(15), 3815. https://doi.org/10.3390/cancers13153815






