Streamlined Schemes for Dosimetry of 177Lu-Labeled PSMA Targeting Radioligands in Therapy of Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
2.3. Dosimetric Data Analysis
2.3.1. Method 1 (M1)
2.3.2. Method 2 (M2)
2.3.3. Method 3 (M3)
2.4. Statistical Analysis
3. Results
3.1. Absorbed Doses Calculated Using Method 1
3.2. Absorbed Doses Calculated Using Method 2
3.3. Absorbed Doses Calculated Using Method 3
3.4. Comparison of Total Absorbed Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Calopedos, R.J.S.; Chalasani, V.; Asher, R.; Emmett, L.; Woo, H.H. Lutetium-177-Labelled Anti-Prostate-Specific Membrane Antigen Antibody and Ligands for the Treatment of Metastatic Castrate-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.I. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-Analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy With (177)Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bogemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior Therapies as Prognostic Factors of Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Treated with [(177)Lu]Lu-PSMA-617. A WARMTH Multicenter Study (the 617 Trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- European Council Directive 2013/59/Euratom on Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. OJ L 13, 17.1. 2014, 1–73.
- Yonekura, Y.; Mattsson, S.; Flux, G.; Bolch, W.E.; Dauer, L.T.; Fisher, D.R.; Lassmann, M.; Palm, S.; Hosono, M.; Doruff, M.; et al. ICRP Publication 140: Radiological Protection in Therapy with Radiopharmaceuticals. Ann. ICRP 2019, 48, 5–95. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bogemann, M.; Rahbar, K. Radioligand Therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: A Pre-VISION Single-Center Analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef] [Green Version]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of (177)Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Barna, S.; Haug, A.R.; Hartenbach, M.; Rasul, S.; Grubmuller, B.; Kramer, G.; Blaickner, M. Dose Calculations and Dose-Effect Relationships in 177Lu-PSMA I&T Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Clin. Nucl. Med. 2020, 45, 661–667. [Google Scholar] [CrossRef]
- Volter, F.; Mittlmeier, L.; Gosewisch, A.; Brosch-Lenz, J.; Gildehaus, F.J.; Zacherl, M.J.; Beyer, L.; Stief, C.G.; Holzgreve, A.; Rubenthaler, J.; et al. Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after (177)Lu-PSMA-617 Radioligand Therapy. Diagnostics 2021, 11, 428. [Google Scholar] [CrossRef]
- Kletting, P.; Thieme, A.; Eberhardt, N.; Rinscheid, A.; D’Alessandria, C.; Allmann, J.; Wester, H.J.; Tauber, R.; Beer, A.J.; Glatting, G.; et al. Modeling and Predicting Tumor Response in Radioligand Therapy. J. Nucl. Med. 2019, 60, 65–70. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM Procedure Guidelines for Radionuclide Therapy with (177)Lu-Labelled PSMA-Ligands ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Bolch, W.E.; Eckerman, K.F.; Sgouros, G.; Thomas, S.R. MIRD Pamphlet No. 21: A Generalized Schema for Radiopharmaceutical Dosimetry--Standardization of Nomenclature. J. Nucl. Med. 2009, 50, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, J.A.; Thomas, S.R.; Stubbs, J.B.; Stabin, M.G.; Hays, M.T.; Koral, K.F.; Robertson, J.S.; Howell, R.W.; Wessels, B.W.; Fisher, D.R.; et al. MIRD Pamphlet No. 16: Techniques for Quantitative Radiopharmaceutical Biodistribution Data Acquisition and Analysis for Use in Human Radiation Dose Estimates. J. Nucl. Med. 1999, 40, 37S–61S. [Google Scholar]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The Second-Generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [PubMed]
- Kletting, P.; Schimmel, S.; Hanscheid, H.; Luster, M.; Fernandez, M.; Nosske, D.; Lassmann, M.; Glatting, G. The NUKDOS Software for Treatment Planning in Molecular Radiotherapy. Z. Med. Phys. 2015, 25, 264–274. [Google Scholar] [CrossRef]
- Santoro, L.; Pitalot, L.; Trauchessec, D.; Mora-Ramirez, E.; Kotzki, P.O.; Bardies, M.; Deshayes, E. Clinical Implementation of PLANET(R) Dose for Dosimetric Assessment after [(177)Lu]Lu-DOTA-TATE: Comparison with Dosimetry Toolkit(R) and OLINDA/EXM(R) V1.0. EJNMMI Res. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Bardies, M.; Zaidi, H. Voxel-Based Dosimetry is Superior to Mean Absorbed Dose Approach for Establishing Dose-Effect Relationship in Targeted Radionuclide Therapy. Med. Phys. 2019, 46, 5403–5406. [Google Scholar] [CrossRef] [Green Version]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lutzen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 Therapy, Dosimetry and Follow-up in Patients with Metastatic Castration-Resistant Prostate Cancer. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Boning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary Experience with Dosimetry, Response and Patient Reported Outcome after 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [Green Version]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- ECIS—European Cancer Information System. European Cancer Observatory (ECO). Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 7 May 2021).
- Zippel, C.; Giesel, F.L.; Kratochwil, C.; Eiber, M.; Rahbar, K.; Albers, P.; Maurer, T.; Krause, B.J.; Bohnet-Joschko, S. PSMA Radioligand Therapy could Pose Infrastructural Challenges for Nuclear Medicine: Results of a Basic Calculation for the Capacity Planning of Nuclear Medicine Beds in the German Hospital Sector. Nuklearmedizin 2021, 60, 216–223. [Google Scholar] [CrossRef]
- Garske, U.; Sandstrom, M.; Johansson, S.; Sundin, A.; Granberg, D.; Eriksson, B.; Lundqvist, H. Minor Changes in Effective Half-Life during Fractionated 177Lu-Octreotate Therapy. Acta Oncol. 2012, 51, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Hanscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Dose Mapping After Endoradiotherapy with (177)Lu-DOTATATE/DOTATOC by a Single Measurement After 4 Days. J. Nucl. Med. 2018, 59, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundlov, A.; Gustafsson, J.; Brolin, G.; Mortensen, N.; Hermann, R.; Bernhardt, P.; Svensson, J.; Ljungberg, M.; Tennvall, J.; Sjogreen Gleisner, K. Feasibility of Simplifying Renal Dosimetry in (177)Lu Peptide Receptor Radionuclide Therapy. EJNMMI Phys. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Willowson, K.P.; Eslick, E.; Ryu, H.; Poon, A.; Bernard, E.J.; Bailey, D.L. Feasibility and Accuracy of Single Time Point Imaging for Renal Dosimetry Following (177)Lu-DOTATATE (‘Lutate’) Therapy. EJNMMI Phys. 2018, 5, 33. [Google Scholar] [CrossRef]
- Madsen, M.T.; Menda, Y.; O’Dorisio, T.M.; O’Dorisio, M.S. Technical Note: Single Time Point Dose Estimate for Exponential Clearance. Med. Phys. 2018, 45, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, M.; Arsenault, F.; Saighi, N.; Zhao, W.; Buteau, F.A.; Celler, A.; Beauregard, J.M. Accuracy and Reproducibility of Simplified QSPECT Dosimetry for Personalized (177)Lu-Octreotate PRRT. EJNMMI Phys. 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Hofman, M.S.; Hicks, R.J.; Scalzo, M.; Violet, J. Radiation Dosimetry in (177)Lu-PSMA-617 Therapy Using a Single Posttreatment SPECT/CT Scan: A Novel Methodology to Generate Time-and Tissue-Specific Dose Factors. J. Nucl. Med. 2020, 61, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Hanscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Absorbed Dose Estimates from a Single Measurement One to Three Days after the Administration of 177Lu-DOTATATE/-TOC. Nuklearmedizin 2017, 56, 219–224. [Google Scholar] [CrossRef]
- Rinscheid, A.; Kletting, P.; Eiber, M.; Beer, A.J.; Glatting, G. Influence of Sampling Schedules on [(177)Lu]Lu-PSMA Dosimetry. EJNMMI Phys. 2020, 7, 41. [Google Scholar] [CrossRef]
- Taieb, D.; Foletti, J.M.; Bardies, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter? J. Nucl. Med. 2018, 59, 747–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The Salivary Glands as a Dose Limiting Organ of PSMA-Targeted Radionuclide Therapy: A Review of the Lessons Learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef]
- Hohberg, M.; Eschner, W.; Schmidt, M.; Dietlein, M.; Kobe, C.; Fischer, T.; Drzezga, A.; Wild, M. Lacrimal Glands May Represent Organs at Risk for Radionuclide Therapy of Prostate Cancer with [(177)Lu]DKFZ-PSMA-617. Mol. Imaging Biol. 2016, 18, 437–445. [Google Scholar] [CrossRef]
- Prive, B.M.; Peters, S.M.B.; Muselaers, C.H.J.; van Oort, I.M.; Janssen, M.J.R.; Sedelaar, J.P.M.; Konijnenberg, M.W.; Zamecnik, P.; Uijen, M.J.M.; Schilham, M.G.M.; et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin. Cancer Res. 2021, 27, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Rahbar, K.; Kurpig, S.; Bogemann, M.; Claesener, M.; Eppard, E.; Gartner, F.; Rogenhofer, S.; Schafers, M.; Essler, M. Early Side Effects and First Results of Radioligand Therapy with (177)Lu-DKFZ-617 PSMA of Castrate-Resistant Metastatic Prostate Cancer: A Two-Centre Study. EJNMMI Res. 2015, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, M.; Celler, A.; Konijnenberg, M.W.; Eckerman, K.F.; Dewaraja, Y.K.; Sjogreen-Gleisner, K.; Committee, S.M.; Bolch, W.E.; Brill, A.B.; Fahey, F.; et al. MIRD Pamphlet No. 26: Joint EANM/MIRD Guidelines for Quantitative 177Lu SPECT Applied for Dosimetry of Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Dewaraja, Y.K.; Frey, E.C.; Sgouros, G.; Brill, A.B.; Roberson, P.; Zanzonico, P.B.; Ljungberg, M. MIRD Pamphlet No. 23: Quantitative SPECT for Patient-Specific 3-Dimensional Dosimetry in Internal Radionuclide Therapy. J. Nucl. Med. 2012, 53, 1310–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.L.; Hennessy, T.M.; Willowson, K.P.; Henry, E.C.; Chan, D.L.; Aslani, A.; Roach, P.J. In Vivo Quantification of (177)Lu with Planar Whole-Body and SPECT/CT Gamma Camera Imaging. EJNMMI Phys. 2015, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, M.; Gleisner, K.S. Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy. Diagnostics 2015, 5, 296–317. [Google Scholar] [CrossRef] [Green Version]
- Stabin, M.G. Fundamentals of Nuclear Medicine Dosimetry; Springer: New York, NY, USA, 2008. [Google Scholar]
- Divoli, A.; Chiavassa, S.; Ferrer, L.; Barbet, J.; Flux, G.D.; Bardies, M. Effect of Patient Morphology on Dosimetric Calculations for Internal Irradiation as Assessed by Comparisons of Monte Carlo Versus Conventional Methodologies. J. Nucl. Med. 2009, 50, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Jousse-Joulin, S. Salivary Glands. In Essential Applications of Musculoskeletal Ultrasound in Rheumatology; Wakefield, R.J., D’Agostino, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Stabin, M.G.; Xu, X.G.; Emmons, M.A.; Segars, W.P.; Shi, C.; Fernald, M.J. RADAR Reference Adult, Pediatric, and Pregnant Female Phantom Series for Internal and External Dosimetry. J. Nucl. Med. 2012, 53, 1807–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabin, M.G.; Konijnenberg, M.W. Re-Evaluation of Absorbed Fractions for Photons and Electrons in Spheres of Various Sizes. J. Nucl. Med. 2000, 41, 149–160. [Google Scholar]
- Stabin, M.G.; Madsen, M.T.; Zaidi, H. Personalized Dosimetry is a Must for Appropriate Molecular Radiotherapy. Med. Phys. 2019, 46, 4713–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergsma, H.; Konijnenberg, M.W.; van der Zwan, W.A.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; Mauff, K.A.; Krenning, E.P.; Kwekkeboom, D.J. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1802–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deasy, J.O.; Moiseenko, V.; Marks, L.; Chao, K.S.; Nam, J.; Eisbruch, A. Radiotherapy Dose-Volume Effects on Salivary Gland Function. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S58–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundmann, O.; Mitchell, G.C.; Limesand, K.H. Sensitivity of Salivary Glands to Radiation: From Animal Models to Therapies. J. Dent. Res. 2009, 88, 894–903. [Google Scholar] [CrossRef]
- Emami, B. Tolerance of Normal Tissue to Therapeutic Radiation. Rep. Radiother Oncol. 2013, 1, 123–127. [Google Scholar]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: A New Radiopharmaceutical for the Treatment of Metastatic Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef]
- Sandstrom, M.; Garske-Roman, U.; Granberg, D.; Johansson, S.; Widstrom, C.; Eriksson, B.; Sundin, A.; Lundqvist, H.; Lubberink, M. Individualized Dosimetry of Kidney and Bone Marrow in Patients Undergoing 177Lu-DOTA-Octreotate Treatment. J. Nucl. Med. 2013, 54, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Filss, C.; Heinzel, A.; Miiller, B.; Vogg, A.T.J.; Langen, K.J.; Mottaghy, F.M. Relevant Tumor Sink Effect in Prostate Cancer Patients Receiving 177Lu-PSMA-617 Radioligand Therapy. Nuklearmedizin 2018, 57, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Wang, H.; Robertson, A.; Armstrong, W.R.; Zaum, R.; Weber, M.; Yagubbayli, F.; Kratochwil, C.; Grogan, T.R.; Nguyen, K.; et al. Tumor Sink Effect in (68)Ga-PSMA-11 PET: Myth or Reality? J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, M.; Telli, T.; Tuncali, M.C.; Karabulut, E. Predictive Factors of Tumor Sink Effect: Insights from (177)Lu-Prostate-Specific Membrane Antigen Therapy. Ann. Nucl. Med. 2021, 35, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, J.M.; Hofman, M.S.; Kong, G.; Hicks, R.J. The Tumour Sink Effect on the Biodistribution of 68Ga-DOTA-Octreotate: Implications for Peptide Receptor Radionuclide Therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The (68)Ga/(177)Lu Theragnostic Concept in PSMA Targeting of Castration-Resistant Prostate Cancer: Correlation of SUVmax Values and Absorbed Dose Estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef]
- Kabasakal, L.; Toklu, T.; Yeyin, N.; Demirci, E.; Abuqbeitah, M.; Ocak, M.; Aygun, A.; Karayel, E.; Pehlivanoglu, H.; Alan Selcuk, N. Lu-177-PSMA-617 Prostate-Specific Membrane Antigen Inhibitor Therapy in Patients with Castration-Resistant Prostate Cancer: Stability, Bio-distribution and Dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Gosewisch, A.; Delker, A.; Tattenberg, S.; Ilhan, H.; Todica, A.; Brosch, J.; Vomacka, L.; Brunegraf, A.; Gildehaus, F.J.; Ziegler, S.; et al. Patient-Specific Image-Based Bone Marrow Dosimetry in Lu-177-[DOTA(0),Tyr(3)]-Octreotate and Lu-177-DKFZ-PSMA-617 Therapy: Investigation of a New Hybrid Image Approach. EJNMMI Res. 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Gosewisch, A.; Ilhan, H.; Tattenberg, S.; Mairani, A.; Parodi, K.; Brosch, J.; Kaiser, L.; Gildehaus, F.J.; Todica, A.; Ziegler, S.; et al. 3D Monte Carlo Bone Marrow Dosimetry for Lu-177-PSMA Therapy with Guidance of Non-Invasive 3D Localization of Active Bone Marrow via Tc-99m-Anti-Granulocyte Antibody SPECT/CT. EJNMMI Res. 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | Value | ||
|---|---|---|---|
| Age (years) | Mean ± SD | 71.7 ± 7.9 | |
| Median Range (min–max) | 72 53–85 | ||
| Previous systemic treatments | Chemotherapy NAAD Chemotherapy and NAAD | 3 6 36 | 6.5% 13.0% 78.3% |
| Other Additional Radium-223-dichlorid | 3 3 | 6.5% 6.5% | |
| Initial PSA (ng/mL) | Mean ± SD | 127.1 ± 33.4 | |
| Median Range | 74 0.5–387 | ||
| Primary Gleason Score | Mean ± SD Median Range | 7.4 ± 1.4 8 3–9 | |
| ECOG | 0 | 20 | 43.5% |
| 1 | 24 | 52.2% | |
| 2 | 2 | 4.3% | |
| eGFR (mL/min/1.73 m2) (pretherapeutic) | Mean ± SD | 82.0 ± 14.2 | |
| Median Range (min–max) | 83.0 43.4–109.4 | ||
| System | Siemens Symbia T6 | Picker/Philips IRIX | GE Discovery 870 CZT |
| Dual head SPECT/CT | Triple-head gamma camera | Dual head SPECT/CT | |
| Collimator | MELP | MEGP | WEHR45 |
| Main Energy Peak | 208 keV ± 7.5% | 208 keV ± 10% | 113 keV ± 7.5% |
| Whole Body Imaging | matrix 1024 × 256 scan speed: 15 cm/min | matrix 1024 × 256 scan speed: 10 cm/min | matrix 1024 × 256 scan speed: 15 cm/min |
| SPECT | # of proj: 120 matrix: 128 × 128 acq dur: 15 s | # of proj: 64 matrix: 64 × 64 acq dur: 15 s | # of proj: 120 matrix: 128 × 128 acq dur: 15 s |
| CT | 120 kV, 50 mAs (DOM), 3 mm slices | 120 kV, 40 mAs (DOM, ASiR reconstruction), 3 mm slices | |
| SPECT-Reconstruction | Hermes Hybrid Recon 3.0 (3D OSEM reconstruction, AC, RR, SC) | ||
| 5it 15ss no post filter | 6it 8ss Post-filter: Butterworth (0.9 cm FWHM) | 5it 15ss no post filter | |
| SPECT Calibration Factor | 9.8 cps/MBq | 6.2 cps/MBq | 14.3 cps/MBq |
| Kidneys | Parotid Glands | |||||
|---|---|---|---|---|---|---|
| Cycle | Effective Half-Life [h] | Absorbed Dose [Gy] | Dose Coefficient [Gy/GBq] | Effective Half-Life [h] | Absorbed Dose [Gy] | Dose Coefficient [Gy/GBq] |
| 1 | 38.2 ± 14.7 (16.1 to 72.6) | 2.9 ± 1.3 (1.8 to 8.6) | 0.49 ± 0.22 (0.30 to 1.44) | 34.2 ± 13.3 (13.8 to 58.4) | 4.8 ± 2.2 (1.2 to 9.0) | 0.79 ± 0.37 (0.19 to 1.50) |
| 2 | 39.1 ± 15.3 (13.2 to 83.2) | 3.3 ± 1.4 (1.6 to 9.0) | 0.55 ± 0.23 (0.28 to 1.51) | 35.8 ± 14.0 (14.5 to 60.0) | 4.6 ± 2.1 (0.8 to 9.9) | 0.77 ± 0.35 (0.18 to 1.59) |
| 3 | 38.7 ± 14.9 (16.9 to 75.8) | 3.5 ± 1.3 (1.8 to 7.2) | 0.59 ± 0.21 (0.30 to 1.21) | 34.0 ± 13.8 (12.6 to 67.3) | 5.3 ± 2.5 (1.2 to 11.0) | 0.86 ± 0.40 (0.22 to 1.69) |
| 4 | 40.0 ± 13.7 (15.2 to 62.8) | 3.4 ± 0.8 (2.1 to 4.9) | 0.57 ± 0.13 (0.36 to 0.83) | 30.5 ± 10.9 (14.9 to 54.0) | 4.8 ± 2.1 (1.3 to 8.4) | 0.81 ± 0.33 (0.23 to 1.29) |
| 5 | 41.5 ± 12.7 (22.4 to 61.5) | 3.5 ± 0.9 (1.5 to 4.8) | 0.59 ± 0.16 (0.26 to 0.81) | 33.8 ± 12.9 (17.3 to 62.1) | 4.3 ± 2.2 (1.2 to 8.7) | 0.72 ± 0.36 (0.22 to 1.37) |
| 6 | 40.9 ± 14.4 (17.5 to 63.4) | 3.7 ± 0.8 (2.5 to 5.2) | 0.62 ± 0.14 (0.42 to 0.86) | 33.9 ± 12.2 (15.1 to 54.5) | 4.8 ± 2.2 (1.2 to 9.3) | 0.81 ± 0.41 (0.21 to 1.73) |
| Kidneys | Parotid Glands | |||||
|---|---|---|---|---|---|---|
| Cycle | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
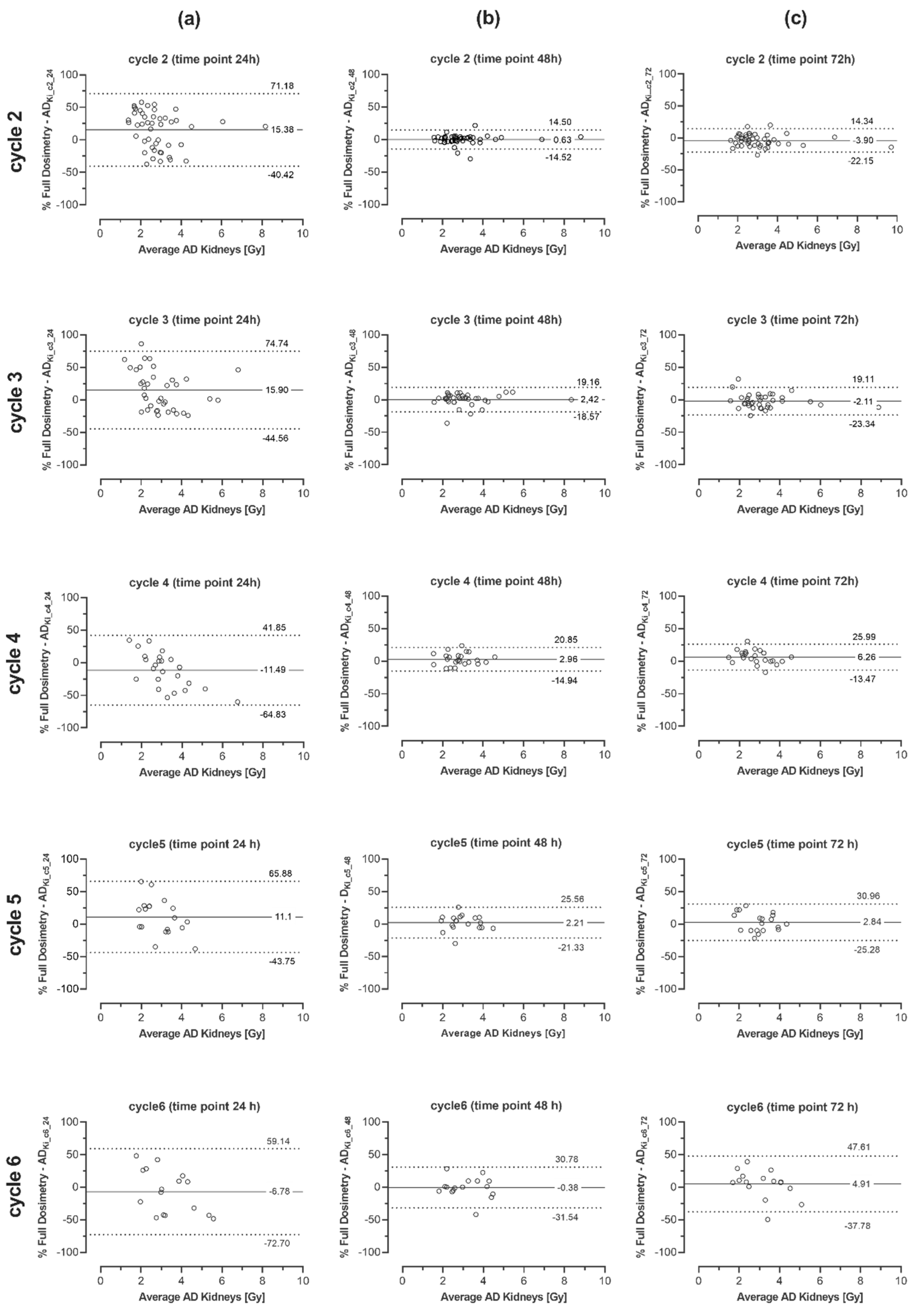
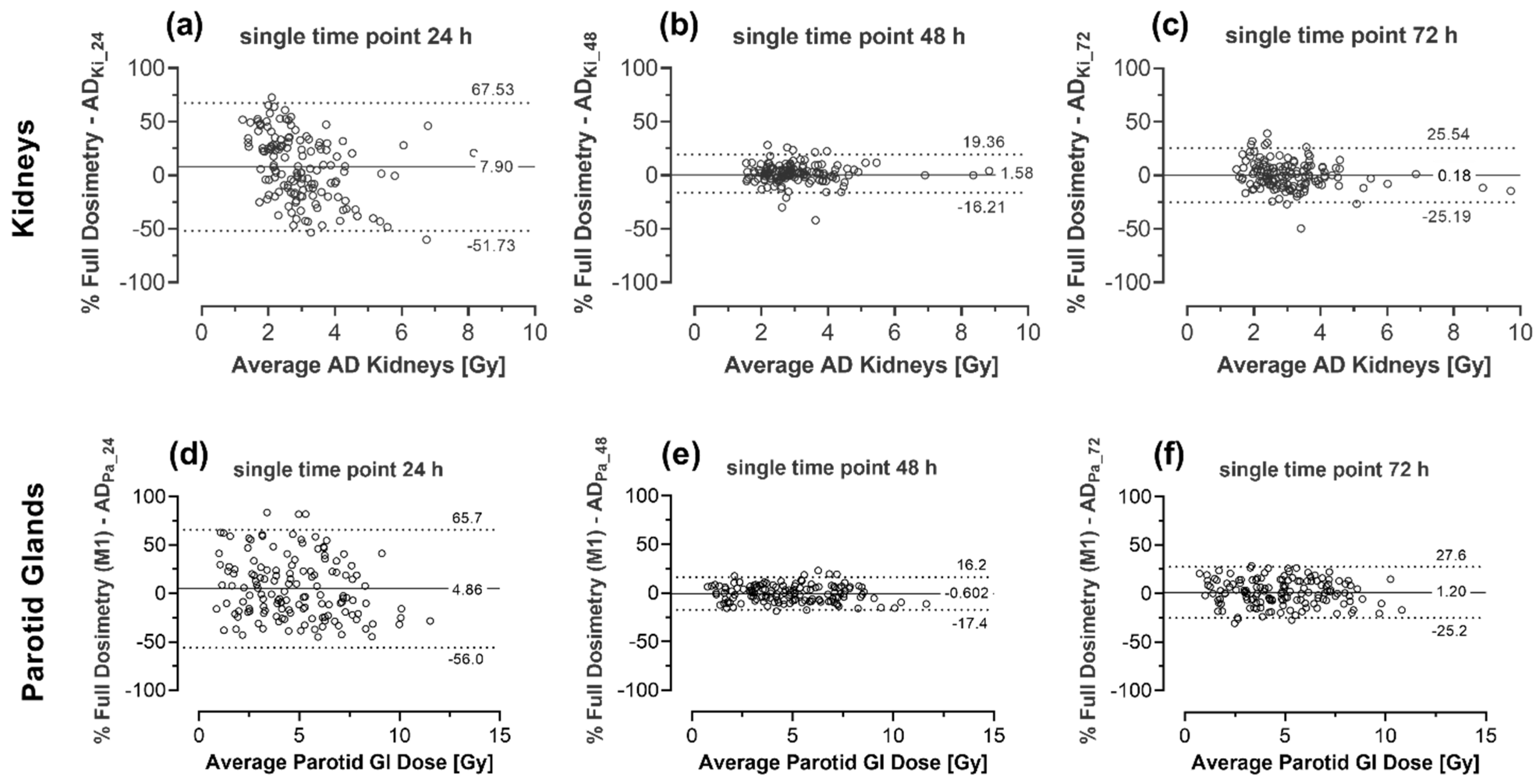
| 2 | 15.4 ± 28.5 (−40.4 to 71.2) | 0.63 ± 5.90 (−10.9 to 12.2) | −3.90 ± 9.31 (−22.1 to 14.3) | −7.13 ± 22.6 (−51.5 to 37.3) | 1.08 ± 7.70 (−14.0 to 16.2) | 3.67 ± 14.84 (−25.4 to 32.8) |
| 3 | 15.1 ± 30.4 (−44.6 to 74.7) | 2.42 ± 6.61 (−10.5 to 15.4) | −2.11 ± 10.8 (−23.3 to 19.1) | 13.0 ± 26.2 (−38.4 to 64.4) | −1.69 ± 7.57 (−16.5 to 13.2) | 4.16 ± 8.58 (−12.7 to 21.0) |
| 4 | −11.5 ± 27.2 (−64.8 to 41.8) | 2.96 ± 9.13 −14.9 to 20.9) | 6.26 ± 10.1 (−13.5 to 26.0) | −6.87 ± 30.4 (−66.4 to 52.6) | −2.57 ± 6.72 (−15.7 to 10.6) | 4.92 ± 11.4 (−17.5 to 27.3) |
| 5 | 11.1 ± 28.0 (−43.8 to 65.9) | 2.12 ± 12.0 (−21.3 to 25.6) | 2.84 ± 14.3 (−25.3 to 31.0) | 19.1 ± 34.3 (−48.2 to 86.4) | −2.79 ± 8.05 (−18.6 to 13.0) | −7.40 ± 12.7 (−32.4 to 17.6) |
| 6 | −6.78 ± 33.6 (−72.7 to 59.1) | −0.38 ± 15.9 (−31.5 to 30.8) | 4.91 ± 21.8 (−37.8 to 47.6) | 12.7 ± 27.6 (−41.4 to 66.7) | 3.22 ± 13.4 (−23.1 to 29.5) | −8.11 ± 14.8 (−37.1 to 20.9) |
| Kidneys | Parotid Glands | |||
|---|---|---|---|---|
| Cycle | Mean AD [Gy] | Mean Difference to M1 [%] | Mean AD [Gy] | Mean Difference to M1 [%] |
| 1 | 2.98 ± 1.32 | 0.00 ± 0.00 | 4.77 ± 2.21 | 0.00 ± 0.00 |
| 2 | 2.91 ± 1.25 | −10.30 ± 18.50 | 4.71 ± 2.20 | 2.30 ± 11.79 |
| 3 | 2.90 ± 1.09 | −16.03 ± 16.90 | 5.00 ± 2.31 | −3.81 ± 16.31 |
| 4 | 2.94 ± 0.78 | −12.43 ± 21.28 | 4.67 ± 2.06 | −2.49 ± 17.18 |
| 5 | 2.81 ± 0.48 | −15.29 ± 22.90 | 4.50 ± 1.95 | 8.80 ± 18.35 |
| 6 | 3.00 ± 0.51 | −17.89 ± 16.10 | 4.73 ± 2.00 | 0.13 ± 13.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurth, J.; Heuschkel, M.; Tonn, A.; Schildt, A.; Hakenberg, O.W.; Krause, B.J.; Schwarzenböck, S.M. Streamlined Schemes for Dosimetry of 177Lu-Labeled PSMA Targeting Radioligands in Therapy of Prostate Cancer. Cancers 2021, 13, 3884. https://doi.org/10.3390/cancers13153884
Kurth J, Heuschkel M, Tonn A, Schildt A, Hakenberg OW, Krause BJ, Schwarzenböck SM. Streamlined Schemes for Dosimetry of 177Lu-Labeled PSMA Targeting Radioligands in Therapy of Prostate Cancer. Cancers. 2021; 13(15):3884. https://doi.org/10.3390/cancers13153884
Chicago/Turabian StyleKurth, Jens, Martin Heuschkel, Alexander Tonn, Anna Schildt, Oliver W. Hakenberg, Bernd J. Krause, and Sarah M. Schwarzenböck. 2021. "Streamlined Schemes for Dosimetry of 177Lu-Labeled PSMA Targeting Radioligands in Therapy of Prostate Cancer" Cancers 13, no. 15: 3884. https://doi.org/10.3390/cancers13153884
APA StyleKurth, J., Heuschkel, M., Tonn, A., Schildt, A., Hakenberg, O. W., Krause, B. J., & Schwarzenböck, S. M. (2021). Streamlined Schemes for Dosimetry of 177Lu-Labeled PSMA Targeting Radioligands in Therapy of Prostate Cancer. Cancers, 13(15), 3884. https://doi.org/10.3390/cancers13153884






