The Rarest of Rare Thymic Lesions: A 10-Year Surgical Pathology Experience
Abstract
Simple Summary
Abstract
1. Introduction
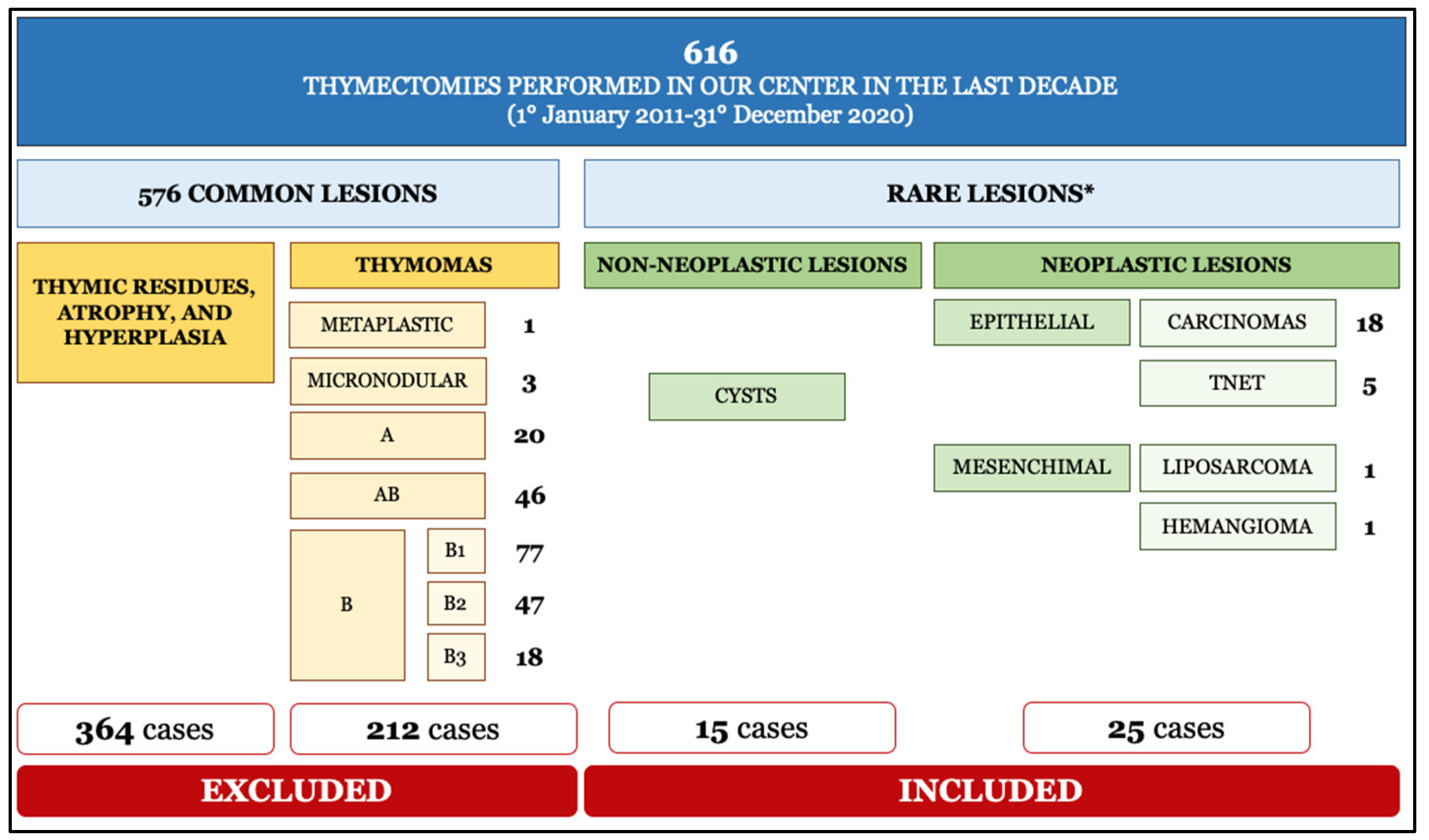
2. Materials and Methods
3. Results
3.1. Thymic Cysts
3.2. Thymic Carcinoma
3.3. Thymic Neuroendocrine Tumors
3.4. Thymic Soft Tissue Neoplasms
3.4.1. Cavernous Hemangioma of the Thymus
3.4.2. Dedifferentiated Thymoliposarcoma
4. Discussion
4.1. Thymic Cysts
4.2. Thymic Carcinoma
4.3. Thymic Neuroendocrine Tumors
4.4. Soft Tissue Thymic Tumors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thapa, P.; Farber, D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2021; Volume 5. [Google Scholar]
- Roden, A.C.; Fang, W.; Shen, Y.; Carter, B.W.; White, D.B.; Jenkins, S.M.; Spears, G.M.; Molina, J.R.; Klang, E.; Segni, M.D.; et al. Distribution of mediastinal lesions across multi-institutional, international, radiology databases. J. Thorac. Oncol. 2020, 15, 568–579. [Google Scholar] [CrossRef]
- de Jong, W.K.; Blaauwgeers, J.L.; Schaapveld, M.; Timens, W.; Klinkenberg, T.J.; Groen, H.J. Thymic epithelial tumours: A population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer 2008, 44, 123–130. [Google Scholar] [CrossRef]
- Weis, C.A.; Yao, X.; Deng, Y.; Detterbeck, F.C.; Marino, M.; Nicholson, A.G.; Huang, J.; Ströbel, P.; Antonicelli, A.; Marx, A.; et al. The impact of thymoma histotype on prognosis in a worldwide database. J. Thorac. Oncol. 2015, 10, 367–372. [Google Scholar] [CrossRef]
- Weigert, C. Pathologisch-anatomischer Beitraz fur erlischen Krankheit (Myasthenia gravis). Neurol. Cent. 1901, 20, 597–601. [Google Scholar]
- Marino, M.; Roden, A.C. The evolution of the histopathologic classification of thymic epithelial tumors. Mediastinum 2018, 2, 9. [Google Scholar] [CrossRef]
- Jaretzki, A.; Wolff, M. “Maximal” thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J. Thorac. Cardiovasc. Surg. 1988, 96, 711–716. [Google Scholar] [PubMed]
- Kaba, E.; Cosgun, T.; Ayalp, K.; Alomari, M.R.; Toker, A. Robotic thymectomy-a new approach for thymus. J. Vis. Surg. 2017, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Monaci, N.; Comacchio, G.M.; Verderi, E.; Marulli, G.; Schiavon, M.; Fortarezza, F.; Pezzuto, F.; Calabrese, F.; Rea, F. Thymic carcinoma with thyroid transcription factor-1 expression: An insidious pitfall. J. Thorac. Oncol. 2019, 14, e68–e70. [Google Scholar] [CrossRef] [PubMed]
- Dubois, P. Gaz. Du diagnostic de la syphilis consideree comme une des causes possibles de la mort du foetus. Med. Paris 1850, 21, 392–395. [Google Scholar]
- Shakerian, B.; Mandegar, M.H. Huge mediastinal thymic cyst in the elderly patient. Cureus 2020, 12, e7240. [Google Scholar] [CrossRef]
- Suster, S.; Rosai, J. Multilocular thymic cyst: An acquired reactive process. Study of 18 cases. Am. J. Surg. Pathol. 1991, 15, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, S.H.; Kim, J.; Lee, K.W.; Choi, Y.R.; Cho, H.; Goo, J.M. Growth of thymic epithelial tumors and thymic cysts: Differential radiological points. Thorac. Cancer 2019, 10, 864–871. [Google Scholar] [CrossRef]
- Syred, K.; Weissferdt, A. Non-neoplastic mediastinal cysts. Adv. Anat. Pathol. 2020, 27, 294–302. [Google Scholar] [CrossRef]
- Prieto-Granada, C.N.; Stevens, T. Multilocular thymic cyst with mucinous differentiation: A mimicker of thymic mucoepidermoid carcinoma. Int. J. Surg. Pathol. 2018, 26, 35–36. [Google Scholar] [CrossRef]
- Veeze-Kuijpers, B.; Van Andel, J.G.; Stiegelis, W.F.; Boldewijn, J.K. Benign thymic cyst following mantle radiotherapy for Hodgkin’s disease. Clin. Radiol. 1987, 38, 289–290. [Google Scholar] [CrossRef]
- Johnsen, N.J.; Bretlau, P. Cervical thymic cysts. Acta Otolaryngol. 1976, 82, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S. Management of incidental anterior mediastinal lesions: Summary of relevant studies. Mediastinum 2019, 3. [Google Scholar] [CrossRef]
- Shakerian, B.; Razavi, N. Incidental thymic cyst in middle mediastinum during emergency coronary artery bypass grafting: Case report. Indian J. Thorac. Cardiovasc. Surg. 2018, 34, 500–501. [Google Scholar] [CrossRef]
- Morollón, N.; Guerrero, P.; Duarte, J. Thymic cyst associated with myasthenia gravis. Neurologia 2016. [Google Scholar] [CrossRef]
- Peacey, S.R.; Belchetz, P.E. Graves’ disease: Associated ocular myasthenia gravis and a thymic cyst. J. R. Soc. Med. 1993, 86, 297–298. [Google Scholar] [PubMed]
- Fongi, E.G.; Gotlieb, D.; Vaamonde, C.A.; Buzzi, A.; Machado, E.; Perianes, I. Myasthenia gravis following excision of a thymic cyst; study of electrolytes during a myasthenic attack. Prensa Med. Argent. 1957, 44, 3754–3760. [Google Scholar]
- Okumura, S.; Ohta, T.; Fujioka, M.; Nakabayashi, H. A case of multilocular thymic cyst with myasthenia gravis. Zasshi J. Nihon Kyobu Geka Gakkai 1995, 43, 917–921. [Google Scholar]
- Mishra, A.K.; Agarwal, S.K.; Pradhan, S.; Agarwal, A. Association of unilocular thymic cyst and myasthenia gravis. Neurol. India 2012, 60, 103–105. [Google Scholar] [PubMed]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019, 18, 259–268. [Google Scholar] [CrossRef]
- Koizumi, T.; Otsuki, K.; Tanaka, Y.; Noguchi, T.; Fukushuima, T.; Kobayashi, T.; Ozawa, T.; Sekiguchi, N.; Hamanaka, K. National incidence and initial therapy for thymic carcinoma in Japan: Based on analysis of hospital-based cancer registry data, 2009–2015. Jpn. J. Clin. Oncol. 2020, 50, 434–439. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, K.; Li, C.; Yang, Y.; Guo, C.; Pu, Y.; Liu, L. Thymic Squamous Cell Carcinoma: A Population-Based Surveillance, Epidemiology, and End Result Analysis. Front. Oncol. 2020, 10, 592023. [Google Scholar] [CrossRef]
- Chalabreysse, L.; Etienne-Mastroianni, B.; Adeleine, P.; Cordier, J.F.; Greenland, T.; Thivolet-Bejui, F. Thymic carcinoma: A clinicopathological and immunohistological study of 19 cases. Histopathology 2004, 44, 367–374. [Google Scholar] [CrossRef]
- Padda, S.K.; Yao, X.; Antonicelli, A.; Riess, J.W.; Shang, Y.; Shrager, J.B.; Korst, R.; Detterbeck, F.; Huang, J.; Burt, B.M.; et al. Paraneoplastic syndromes and thymic malignancies: An examination of the international thymic malignancy interest group retrospective database. J. Thorac. Oncol. 2018, 13, 436–446. [Google Scholar] [CrossRef]
- Scorsetti, M.; Leo, F.; Trama, A.; D’Angelillo, R.; Serpico, D.; Macerelli, M.; Zucali, P.; Gatta, G.; Garassino, M.C. Thymoma and thymic carcinomas. Crit. Rev. Oncol. Hematol. 2016, 99, 332–350. [Google Scholar] [CrossRef]
- Berghmans, T.; Durieux, V.; Holbrechts, S.; Jungels, C.; Lafitte, J.J.; Meert, A.P.; Moretti, L.; Ocak, S.; Roelandts, M.; Girard, N. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer 2018, 126, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Yao, X.; Ruffini, E.; Ahmad, U.; Antonicelli, A.; Huang, J.; Guerrera, F.; Venuta, F.; van Raemdonck, D.; Travis, W.; et al. Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: A joint analysis of the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group. Eur. J. Cardiothorac. Surg. 2016, 50, 766–771. [Google Scholar] [CrossRef]
- Ahmad, U.; Yao, X.; Detterbeck, F.; Huang, J.; Antonicelli, A.; Filosso, P.L.; Ruffini, E.; Travis, W.; Jones, D.R.; Zhan, Y.; et al. Thymic carcinoma outcomes and prognosis: Results of an international analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 95–101. [Google Scholar] [CrossRef]
- Roden, A.C.; Szolkowska, M. Common and rare carcinomas of the thymus. Virchows Arch. Int. J. Pathol. 2021, 478, 111–128. [Google Scholar] [CrossRef]
- Hishida, T.; Nomura, S.; Yano, M.; Asamura, H.; Yamashita, M.; Ohde, Y.; Kondo, K.; Date, H.; Okumura, M.; Nagai, K.; et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: Results of 306 cases from a Japanese Nationwide Database Study. Eur. J. Cardiothorac. Surg. 2016, 49, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Shen, R.; Guo, T.; Zakowski, M.F.; Heguy, A.; Riely, G.J.; Huang, J.; Lau, C.; Lash, A.E.; Ladanyi, M.; et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. 2009, 15, 6790–6799. [Google Scholar] [CrossRef]
- Wang, Y.; Thomas, A.; Lau, C.; Rajan, A.; Zhu, Y.; Killian, J.K.; Petrini, I.; Pham, T.; Morrow, B.; Zhong, X.; et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci. Rep. 2014, 4, 7336. [Google Scholar] [CrossRef]
- Kishibuchi, R.; Kondo, K.; Soejima, S.; Tsuboi, M.; Kajiura, K.; Kawakami, Y.; Kawakita, N.; Sawada, T.; Toba, H.; Yoshida, M.; et al. DNA methylation of GHSR, GNG4, HOXD9 and SALL3 is a common epigenetic alteration in thymic carcinoma. Int. J. Oncol. 2020, 56, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Ströbel, P.; Hartmann, M.; Jakob, A.; Mikesch, K.; Brink, I.; Dirnhofer, S.; Marx, A. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N. Engl. J. Med. 2004, 350, 2625–2626. [Google Scholar] [CrossRef]
- Rossi, V.; Donini, M.; Sergio, P.; Passalacqua, R.; Rossi, G.; Buti, S. When a thymic carcinoma “becomes” a GIST. Lung Cancer 2013, 80, 106–108. [Google Scholar] [CrossRef]
- Schirosi, L.; Nannini, N.; Nicoli, D.; Cavazza, A.; Valli, R.; Buti, S.; Garagnani, L.; Sartori, G.; Calabrese, F.; Marchetti, A.; et al. Activating c-KIT mutations in a subset of thymic carcinoma and response to different c-KIT inhibitors. Ann. Oncol. 2012, 23, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Suster, S.; Moran, C.A. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am. J. Surg. Pathol. 1996, 20, 1469–1480. [Google Scholar] [CrossRef]
- Weissferdt, A.; Moran, C.A. Thymic carcinoma associated with multilocular thymic cyst: A clinicopathologic study of 7 cases. Am. J. Surg. Pathol. 2011, 35, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Detterbeck, F.; Van Raemdonck, D.; Rocco, G.; Thomas, P.; Weder, W.; Brunelli, A.; Guerrera, F.; Keshavjee, S.; Altorki, N.; et al. Thymic carcinoma: A cohort study of patients from the European society of thoracic surgeons database. J. Thorac. Oncol. 2014, 9, 541–548. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int. J. Cancer 2003, 105, 546–551. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; Shen, G.; Chai, Y.; Liang, C. Association between Epstein-Barr virus and Thymic epithelial tumors: A systematic review. Infect. Agents Cancer 2019, 14, 32. [Google Scholar] [CrossRef]
- Hu, W.M.; Jin, J.T.; Wu, C.Y.; Lu, J.B.; Zhang, L.H.; Zeng, J.; Lin, S.X. Expression of P63 and its correlation with prognosis in diffuse large B-cell lymphoma: A single center experience. Diagn. Pathol. 2019, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Pyo, J.S.; Kim, N.Y.; Kang, D.W. Diagnostic roles of immunohistochemistry in thymic tumors: Differentiation between thymic carcinoma and thymoma. Diagnostics 2020, 10, 460. [Google Scholar] [CrossRef]
- Takikita, M.; Altekruse, S.; Lynch, C.F.; Goodman, M.T.; Hernandez, B.Y.; Green, M.; Cozen, W.; Cockburn, M.; Sibug Saber, M.; Topor, M.; et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009, 69, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Suster, S. Mucoepidermoid carcinomas of the thymus. A clinicopathologic study of six cases. Am. J. Surg. Pathol. 1995, 19, 826–834. [Google Scholar] [CrossRef]
- Wu, S.G.; Li, Y.; Li, B.; Tian, X.Y.; Li, Z. Unusual combined thymic mucoepidermoid carcinoma and thymoma: A case report and review of literature. Diagn. Pathol. 2014, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Erickson-Johnson, M.R.; Yi, E.S.; García, J.J. Analysis of MAML2 rearrangement in mucoepidermoid carcinoma of the thymus. Hum. Pathol. 2013, 44, 2799–2805. [Google Scholar] [CrossRef]
- Wick, M.R.; Rosai, J. Neuroendocrine neoplasms of the thymus. Pathol. Res. Pract. 1988, 183, 188–199. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Lausi, P.O.; Refai, M.; Filosso, P.L.; Ruffini, E.; Oliaro, A.; Guerrera, F.; Brunelli, A. Thymic neuroendocrine tumors. Thorac. Surg. Clin. 2014, 24, 327–332. [Google Scholar] [CrossRef]
- Teh, B.T.; McArdle, J.; Chan, S.P.; Menon, J.; Hartley, L.; Pullan, P.; Ho, J.; Khir, A.; Wilkinson, S.; Larsson, C.; et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine 1997, 76, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.C.; Tan, M.H.; Eng, C.; Teh, B.T.; Rajasoorya, R.C. Thymic carcinoid in multiple endocrine neoplasia 1: Genotype-phenotype correlation and prevention. J. Intern. Med. 2006, 259, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Ströbel, P.; Zettl, A.; Shilo, K.; Chuang, W.Y.; Nicholson, A.G.; Matsuno, Y.; Gal, A.; Laeng, R.H.; Engel, P.; Capella, C.; et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: A multi-institutional clinicopathologic study. Genes Chromosom. Cancer 2014, 53, 738–749. [Google Scholar] [CrossRef]
- Filosso, P.L.; Ruffini, E.; Solidoro, P.; Roffinella, M.; Lausi, P.O.; Lyberis, P.; Oliaro, A.; Guerrera, F. Neuroendocrine tumors of the thymus. J. Thorac. Dis. 2017, 9, S1484–S1490. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.A.; Kornstein, M.J.; Cohen, C.; Duarte, I.G.; Miller, J.I.; Mansour, K.A. Neuroendocrine tumors of the thymus: A clinicopathological and prognostic study. Ann. Thorac. Surg. 2001, 72, 1179–1182. [Google Scholar] [CrossRef]
- Cardillo, G.; Treggiari, S.; Paul, M.A.; Carleo, F.; De Massimi, A.R.; Remotti, D.; Graziano, P.; Martelli, M. Primary neuroendocrine tumours of the thymus: A clinicopathologic and prognostic study in 19 patients. Eur. J. Cardiothorac. Surg. 2010, 37, 814–818. [Google Scholar] [CrossRef]
- Gaur, P.; Leary, C.; Yao, J.C. Thymic neuroendocrine tumors: A SEER database analysis of 160 patients. Ann. Surg. 2010, 251, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Yao, X.; Ahmad, U.; Zhan, Y.; Huang, J.; Ruffini, E.; Travis, W.; Lucchi, M.; Rimner, A.; Antonicelli, A.; et al. Outcome of primary neuroendocrine tumors of the thymus: A joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J. Thorac. Cardiovasc. Surg. 2015, 149, 103–109.e2. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Rosado-de-Christenson, M.; Suster, S. Thymolipoma: Clinicopathologic review of 33 cases. Mod. Pathol. 1995, 8, 741–744. [Google Scholar] [PubMed]
- Rieker, R.J.; Schirmacher, P.; Schnabel, P.A.; Moser, K.; Hoffmann, H.; Dienemann, H.; Pfannschmidt, J. Thymolipoma. A report of nine cases, with emphasis on its association with myasthenia gravis. Surg. Today 2010, 40, 132–136. [Google Scholar] [CrossRef]
- Hudacko, R.; Aviv, H.; Langenfeld, J.; Fyfe, B. Thymolipoma: Clues to pathogenesis revealed by cytogenetics. Ann. Diagn. Pathol. 2009, 13, 185–188. [Google Scholar] [CrossRef]
- Boland, J.M.; Colby, T.V.; Folpe, A.L. Liposarcomas of the mediastinum and thorax: A clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am. J. Surg. Pathol. 2012, 36, 1395–1403. [Google Scholar] [CrossRef]
- Paquette, M.; Truong, P.T.; Hart, J.; Jones, S.O.; Martens, B.; Christie, J.L.; Alexander, C.; Joe, H. Primary sarcoma of the mediastinum: A report of 16 cases referred to the British Columbia Cancer Agency. J. Thorac. Oncol. 2010, 5, 898–906. [Google Scholar] [CrossRef]
- den Bakker, M.A.; Marx, A.; Mukai, K.; Ströbel, P. Mesenchymal tumours of the mediastinum—Part I. Virchows Arch. Int. J. Pathol. 2015, 467, 487–500. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Moran, C.A.; Perino, G.; Koss, M.N.; Rosai, J. Liposarcoma of the anterior mediastinum and thymus. A clinicopathologic study of 28 cases. Am. J. Surg. Pathol. 1995, 19, 782–791. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.C.; et al. Vascular anomalies classification: Recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, F.; Tu, S.; Zhang, X.; Zhao, C. Cavernous hemangioma of the thymus: A case report and review of the literature. Medicine 2018, 97, e11698. [Google Scholar] [CrossRef]
- Tsubochi, H.; Endo, T.; Sogabe, M.; Endo, S.; Morinaga, S.; Dobashi, Y. Solitary fibrous tumor of the thymus with variegated epithelial components. Int. J. Clin. Exp. Pathol. 2014, 7, 7477–7484. [Google Scholar]
- Licci, S.; Puma, F.; Sbaraglia, M.; Ascani, S. Primary intrathymic lymphangioma. Am. J. Clin. Pathol. 2014, 142, 683–688. [Google Scholar] [CrossRef]
- Jenks, J.D.; Katsivas, T.F. Cystic mediastinal thymic lymphangioma mimicking echinococcal cyst. J. Thorac. Oncol. 2015, 10, 1498–1499. [Google Scholar] [CrossRef]
- Weissferdt, A.; Kalhor, N.; Suster, S.; Moran, C.A. Primary angiosarcomas of the anterior mediastinum: A clinicopathologic and immunohistochemical study of 9 cases. Hum. Pathol. 2010, 41, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Han, J.; Lee, H.W.; Cho, S.Y.; Kim, H.K. A rare case of thymic gangliocytic paraganglioma. J. Pathol. Transl. Med. 2016, 50, 165–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueda, Y.; Omasa, M.; Taki, T.; Okabe, R.; Cho, H.; Shoji, T.; Huang, C.L.; Yuba, Y. Thymic neuroblastoma within a thymic cyst in an adult. Case Rep. Oncol. 2012, 5, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Erlandson, R.A.; Rosai, J. Thymic neuroblastoma in adults: Report of three cases with special emphasis on its association with the syndrome of inappropriate secretion of antidiuretic hormone. Am. J. Clin. Pathol. 1997, 108, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Marutsuka, K.; Mitsukawa, T.; Kuribayashi, T.; Taniguchi, S.; Sumiyoshi, A. Ganglioneuroblastoma of the thymus: An adult case with the syndrome of inappropriate secretion of antidiuretic hormone. Hum. Pathol. 1996, 27, 506–509. [Google Scholar] [CrossRef]
- Ogawa, F.; Amano, H.; Iyoda, A.; Satoh, Y. Thymic neuroblastoma with the syndrome of inappropriate secretion of antidiuretic hormone. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 903–905. [Google Scholar] [CrossRef] [PubMed]





| Case | Age | Sex | Clinic | Size (cm) | Cyst Histology | Thymus Histology |
|---|---|---|---|---|---|---|
| 1 | 65 | M | Asymptomatic | 5 | Unilocular | Fatty involution |
| 2 | 39 | M | MG | 4 | Multilocular with CG | Follicular hyperplasia |
| 3 | 61 | M | Asymptomatic | 3.5 | Unilocular with CG | Fatty involution |
| 4 | 70 | M | Asymptomatic | 8 | Multilocular with CG and calcification | Fatty involution |
| 5 | 41 | F | MG | 6 | Multilocular | Follicular hyperplasia |
| 6 | 40 | M | Asymptomatic | 6 | Unilocular with epithelial mucinous metaplasia | Fatty involution |
| 7 | 57 | F | Asymptomatic | 8 | Multilocular with granulation tissue | Epithelial hyperplasia |
| 8 | 44 | F | Dyspnea | 6.5 | Unilocular with CG | Follicular hyperplasia |
| 9 | 49 | F | Asymptomatic | 4 | Multilocular with CG | Fatty involution |
| 10 | 70 | M | MG | 3 | Unilocular | Epithelial hyperplasia |
| 11 | 51 | M | Asymptomatic | 1 | Unilocular with stratified cuboidal epithelium | Fatty involution |
| 12 | 69 | M | MG | 1.4 | Multilocular with CG | Fatty involution |
| 13 | 26 | F | Chest pain | 3.7 | Multilocular with CG | Epithelial hyperplasia |
| 14 | 62 | M | Asymptomatic | 2 | Unilocular | Fatty involution |
| 15 | 30 | F | Asymptomatic | 5.1 | Unilocular with CG | Epithelial hyperplasia |
| Case | Age | Sex | Histotype | Immunohistochemistry | M–K | TNM (Stage) | Recurrence | Follow-Up † | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | F | SCC | CK+; CD5+−; CD117+; p63+ | IVb | T3N0M1a (IVA) | Yes | 60 | DOD |
| 2 | 62 | M | BC | CK+; CD5+−; CD117+; CGA−; Syn−; p63+ | IIb | T1aN0 (I) | Yes | 113 | AWD |
| 3 | 65 | M | SCC | CK+; CD5+; CD117+; p63+ | IVb | T1bN1M1b (IVB) | Yes | 22 | DOD |
| 4 | 60 | F | SCC | CK+; CD5+; CD117+; p63+ | IVb | T3N2M0 (IVA) | Yes | 18 | DOD |
| 5 | 73 | M | LEC | CK+; CD5+; CD117+; p63+; Syn+−; EBER+ | IIb | T1aN0Mx (I) | No | 77 | NED |
| 6 | 63 | M | SCC | CK+; CD5+; CD117+; p63+ | III | T2N0M0 (II) | Yes | 75 | AWD |
| 7 | 84 | M | SCC | CK+; CD5+−; CD117+−; p63+; GLUT1+ | III | T3N0M0 (IIIA) | No | 66 | NED |
| 8 | 73 | M | SCC | CK+; CD5+−; CD117+; p63+; GLUT1+ | IIb | T1aN0M0 (I) | No | 16 | DOC |
| 9 | 75 | M | c-SCC ‡ | CK+−; CD5+−; CD117+; p63+; GLUT1+− | IVb | T3N1M1a (IVA) | No | 63 | NED |
| 10 | 72 | M | SCC | CK+; CD5+−; CD117+; p63+; GLUT1+ | III | T3N0M0 (III) | No | 61 | NED |
| 11 | 67 | M | SCC | CK+; CD5+; CD117+; p63+; p40+; GLUT1+ | III | T3N0M0 (III) | Yes | 29 | AWD |
| 12 | 62 | M | SCC | CK+; CD5+−; CD117+; p40+; GLUT1+− | III | T3N0M0 (III) | No | 23 | NED |
| 13 | 56 | F | LGPA | CK+; CD5+−; CD117−; GLUT1+; TTF1−; CDX2−, p40− | IIa | T1aN0M0 (I) | No | 21 | NED |
| 14 | 70 | M | SCC | CK+; CD5−; CD117+; p63+; GLUT1+ | III | T1bN0M0 (I) | No | 18 | NED |
| 15 | 62 | M | MEC | CK+; p63+−; CK7+−, CD117−; CD5−; GLUT1− | IVb | T3NxM1b (IVB) | Yes | 23 | AWD |
| 16 | 34 | M | LEC | CK+; CD5+; CD117+; p40+; GLUT1−; EBER+ | IVb | T3N0M1b (IVB) | Yes | 11 | AWD |
| 17 | 58 | M | TC, NOS | CK+; CD5−; CD117+; p63+; GLUT1+ | IIa | T1aN0M0 (I) | No | 9 | NED |
| 18 | 49 | M | TC, NOS | CK+; CD5+; CD117+; p63−; GLUT1+−; TTF1+ | IVb | T3N2M0 (IVA) | Yes | 17 | AWD |
| Case | Age | Sex | Histotype | Immunohistochemistry | M–K | TNM (Stage) | Recurrence | Follow-Up † | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | AC | CK+, CGA+; Syn+; CD117+; Ki67: 10% | II | T1aN0M0 (I) | Yes | 75 | DOD |
| 2 | 44 | F | SCC | CK+, CGA−; Syn+; CD117+; Ki67: 70% | IVb | T3N1M1a (IVA) | No | 0 | DOD |
| 3 | 47 | F | AC | CK+, CGA−; Syn+; CD117+; Ki67: 20% | IVb | T3N1M1a (IVA) | Yes | 51 | DOD |
| 4 | 46 | F | TyC | CK+, CGA+; Syn+; CD117+; Ki67: 4% | IIa | T1aN0M0 (I) | No | 51 | NED |
| 5 | 27 | F | c-NET ‡ | CK+, CGA+; Syn−; CD117−; Ki67: 1% | IVa | T3N0M1a (IVA) | Yes | 28 | AWD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, F.; Fortarezza, F.; Pezzuto, F.; Lunardi, F.; Comacchio, G.; Sbaraglia, M.; Pasello, G.; Marulli, G.; Dei Tos, A.P.; Rea, F. The Rarest of Rare Thymic Lesions: A 10-Year Surgical Pathology Experience. Cancers 2021, 13, 4056. https://doi.org/10.3390/cancers13164056
Calabrese F, Fortarezza F, Pezzuto F, Lunardi F, Comacchio G, Sbaraglia M, Pasello G, Marulli G, Dei Tos AP, Rea F. The Rarest of Rare Thymic Lesions: A 10-Year Surgical Pathology Experience. Cancers. 2021; 13(16):4056. https://doi.org/10.3390/cancers13164056
Chicago/Turabian StyleCalabrese, Fiorella, Francesco Fortarezza, Federica Pezzuto, Francesca Lunardi, Giovanni Comacchio, Marta Sbaraglia, Giulia Pasello, Giuseppe Marulli, Angelo Paolo Dei Tos, and Federico Rea. 2021. "The Rarest of Rare Thymic Lesions: A 10-Year Surgical Pathology Experience" Cancers 13, no. 16: 4056. https://doi.org/10.3390/cancers13164056
APA StyleCalabrese, F., Fortarezza, F., Pezzuto, F., Lunardi, F., Comacchio, G., Sbaraglia, M., Pasello, G., Marulli, G., Dei Tos, A. P., & Rea, F. (2021). The Rarest of Rare Thymic Lesions: A 10-Year Surgical Pathology Experience. Cancers, 13(16), 4056. https://doi.org/10.3390/cancers13164056








