The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. The Prevalence of Prostate Cancer
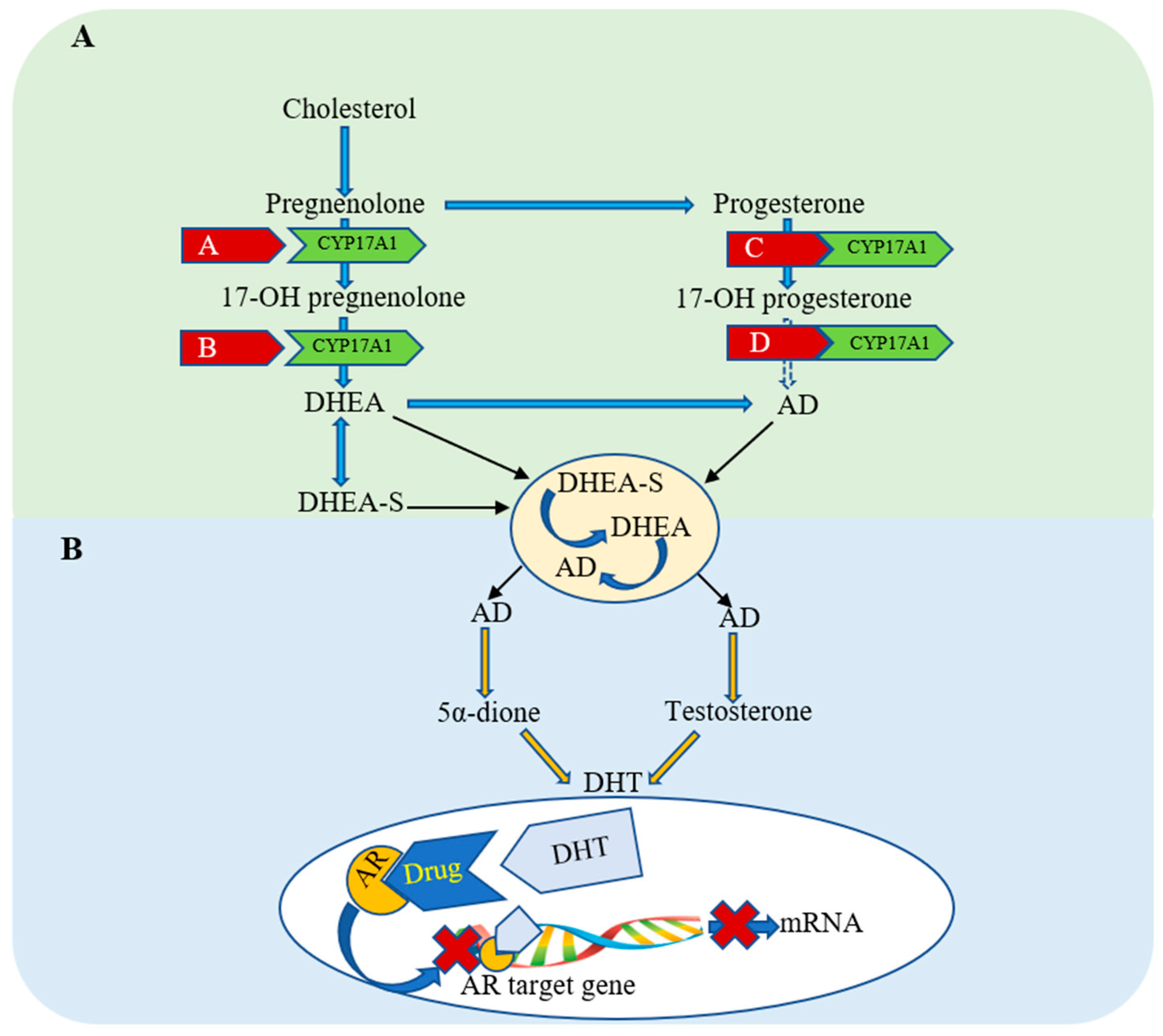
1.2. Current Treatments in Prostate Cancer
1.3. Cannabis sativa and Cannabinoids
2. Cannabinoids and the Entourage Effect
3. Cannabinoids as Pharmacological Effectors
3.1. Cell Signaling Mediated by Cannabinoid Receptors
3.1.1. Cannabinoid-Induced Inhibition of Cell Proliferation
3.1.2. Cannabinoid-Induced Apoptosis
3.1.3. Cannabinoid-Induced Inhibition of Cell Motility
4. Current Clinical Trials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2A | Adenosine |
| ADT | Androgen-deprivation therapy |
| AMPK | AMP-activated protein kinase |
| AR | Androgen receptor |
| O2− | Anion superoxide |
| AMPK | AMP-activated protein kinase |
| 2-AG | 2-arachidonoylglycerol |
| cAMPK | Cyclic adenosine monophosphate |
| CBD | Cannabidiol |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| DHEA | Dehydroepiandrosterone |
| D2 | Dopamine |
| DHT | Dihydrotestosterone |
| ECS | Endocannabinoid system |
| ER | Endoplasmic reticulum |
| ERK | Extracellular-signal-regulated-kinase |
| EGFR | Epidermal growth factor receptor |
| GPCRs | G protein-coupled receptors |
| OH. | Hydroxyl radical |
| H2O2 | Hydrogen peroxide |
| 2-Lino-Gl | 2-linoleoyl-glycerol |
| mTOR | Mammalian target of rapamycin |
| MET | (R)-methanandamide |
| MAPK | Mitogen-activated protein kinase |
| 2-Palm-Gl | 2-palmitoyl-glycerol |
| PI3K | Phosphatidylinositol 3-kinase |
| PKA | Protein kinase A |
| PKB | Protein kinase B |
| ROS | Reactive oxygen species |
| 5HT | Serotonin |
| SC | Synthetic cannabinoids |
| ∆9-THC | ∆9-tetrahydrocannabinol |
| TRPM8 | Transient receptor potential melastatin type-8 |
| TRIB3 | Tribbles homolog 3-dependent signaling |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdana, N.R.; Mochtar, C.A.; Umbas, R.; Hamid, A. The risk factors of prostate cancer and its prevention: A literature review. Acta Med. Indones. 2016, 48, 228–238. [Google Scholar] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kirby, R.S.; Patel, M.I. Fast Facts: Prostate Cancer; Health Press Limited: Oxford, UK, 2009. [Google Scholar]
- Ramon, J.; Denis, L. Prostate Cancer (Recent Results in Cancer Research); Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Dasgupta, P.; Kirby, R.S. ABC of Prostate Cancer; John Wiley & Sons: Oxford, UK, 2011; Volume 159. [Google Scholar]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current treatment options for metastatic hormone-sensitive prostate cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [Green Version]
- Makowiecka, J.; Wielgus, K. Therapeutic Potential of Cannabinoids—Retrospective and Historical Developments. J. Nat. Fibers 2014, 11, 185–198. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, M. History of Therapeutic Cannabis; McFarland & Company, Inc., Publishers: Jefferson, CO, USA, 1997; pp. 35–55. [Google Scholar]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for cancer treatment: Progress and promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Bridgeman, M.B.; Abazia, D.T. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharm. Ther. 2017, 42, 180. [Google Scholar]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef] [Green Version]
- Kocis, P.T.; Vrana, K.E. Delta-9-tetrahydrocannabinol and cannabidiol drug-drug interactions. Med. Cannabis Cannabinoids 2020, 3, 12–24. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [Green Version]
- Cridge, B.J.; Rosengren, R.J. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag. Res. 2013, 5, 301. [Google Scholar]
- Ladin, D.A.; Soliman, E.; Griffin, L.; van Dross, R. Preclinical and clinical assessment of cannabinoids as anti-cancer agents. Front. Pharmacol. 2016, 7, 361. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; de Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecksel, R.; LaVigne, J.; Streicher, J.M. In Defense of the “Entourage Effect”: Terpenes Found in Cannabis sativa Activate the Cannabinoid Receptor 1 In vitro. FASEB 2020, 34, 1. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.; Hecksel, R.; Streicher, J.M. In Defense of the “Entourage Effect”: Terpenes Found in Cannabis sativa Activate the Cannabinoid Receptor 1 In vivo. FASEB 2020, 34, 1. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.A.; Bianco, F.J. The role of cannabinoids in prostate cancer: Basic science perspective and potential clinical applications. Indian J. Urol. 2012, 28, 9–14. [Google Scholar] [CrossRef]
- Malhotra, P.; Casari, I.; Falasca, M. Therapeutic potential of cannabinoids in combination cancer therapy. Adv. Biol. Regul. 2021, 79, 100774. [Google Scholar] [CrossRef]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.G.; Scheau, A.E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis. Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef] [Green Version]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23. [Google Scholar] [CrossRef] [Green Version]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Vayalil, P.K. Mitochondrial oncobioenergetics of prostate tumorigenesis. Oncol. Lett. 2019, 18, 4367–4376. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casanovas, M.; Pérez-Olives, C.; Ferreiro-Vera, C.; Navarro, G.; Sánchez de Medina, V.; Nadal, X. Pharmacological potential of varinic-, minor-, and acidic phytocannabinoids. Pharmacol. Res. 2020, 158, 104801. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Reggio, P.H. An Update on Non-CB1, Non-CB2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Salon, J.A.; Lodowski, D.T.; Palczewski, K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol. Rev. 2011, 63, 901–937. [Google Scholar] [CrossRef] [Green Version]
- Ferré, S.; Casadó, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, X. G protein-coupled receptor oligomerization revisited: Functional and pharmacological perspectives. Pharmacol. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef] [Green Version]
- Glass, M.; Felder, C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997, 17, 5327–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventura, J.; Rico, A.J.; Moreno, E.; Sierra, S.; Sánchez, M.; Luquin, N.; Farré, D.; Müller, C.E.; Martínez-Pinilla, E.; Cortés, A.; et al. L-DOPA-treatment in primates disrupts the expression of A(2A) adenosine-CB(1) cannabinoid-D(2) dopamine receptor heteromers in the caudate nucleus. Neuropharmacology 2014, 79, 90–100. [Google Scholar] [CrossRef]
- Jin, J.; Momboisse, F.; Boncompain, G.; Koensgen, F.; Zhou, Z.; Cordeiro, N.; Arenzana-Seisdedos, F.; Perez, F.; Lagane, B.; Kellenberger, E.; et al. CCR5 adopts three homodimeric conformations that control cell surface delivery. Sci. Signal. 2018, 11, eaal2869. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, W.; Yamanishi, Y.; Limviphuvadh, V.; Saito, A.; Toh, H. GGIP: Structure and sequence-based GPCR-GPCR interaction pair predictor. Proteins 2016, 84, 1224–1233. [Google Scholar] [CrossRef]
- Cortes, A.; Moreno, E.; Rodriguez-Ruiz, M.; Canela, E.I.; Casado, V. Targeting the dopamine D3 receptor: An overview of drug design strategies. Expert Opin. Drug Discov. 2016, 11, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Vera, T.M.; Vanhauwe, J.; Thomas, T.O.; Medkova, M.; Preininger, A.; Mazzoni, M.R.; Hamm, H.E. Insights into G protein structure, function, and regulation. Endocr. Rev. 2003, 24, 765–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB(1) and CB(2) Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.H.; Sim-Selley, L.J.; Selley, D.E. Cannabinoid CB1 receptor-interacting proteins: Novel targets for central nervous system drug discovery? Br. J. Pharmacol. 2010, 160, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotter, E.; Graham, S.; Glass, M. Cannabinoid Receptor Signal Transduction Pathways. In The Cannabinoid Receptors; Reggio, P.H., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 153–171. [Google Scholar]
- Ibsen, M.S.; Finlay, D.B.; Patel, M.; Javitch, J.A.; Glass, M.; Grimsey, N.L. Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front. Pharmacol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Dalton, G.D.; Howlett, A.C. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br. J. Pharmacol. 2012, 165, 2497–2511. [Google Scholar] [CrossRef] [Green Version]
- Neves, L.M.S.; Gonçalves, E.C.D.; Cavalli, J.; Vieira, G.; Laurindo, L.R.; Simões, R.R.; Coelho, I.S.; Santos, A.R.S.; Marcolino, A.M.; Cola, M.; et al. Photobiomodulation Therapy Improves Acute Inflammatory Response in Mice: The Role of Cannabinoid Receptors/ATP-Sensitive K(+) Channel/p38-MAPK Signalling Pathway. Mol. Neurobiol. 2018, 55, 5580–5593. [Google Scholar] [CrossRef]
- De Assis Lima, I.V.; Bellozi, P.M.; Batista, E.M.; Vilela, L.R.; Brandão, I.L.; Ribeiro, F.M.; Moraes, M.F.D.; Moreira, F.A.; de Oliveira, A.C. Cannabidiol anticonvulsant effect is mediated by the PI3Kγ pathway. Neuropharmacology 2020, 176, 108156. [Google Scholar] [CrossRef]
- Malenczyk, K.; Jazurek, M.; Keimpema, E.; Silvestri, C.; Janikiewicz, J.; Mackie, K.; Di Marzo, V.; Redowicz, M.J.; Harkany, T.; Dobrzyn, A. CB1 cannabinoid receptors couple to focal adhesion kinase to control insulin release. J. Biol. Chem. 2013, 288, 32685–32699. [Google Scholar] [CrossRef] [Green Version]
- Cheratta, A.R.; Thayyullathil, F.; Pallichankandy, S.; Subburayan, K.; Alakkal, A.; Galadari, S. Prostate apoptosis response-4 and tumor suppression: It’s not just about apoptosis anymore. Cell Death Dis. 2021, 12, 47. [Google Scholar] [CrossRef]
- Dixit, V.; Sharma, V.; Lohiya, N. The effect of chronically administered cannabis extract on the testicular function of mice. Eur. J. Pharmacol. 1974, 26, 111–114. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, A.; Srivastava, P.; Turner, H.; Krishna, A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 195–205. [Google Scholar] [CrossRef]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14. [Google Scholar] [CrossRef]
- Pietrovito, L.; Iozzo, M.; Bacci, M.; Giannoni, E.; Chiarugi, P. Treatment with Cannabinoids as a Promising Approach for Impairing Fibroblast Activation and Prostate Cancer Progression. Int. J. Mol. Sci. 2020, 21, 787. [Google Scholar] [CrossRef] [Green Version]
- Roberto, D.; Klotz, L.H.; Venkateswaran, V. Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. Prostate 2019, 79, 151–159. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Louka, S.; Neophytou, C.; Constantinou, A. Abstract 4030: Synthetic cannabinoids AM-251 and AM-1241 induce cell death in prostate cancer cells. Cancer Res. 2020, 80, 4030. [Google Scholar]
- Nithipatikom, K.; Endsley, M.P.; Isbell, M.A.; Falck, J.R.; Iwamoto, Y.; Hillard, C.J.; Campbell, W.B. 2-Arachidonoylglycerol: A novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 2004, 64, 8826–8830. [Google Scholar] [CrossRef] [Green Version]
- Morell, C.; Bort, A.; Vara, D.; Ramos-Torres, A.; Rodriguez-Henche, N.; Diaz-Laviada, I. The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2016, 19, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Serradell, O.; Poblete, C.; Sanchez, C.; Castellon, E.; Gallegos, I.; Huidobro, C.; Llanos, M.; Contreras, H. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015, 33, 1599–1608. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Vara, D.; Goméz-Cañas, M.; Zúñiga, M.C.; Olea-Azar, C.; Goya, P.; Fernández-Ruiz, J.; Díaz-Laviada, I.; Jagerovic, N. Synthetic cannabinoid quinones: Preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur. J. Med. Chem. 2013, 70, 111–119. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Gomez-Granados, A.D.; Tang, A.T.; Pfeiffer, A.W.; Williams, C.L.; Campbell, W.B. Cannabinoid receptor type 1 (CB1) activation inhibits small GTPase RhoA activity and regulates motility of prostate carcinoma cells. Endocrinology 2012, 153, 29–41. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer Res. 2011, 31, 3799–3807. [Google Scholar]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Diaz-Laviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R (+)-Methanandamide and JWH-015: Involvement of CB 2. Br. J. Cancer 2009, 101, 940–950. [Google Scholar] [CrossRef] [Green Version]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Lorente, M.; Egia, A.; Blázquez, C.; García, S.; Giroux, V.; Malicet, C.; Villuendas, R.; Gironella, M.; González-Feria, L. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 2006, 9, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer. 2003, 3, 745–755. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar]
- Signorile, A.; Ferretta, A.; Ruggieri, M.; Paolicelli, D.; Lattanzio, P.; Trojano, M.; de Rasmo, D. Mitochondria, Oxidative Stress, cAMP Signalling and Apoptosis: A Crossroads in Lymphocytes of Multiple Sclerosis, a Possible Role of Nutraceutics. Antioxidants 2020, 10, 21. [Google Scholar] [CrossRef]
- Perillo, B.; di Donato, M.; Pezone, A.; di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar]
- Dando, I.; Donadelli, M.; Costanzo, C.; Dalla Pozza, E.; D’alessandro, A.; Zolla, L.; Palmieri, M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013, 4, e664. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, T.; Grabiec, U.; Ghadban, C.; Feese, K.; Dehghani, F. The influence of biomechanical properties and cannabinoids on tumor invasion. Cell Adh. Migr. 2017, 11, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, T.; Feese, K.; Ghadban, C.; Dehghani, F.; Grabiec, U. On the influence of cannabinoids on cell morphology and motility of glioblastoma cells. PLoS ONE 2019, 14, e0212037. [Google Scholar] [CrossRef]
- Dalton, G.D.; Peterson, L.J.; Howlett, A.C. CB₁ cannabinoid receptors promote maximal FAK catalytic activity by stimulating cooperative signaling between receptor tyrosine kinases and integrins in neuronal cells. Cell. Signal. 2013, 25, 1665–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Government. Clinical Trial Cancer—Australia. Available online: https://www.australianclinicaltrials.gov.au (accessed on 24 June 2021).
- Clinical Trial Breast Cancer—USA. Available online: https://clinicaltrials.gov/ct2/show/NCT04482244 (accessed on 24 June 2021).
- Myint, Z.; St Clair, W.H.; Strup, S.; Wang, P.; James, A.C.; Yan, D.; Allison, D.B.; Ellis, C.S.; Otto, D.E.; Kolesar, J.; et al. A phase I, dose-expansion cohort study on the safety of a cannabidiol for biochemical recurrence in prostate cancer patients. J. Clin. Oncol. 2021, 39, TPS263. [Google Scholar] [CrossRef]
- Clinical Trial Prostate Cancer—USA. Available online: https://clinicaltrials.gov/ct2/show/NCT04428203 (accessed on 24 June 2021).
- Bachari, A.; Piva, T.J.; Salami, S.A.; Jamshidi, N.; Mantri, N. Roles of Cannabinoids in Melanoma: Evidence from In vivo Studies. Int. J. Mol. Sci. 2020, 21, 6040. [Google Scholar] [CrossRef]
- Singh, K.; Jamshidi, N.; Zomer, R.; Piva, T.J.; Mantri, N. Cannabinoids and Prostate Cancer: A Systematic Review of Animal Studies. Int. J. Mol. Sci. 2020, 21, 6265. [Google Scholar] [CrossRef]
- Roberto, D.; Klotz, L.H.; Venkateswaran, V. Cannabinoids as an Anticancer Agent for Prostate Cancer. J. Urol. Res. 2017, 4, 1090–1097. [Google Scholar]


| Cannabinoids | Cannabinoid Receptors | Prostate Cancer Cell Type | Mechanism of Action | Anticancer Effect | In Vitro/In Vivo | Citations |
|---|---|---|---|---|---|---|
| WIN 55-212.2, CBD | CB1, CB2 | CAFs, PC3, DU145, LNCaP/PNT-1 | Downregulates α-smooth muscle actin and matrix metalloprotease-2 expression, Inhibits CAFs migration | Cannabinoid inhibits CAF migration, impairs the activation and the reactivity of CAFs WIN 55-212.2 ≥ 5 µM and CBD 5 µM induces cell death in prostate cancer cell lines, without affecting healthy prostate epithelial cells | In vitro | [70] |
| AM-251/ AM-1241 | PC3, DU145 | Induction of caspase-dependent apoptosis in DU145 cells and autophagy in PC3 cells | Inhibition of the proliferation and reducing viable cell number | In vitro | [73] | |
| WIN55,212-2 | CB2 | PC3, DU145, LNCaP | Reduction in phosphorylated retinoblastoma (pRb) and Cdk4 expression in a dose-dependent manner; Increase in p27 expression compared to control; WIN55,212-2 exert its anti-proliferative effects partially through the CB2 receptor. | Cannabinoid Induces cell cycle arrest, apoptosis and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer | In vivo/In vitro | [71] |
| WIN55,212-2 | CB2 | LNCaP | Downregulated the PI3K/Akt/mTOR signaling pathway; Activation of AMP | Inhibition of neuroendocrine differentiation (NE) and reduction in tumor size | In vitro/In vivo | [75] |
| AEA, 2-AG, MET | CB1 | PC3, Primary tissue samples from patients | Activated caspase-3-Down regulation of Bcl-2- Activated the Erk pathway; Decrease in the activation levels of the Akt pathway; Activation of apoptotic pathway without alteration in cell cycle | Inhibition of cell growth | In vitro | [76] |
| CBD | LNCaP-TRPM8 PC3–CB1, CB2 DU145–TRPV1 22RV1-TRPV1 | LNCaP, 22RV1, DU145, PC3 | CBD induces intrinsic apoptotic pathway and upregulated PUMA in all cell lines and AR in LNCaP, 22RV1 Increased expression of p27 and p21, G1/S phase transition in LNCaP, 22RV1, DU145 and PC3 CBD-BDS dose-dependently inhibited the growth of xenografts from LNCaP, but not DU145 cells CBD-BDS dose-dependently inhibited the growth of xenografts from LNCaP but not DU145 cells | Inhibition of cell viability and tumor growth | In vitro/In vivo | [77] |
| PM49 (synthetic cannabinoid quinone) | PPARγ receptor and partially CB1 | LNCaP | ROS production, Cell cycle arrest in G0/G1 phase; Apoptosis induction | Inhibition of cell viability Reduction in tumor growth | In vitro/In vivo | [78] |
| WIN55212-2 | CB1 | PC3, DU145 | Inhibition of small GTPase RhoA activity and increases the Rac1 and Cdc42 activity; Loss of actin/myosin microfilaments, cell spreading, and cell migration | Decreased cell motility | In vitro | [79] |
| WIN55212-2, CBD | CB1, CB2 | LNCaP | WIN and CBD activate PARP cleavage and induce apoptosis; WIN effects are CB receptor independent; CBD effects are CB1 and CB2 receptor dependent | Cannabinoid induce phosphatases and phosphatase-dependent apoptosis in cancer cell lines. Inhibition of proliferation Inhibition of cell growth | In vitro | [80] |
| JWH-015, MET | CB2 | PC3, DU145, LNCaP | Inhibits Akt-mTOR pathway; Induction of de novo synthesis of ceramide and ER stress- proapoptotic effect–Included JNK activation | Inhibition of cell growth Reduction of tumor growth | In vitro/In vivo | [81] |
| WIN55,212-2 | CB1, CB2 | LNCaP, PC3 | Induction of p53 and p27/KIP1, Down-regulation of cyclins D1, D2, E and E2F1; Decrease in the expression of cdk-2, -4, -6, pRb, DP1 and DP2; Up-regulation of ERK1/2 and inhibition of PI3k/Akt pathways; Increase in Bax/Bcl-2 ratio- Induction of apoptosis; G0/G1 phase cell cycle arrest | Inhibition of cell growth Induction of apoptosis | In vitro | [37] |
| 2AG | CB1 | PC3, DU145 | Inhibits adenylyl cyclase and decreases activity of PKA; Inhibition of invasion | Inhibition of invasion of prostate cancer cells | In vitro | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers 2021, 13, 4107. https://doi.org/10.3390/cancers13164107
Singh K, Nassar N, Bachari A, Schanknecht E, Telukutla S, Zomer R, Piva TJ, Mantri N. The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers. 2021; 13(16):4107. https://doi.org/10.3390/cancers13164107
Chicago/Turabian StyleSingh, Kanika, Nazim Nassar, Ava Bachari, Ellen Schanknecht, Srinivasareddy Telukutla, Roby Zomer, Terrence J. Piva, and Nitin Mantri. 2021. "The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer" Cancers 13, no. 16: 4107. https://doi.org/10.3390/cancers13164107
APA StyleSingh, K., Nassar, N., Bachari, A., Schanknecht, E., Telukutla, S., Zomer, R., Piva, T. J., & Mantri, N. (2021). The Pathophysiology and the Therapeutic Potential of Cannabinoids in Prostate Cancer. Cancers, 13(16), 4107. https://doi.org/10.3390/cancers13164107








