Simple Summary
Multidisciplinary team meetings have increasingly been implemented in cancer care worldwide to ensure timely, accurate and evidence-based diagnosis, and treatment plans. Nowadays, multidisciplinary team meetings are generally considered indispensable. However, they are considered time-consuming and expensive, while the effects of multidisciplinary team meetings are not yet fully understood. The aim of this systematic review is to update and summarize the literature and create an overview of the existing knowledge. Cancer types such as colorectal, lung, prostate and breast cancer with rapidly increasing incidence rates will inevitably impact the workload of clinicians. Understanding the effects of the widely implemented multidisciplinary team meetings in oncology care is fundamental in order to optimize care pathways and allocate resources in the rapidly diversifying landscape of cancer therapies.
Abstract
Objective: The aim of our systematic review is to identify the effects of multidisciplinary team meetings (MDTM) for lung, breast, colorectal and prostate cancer. Methods: Our systematic review, performed following PRISMA guidelines, included studies examining the impact of MDTMs on treatment decisions, patient and process outcomes. Electronic databases PUBMED, EMBASE, Cochrane Library and Web of Science were searched for articles published between 2000 and 2020. Risk of bias and level of evidence were assessed using the ROBINS-I tool and GRADE scale. Results: 41 of 13,246 articles were selected, evaluating colorectal (21), lung (10), prostate (6) and breast (4) cancer. Results showed that management plans were changed in 1.6–58% of cases after MDTMs. Studies reported a significant impact of MDTMs on surgery type, and a reduction of overall performed surgery after MDTM. Results also suggest that CT and MRI imaging significantly increased after MDTM implementation. Survival rate increased significantly with MDTM discussions according to twelve studies, yet three studies did not show significant differences. Conclusions: Despite heterogeneous data, MDTMs showed a significant impact on management plans, process outcomes and patient outcomes. To further explore the impact of MDTMs on the quality of healthcare, high-quality research is needed.
1. Introduction
Multidisciplinary care has increasingly been implemented in cancer care pathways worldwide, with oncology multidisciplinary team meetings (MDTMs) as a central platform for coordinated care delivery. An MDTM can be defined as “a group of healthcare professionals with different specialties who meet periodically (e.g., weekly) to discuss patient cases, diagnosis and treatment recommendations” [1]. MDTMs are often tailored as disease-specific and therefore differ in organization, i.e., in meeting frequency, duration or core team. The goal of the MDTM is to ensure timely, accurate and evidence-based diagnosis, treatment plans and follow-up for all discussed patients [2]. In 1995, Calman-Hine showed a positive correlation between multidisciplinary care and optimal decision making for cancer patients [3]. Since the publication of the Calman-Hine report, MDTMs have increasingly been adopted as part of routine cancer care pathways and are nowadays generally considered indispensable [2]. However, at the same time, MDTMs are considered time-consuming and expensive. The total workload of clinicians, occupied by MDTMs, is expected to increase, especially for cancer types with continuously increasing incidence rates, such as colorectal, lung, prostate and breast cancer [4].
These cancer types together constitute the top four cancer types in terms of global annual incidence [5]. Therefore, it is of great importance that the impact of MDTMs on different aspects of the clinical pathway and patient outcomes are well-understood. Previous systematic reviews showed weak evidence of impact on diagnosis and management plans. However, these studies found little evidence that MDTMs improve clinical outcomes [6,7,8]. We are the first to report on multiple cancer types in detail, to compare the effects of MDTMs in the four cancer types (colorectal, lung, prostate and breast cancer) that are expected to have a high impact on global healthcare. The aim of this systematic review is to update and summarize the literature and create an overview of the existing knowledge regarding the effects of MDTMs for colorectal, lung, prostate and breast cancer and to identify their value in these patient care pathways.
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane Collaboration’s double-data collection and extraction methodology [9,10].
2.1. Protocol and Registration
The protocol of this systematic review is registered in the PROSPERO database (CRD42019127476) [11].
2.2. Search Strategy
Relevant studies were searched in the following electronic databases: (1) PUBMED, (2) EMBASE, (3) Cochrane Library and (4) Web of Science. A librarian was consulted for the search strategy, and the search strategy combined variations for ‘multidisciplinary team meetings’ and ‘colorectal cancer’, ‘lung cancer’, ‘prostate cancer’ or ‘breast cancer’, (Supplementary Tables S1 and S2). No language restrictions were applied. Time limits were from 1 January 2000 to 31 December 2020. In addition, reference lists of relevant systematic reviews were screened to identify additional studies.
2.3. Study Selection Criteria
The inclusion criteria consisted of randomized controlled trials (RCT), non-randomized controlled trials, observational studies and before-and-after studies. Typically, observational studies evaluated plans prior to and after MDTM discussion and before-and-after studies compared a cohort of patients that were discussed in MDTM with a control cohort.
Studies were included if the impact of MDTMs was examined for colorectal cancer, lung cancer, prostate cancer or breast cancer. Studies that evaluated mixed cohorts, such as urological cancers, were included if extracting specific data on prostate cancer was feasible. MDTMs were defined as “regular meetings where a multidisciplinary team of specialists attend and discuss diagnosis and treatment recommendations for patients” [1].
Studies that did investigate MDTMs for any of the four cancer types were critically evaluated and excluded if they met any of the following exclusion criteria. Study designs that were excluded were qualitative studies and studies without control groups. Studies were also excluded if they investigated: (1) the effect of another intervention, implemented in addition to the MDTM (e.g., telecommunication), (2) the implementation process, (3) opinions of healthcare professionals or (4) adherence to MDT advice.
2.4. Data Collection and Extraction
Abstracts of articles yielded from the search were imported in Endnote, and duplicates were removed [12]. Thereafter, the web application Rayyan QCRI was used for the screening process [13]. Two reviewers (L.K. and H.W.) independently performed a title–abstract screening, and articles that met the inclusion criteria were selected for full-text screening. Subsequently, the full-text articles were retrieved and reviewed (L.K. and H.W.). Disagreements on selection were discussed regularly and resolved by consensus. If no agreement could be reached, a third independent investigator (R.M.) could be consulted. Cohen’s kappa was calculated to determine the inter-observer variability of full-text screening. The data were extracted independently by two reviewers to ensure correct extraction (L.K. and H.W.). Meta-analysis in general was not possible due to the heterogeneity of the data, however a few parameters were calculated. A weighted average was calculated for changes in management plans per cancer type, weighing the percentage of changed plans with the number of included patients. Studies that did not perform statistics were not included in the analysis of data. Summative tables were created to present the data.
2.5. Quality Assessment
Two researchers (L.K. and H.W.) independently assessed the risk of bias, using the ROBINS-I tool for non-randomized studies [14]. In addition, the quality of evidence of the studies was assessed with the GRADE scale [15] by the same researchers (L.K. and H.W.). Any disagreements were first discussed between the reviewers, and if required, a third researcher (R.M.) could be consulted.
3. Results
A total of 20,912 articles were retrieved from electronic database searches (Figure 1). After removal of duplicates, 13,246 studies were evaluated in the title–abstract screening. Following title-abstract screening, 165 full-text articles were assessed for eligibility, and 41 studies were included in the final analysis. The measured Cohen’s Kappa for the inter-observer agreement between reviewers was 0.656, indicating substantial agreement [16]. It was not necessary to consult a third reviewer during title–abstract and full-text screening, as no disagreements remained after discussion.
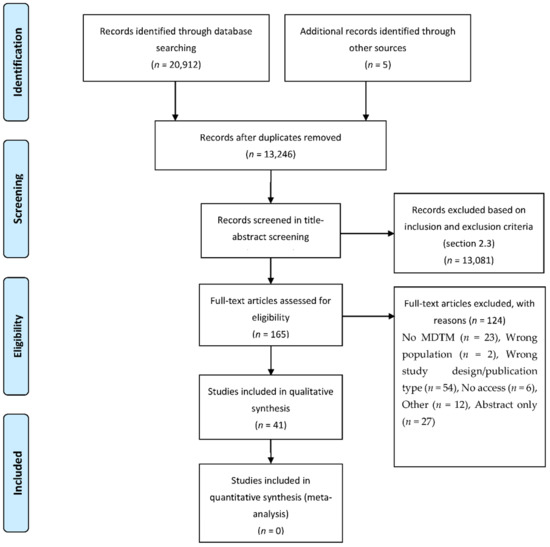
Figure 1.
PRISMA-P 2015 flowchart.
3.1. Study Characteristics
Forty-one articles investigated the effect of MDTMs on cancer care pathways in colorectal cancer (21/41) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], lung cancer (10/41) [38,39,40,41,42,43,44,45,46,47], prostate cancer (6/41) [48,49,50,51,52,53] and breast cancer (4/41) [54,55,56,57] (Table 1). All studies were conducted in adults (total n = 82,073, range 42–32,569 study subjects per study, mean 2002), with studies originating from in Oceania (8/41) [20,26,29,38,39,43,45,52], Asia (9/41) [18,21,23,35,41,46,50,56,57], Europe (13/41) [17,19,24,25,27,31,32,34,36,37,44,48,49], North America (10/41) [22,28,30,33,40,42,47,51,53,54] or Africa (1/41) [55]. Studies were performed in multicenter (6/41) [34,37,38,40,41,56] or single-center (35/41) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,35,36,39,42,43,44,45,46,47,48,49,50,51,52,53,54,55,57] settings and according to hospital types: general hospitals (12/41) [19,24,25,29,32,36,38,42,46,48,54,55,57], university hospitals (16/41) [17,18,22,26,27,28,30,33,34,35,43,44,47,50,51,53], tertiary hospitals (8/41) [20,21,23,31,39,45,49,52] or unspecified (4/41) [37,40,41,56].

Table 1.
Study characteristics.
Overall, MDTM characteristics were similar across all included articles. In general, members of MDTMs include surgeons, (radiation) oncologists, radiologists, pathologists, residents and nurses. The frequency of MDTMs ranged from 4 times a week [21], to weekly [17,19,20,22,23,24,25,29,33,34,35,36,38,39,42,43,44,45,46,49,52,53,54,55] to biweekly [26,28,30,40,47,48]. Ten articles provided limited or no information on the frequency of MDTMs [18,27,31,32,37,41,50,51,56,57]. The outcomes measured in the articles were changes in or differences between: (1) process outcomes (9/41), (2) patient management (34/41) and (3) patient outcomes (18/41). Studies using a before–after study design (12/41) [17,23,24,26,28,32,34,35,36,44,46,55] or a case-control study design (13/41) [18,20,25,27,31,33,37,38,39,40,43,47,56] investigated a combination of all three outcome groups. When a cohort study design (16/41) [19,21,22,29,30,41,42,45,48,49,50,51,52,53,54,57] was used, most investigated patient management (13/16).
3.2. Risk of Bias and Quality of Evidence Assessment
With the use of the Robins-I tool, the risk of bias was rated critical in 22 studies (22/41) [17,18,19,20,21,22,23,25,26,27,30,31,32,33,34,35,42,44,46,49,50,53] and serious in 17 studies (17/41) [24,28,29,36,37,38,39,40,41,43,45,47,48,52,55,56,57]. Two studies showed no clear indication of serious or critical risk of bias but lacked information in one or more key domains of bias that hampered proper determination of overall risk of bias [51,54]. Based on the GRADE scale, the level of evidence had to be rated low in 2 studies [51,54], and very low in 39 studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,53,55,56,57] (Supplementary Figure S1).
Nine studies assessed the effect of MDTMs on process outcomes. Reported process outcomes included time to treatment, time to diagnosis, costs and other process outcomes (Table 2).

Table 2.
Results of process outcomes.
For colorectal cancer, two studies showed significantly increased length of time to surgery in the MDTM cohort [26,37]. Chinai et al., estimated the annual costs of colorectal MDTMs, including direct and overhead costs, at £162,734 [19].
For lung cancer, Boxer et al., found a significantly increased time to start chemotherapy with palliative intent, while time to all other treatments remained unchanged [38]. Freeman et al., however showed a significantly shorter time to treatment when patients were discussed in the MDTM. They also assessed the mean cost of care and found that mean costs reduced significantly in the MDTM group compared to the control group. Furthermore, they also showed a significantly increased adherence to national guidelines and research participation [40]. Muthukrishnan et al. found that the MDTM group showed a significantly longer time to complete staging, time from imaging to diagnosis, staging to therapy and imaging to therapy. However, for a subgroup with stage I–III, only the time from staging to therapy was significantly longer in the MDTM group [47].
Brandão et al. showed that time from diagnosis to treatment for breast cancer patients was not significantly different between control and MDTM groups. Furthermore, they confirmed the cost-effectiveness of MDTM implementation [55].
Two articles reporting on process outcomes did not perform statistical analysis [29,43].
3.3. Patient Management
Changes in Overall Management Plans
In total, fourteen studies investigated the effect of MDTMs on overall management plans (treatment and/or diagnostic procedures) in a cohort study design. These studies compared definitive MDTM plans to those determined prior to MDTM case evaluation (Table 3) [19,20,21,22,30,42,45,48,49,50,51,52,53,54]. Overall, these studies reported an effect on management plans that were changed in 1.6–58% of all cases. In colorectal cancer, MDTMs affected overall management in 6–29% of the cases, resulting in a weighted average change of 16.2% [19,20,21,22,30], whereas lung cancer MDTMs changed management plans in 53.2% on average, ranging between 53% and 58% [42,45]. In prostate cancer, MDTMs changed management plans in 27.1% on average, ranging from 1.6–43% [48,49,50,51,52,53]. Breast cancer was only reported in one study, showing a total change of 42.1% of the management plans [54].

Table 3.
Results for changes in management plans.
Nine studies investigated the type of changes. In six studies (all cancer types), researchers showed that treatment plans were changed more often (range 12.9–94.9%, mean 35.0%) than diagnostic plans (range 4–71.0%, mean 25.9%) [20,21,22,30,51,54]; in contrast, two studies (colorectal and lung cancer) showed the opposite effect [42,45]. One study on prostate cancer showed that changes in treatment plans occurred most often for patients with local disease, while changes in both diagnostic and treatment plans were more common in patients with advanced disease [49].
Six articles subdivided the whole cohort according to disease stage. For colorectal cancer (CRC) patients, one study showed that the percentage of management plans changed less often in newly diagnosed CRC cases (7.6%) compared to recurred CRC cases (16.4%) [21]. In prostate cancer, four articles reported changes in management plans subdivided according to cancer characteristics, presenting conflicting results. De Luca et al. showed that management plans changed more often in advanced (46.9%) and metastatic (33.4%) disease compared to local disease (23.2%) [49]. Similarly, Rao et al. showed changes in management plans in 23% of localized and 38% of metastatic prostate cancer cases [52]. El Khoury et al. and Kurpad et al., showed no trends when subdividing the patients according to Gleason score and disease stage, respectively [50,51]. Murthy et al., showed that changes were more common in early breast cancer patients (Stage 0–IIB: 8–27%) than in advanced patients (stage IIIA–IV: 0–7%) [54].
One article measured other outcomes in colorectal cancer patients, i.e., the type of MDT presentation (preoperative, follow-up, etc.) and whether the initial plan was tentative or definitive. Postoperative management plans (6.3%) changed significantly less often compared to initial (32.4%) or follow-up plans (35.2%). Tentative management plans (45.5%) changed significantly more than definitive plans (9.6%) [22].
3.4. Effect on Diagnostics and Treatment
Twenty-three studies investigated the effects of MDTMs on diagnostics and/or treatments using either a before–after or a case-control study design. In contrast to management plans, these studies focused on the diagnostics and/or treatments the patients actually received. These outcomes were not reported for prostate cancer.
3.4.1. Diagnostics
Six studies investigated the effect of MDTMs on diagnostics received by colorectal cancer patients, i.e., MRI, CT, ultrasound or colonoscopies (Table 4). Of these six studies, three measured MRI imaging, and all showed a significant increase in the MDTM cohort, compared to the control group [17,20,36]. CT imaging was shown to increase significantly by four studies [17,20,35,37], and one study showed no significant effect [36]. In addition, Fernando et al., showed that the use of chest CT significantly increased, while CT of the abdomen did not change significantly in the MDTM group [20]. Three studies found no significant difference in ultrasound imaging [17,20,36]. Of the four studies investigating colonoscopies, three showed no significant difference between MDTM and control groups [20,28,36].

Table 4.
Results for diagnostics, treatment and palliative care.
Two studies did not perform statistical analyses on (all) of these outcomes [17,27].
3.4.2. Surgery
Fourteen studies investigated the preferred surgical type and whether surgery was performed (Table 4).
In colorectal cancer, all studies reporting on resection of primary tumors indicated a reduction in the MDTM cohort [23,37], and all studies reporting on surgical type showed a significant effect of MDTMs on the preferred surgical type [27,28,33]. Foucan et al., showed that colorectal cancer patients with advanced disease received different treatments depending on the MDTM status, unlike patients with early-stage disease. Stage III and IV patients without MDTM discussion most often received surgery alone, whereas patients in the MDTM group received mostly surgery followed by chemotherapy [37]. In lung cancer, Boxer et al., showed no significant effect on the number of performed surgeries [38], however Freeman et al., showed a significant reduction in (non-therapeutic) surgical procedures [40]. Tamburini et al., showed a significant effect on preferred surgical type [44]. For breast cancer, no significant increases in surgery or surgery type were identified [55].
3.4.3. Radiotherapy, Chemotherapy and Palliative Care
Twelve studies investigated the effects of MDTMs on how many patients received chemotherapy, radiotherapy and palliative care (Table 4).
In colorectal cancer, Lan et al., showed a significant increase in the use of radiotherapy in the MDTM cohort [23], while MacDermid et al., showed no significant effect of MDTMs [24]. Similarly, in lung cancer, Boxer et al., showed a significant increase in the use of radiotherapy in the MDTM cohort [38], while Bydder et al., showed no significant effect of MDTMs [39]. Brandão et al., found no significant changes in radiotherapy for breast cancer patients [55] (Table 4).
According to Lan et al., the number of colorectal cancer patients who received chemotherapy was significantly higher in the MDTM cohort [23]. However, Ye et al., showed a significant decrease in overall cohort and stages I and IIA colorectal cancer, while stages IIB–IV showed no significant differences between the cohorts [35]. In lung cancer, Boxer et al., showed a significant increase of chemotherapy in the MDTM cohort [38], however Bydder et al., found no significant difference [39] (Table 4). In breast cancer, Brandão et al., showed that MDTM implementation did not lead to significant changes in the use of (neoadjuvant) chemotherapy or endocrine therapy [55].
Lan et al., showed a significant increase in the number of colorectal cancer patients in MDTM cohorts who received palliative chemotherapy, however MacDermid et al., showed no significant difference [23,24]. In lung cancer, two studies also showed a significant increase in palliative care [38,40], however three additional studies showed no significant difference between MDTM cohorts and control groups [39,43] (Table 4).
Finally, Tsai et al., found no significant differences between MDTM and control groups in any treatment combinations for breast cancer patients [56].
Muthukrishnan et al., provided no statistical analysis on these specific outcomes [47].
3.4.4. Patient Outcomes
Eighteen studies reported on patient outcomes, i.e., survival, recurrence or metastasis, mortality and other patient-related outcomes (Table 5). These outcomes were not measured for prostate cancer.

Table 5.
Results of patient outcomes.
Six out of eight studies that reported on survival showed a significant increase in survival of colorectal cancer patients that were discussed in MDTMs [18,23,24,25,35,37]. Of which, three studies only showed a significant effect of MDTMs on specific subgroups [24,25,37]. MacDermid et al., showed a significant increase in survival of patients with Dukes C disease while remaining unchanged for patients with Dukes B disease [24]. Similarly, Munro et al., showed that 5-year cause-specific survival for advanced cases increased significantly with MDTMs, while it did not change for early disease cases [25]. Foucan et al., showed a significantly increased survival duration after diagnosis, but not after surgery [37]. One out of eight studies reporting on survival did not show significant effects of MDTMs in colorectal cancer pathways [34], while one other study did not perform statistical analysis on the survival outcomes [27].
Besides survival, other patient-related outcomes were measured. Swellengrebel et al., showed no significant effect on resection margin rates [31]. Wille-Jørgensen et al. and Lan et al., showed that postoperative mortality decreased significantly after implementation of MDTMs in colorectal cancer [23,34], while recurrence and metastasis rates were not significantly affected [34]. Ye et al., showed a significantly lower tumor recurrence and longer time to recurrence for colorectal cancer patients who were discussed during MDTMs [35]. Chen et al. and Munro et al., both identified a significantly lower hazard ratio (HR) of death in the MDTM groups [18,25].
All five studies reporting on survival of lung cancer patients showed a significant improvement in survival of patients discussed during MDTMs [39,41,43,44,46]. Pan et al., showed a significantly lower adjusted hazard ratio (HR) of death in patients with stage III and IV non-small cell lung cancer that were discussed in MDTMs [41]. In particular, Hung et al., showed a prolonged length of survival for stage III lung cancer patients [46]. Quality of surgery was measured by Tamburini et al. and showed no significant differences between non-MDTM and MDTM groups, while overall mortality was significantly decreased [44].
In total, three studies reported on patient outcomes for breast cancer, of which all found a higher overall survival rate in the MDTM group compared to the control group [55,56,57]. Brandão et al., found a significantly higher survival rate in early breast cancer (Stage 0–III), while MDTM discussion in patients with metastatic breast cancer did not lead to a survival benefit. Furthermore, no significant increases in the proportion of clean surgical margins and complete axillary surgery were identified [55]. Brandão et al., found no significant differences in recurrence rate, while Tsai et al., showed a significant decrease in the MDTM group [55,56]. Yang et al., showed that survival was significantly higher in patients compliant with MDTM recommendations compared to the non-compliant group [57].
4. Discussion
4.1. Summary of Evidence
We systematically reviewed scientific literature and identified the impact MDTMs can have on colorectal, lung, prostate and breast cancer care. Overall, results showed that the implementation of MDTMs can have a significant impact on treatment decisions, patient outcomes and process outcomes (Table 6). However, all studies showed a low to very low quality of evidence and a critical or serious risk of bias. While our review suggests benefits of MDTMs in colorectal, lung, prostate and breast cancer care, there is need for more high-quality research.

Table 6.
Summary of main outcomes per cancer type.
Studies reporting on process outcomes such as cost- or time-related components are limited. Results suggest that MDTMs can have an effect on these process outcomes, however due to the limited evidence, no solid conclusions can be drawn. The systematic review by Ke et al., suggested that the investments in MDTMs are justified, but similar to our findings, Ke et al., also stated that there is a need for more rigorous studies on cost-effectiveness [58]. Some of the studies suggested that effects of MDTMs on management are less in early-stage (or non-recurrent) disease, which will typically constitute cases where the recommended treatment is more standardized and benefits of MDTM discussion may be limited [21,24,37,49,52]. This suggests a focus of future research on the cost-effectiveness of specific patient subgroups, i.e., early and advanced disease.
Results indicated that the impact on changes in management plans are different per cancer type and per hospital type (e.g., general hospital, university hospital). Overall, MDTM discussion resulted in more changes in lung cancer management plans (53–58%), compared to colorectal (6–29%), prostate (1.6–43%) and breast cancer (42.1%). This might be explained by the following aspects of lung cancer care. Typically, guidelines for lung cancer are less comprehensive and more frequently updated compared to, for example, prostate and breast cancer. In addition, management plans must be comprised in a short timespan due to the high mortality of lung cancer [59]. Of the 14 studies that reported on changes in management plans, 5 were performed in a university hospital, 5 were performed a tertiary referral cancer center and 4 in a general hospital. In all cancer types, general hospitals had the lowest percentage of changes in management plans, suggesting less impact of MDTMs on management plans in general hospitals. The latter might be the result of different case mix between general hospitals and tertiary referral centers or university hospitals. In addition, clinical trials might offer more diagnostic and therapy opportunities to be considered in university hospitals. Furthermore, due to the researchers’ affiliations with teaching and academic hospitals, more research is conducted there instead of general hospitals [1].
Studies that reported on changes in management plans generally showed a high risk of bias in measurement of outcomes, for several reasons. First, physicians who formulated the management plans prior to MDTMs often attended the meeting, and potentially affected the final recommendation with their opinion. Second, the final MDTM recommendation might also be influenced by knowledge of the initial plans, even when the physician who developed the initial plan did not attend the MDTM. Third, in some studies, the physicians who formulated the initial MDTM plans were also the outcome assessors that subjectively evaluated the changes made during the MDTM. A few studies minimized this outcome bias by blinding the MDTM to the initial plans or had an independent physician draw up the initial plans [29,45].
Studies that compared MDTM groups to (historical) control groups in terms of the number of patients that received certain types of diagnostics and/or treatment were focused on colorectal, lung and breast cancer patients. These outcomes were not reported for prostate cancer. All papers reporting on number of MRI scans and most papers on CT scans showed a significant increase in the MDTM cohort, suggesting more accurate staging. The systematic review of Pillay et al., showed similar outcomes, and also concluded that patients discussed at an MDTM were more likely to receive appropriate staging [7]. The results suggest that MDTM discussion often affects the treatment that patients received, typically less so for surgery, and different surgical types. Radiotherapy, chemotherapy and palliative care were chosen equally or more often. However, it is uncertain whether these trends are completely the result of MDTM discussion. Studies that reported on the impact of MDTMs on treatment patients received often compared the MDTM cohort with a control group over a long time period. For example, Lan et al., measured from 2001 to 2010, while MDTM was introduced in 2007. Therefore, differences in MDTM and control groups might also be affected by changes in techniques, guidelines and clinical practice. Lan et al., stated that there were significant differences in many aspects of the diagnosis and treatment during their measurement time, i.e., the introduction of targeted therapy. Thus, the impact of MDTMs might be overestimated.
Most studies investigating the effects of MDTMs on survival showed a significantly improved survival rate for colorectal, lung and breast cancer patients. A few studies identified the effects of MDTMs in certain patient subgroups based on disease stage and/or treatment combination [18,24,25,41,55,57]. Often, the overall survival rate was significantly improved, while some subgroups did not show significant differences in survival. Similar to the improved staging mentioned earlier, the improved survival might be partially explained by improvements in techniques, guidelines and clinical practice during the long measurement time of the studies.
Overall, most of the results in this systematic review are in agreement with previously published work. Results showed evidence for improved survival for colorectal, lung and breast cancer. Changes in clinical diagnostic and treatment decision making for colorectal, prostate and breast cancer was identified but rated as weak [7,8,60,61,62]. The reported patient and process outcomes in this systematic review were investigated in colorectal, lung and breast cancer, and not in prostate cancer. The review of Holmes et al., also concluded that the number of articles that studied the effect of MDTMs in prostate cancer is limited. Similar to our findings, Holmes et al., did encounter many abstracts, suggesting potential future publications on the topic [63]. For breast cancer, Blackwood et al., also showed evidence that an MDT approach is associated with improved clinical outcomes, however they did not report on the effect of MDTMs in particular [64].
4.2. Strengths and Limitations
Our systematic review has several strengths. Two independent researchers screened over 13,000 articles in a title–abstract screening and 165 articles in full-text, following the PRISMA-P 2015 and the Cochrane Collaboration’s double-date collection and extraction methodology [9,10]. We are the first to specifically evaluate and compare the effect of MDTMs on colorectal, lung, prostate and breast cancer care.
Our review also has some limitations. First, we acknowledge that selective reporting and publication bias cannot be ruled out. During the screening process, 27 abstracts met the inclusion criteria but were excluded due to a lack of information. In most cases, these abstracts did not result in a published paper, which might indicate a publication bias. Second, during the screening process, MDTMs might be misclassified as ‘multidisciplinary (MDT) approach’ due to limited or unclear information and the lack of a consistent definition for multidisciplinary team meetings [61,65]. Articles reporting the effect of ‘MDT approach’ were excluded, because ‘MDT approach’ is a broad term for collaboration between medical specialists, that may or may not include MDTMs. Subsequently, we cannot rule out exclusion of misclassified MDTMs. Third, general observations on MDTMs are limited due to small numbers of articles, inadequate statistical analysis and heterogeneity of patient and process outcomes. Finally, all studies included in this review had an observational study design. According to the GRADE scale, observational studies without special strengths or important limitations provide low quality of evidence. Therefore, the level of evidence of the included studies had to be rated low to very low. In contrast, an RCT without important limitations provides high-quality evidence according to the GRADE scale. However, MDTMs are often considered mandatory, and denying patients an MDTM discussion might be considered unethical. Therefore, an RCT might not be feasible in the evaluation of the effect of MDTMs. In conclusion, before discarding the low level of evidence, the level of evidence might never be higher than this.
4.3. Future Research
An unequal distribution of cancer types was identified in the literature with regard to the effects of MDTMs on treatment decisions, patient outcomes and process outcomes. As a result, current evidence of the potential benefits of MDTMs differs per cancer type, yet none of the evidence presented in the included studies is strong. In particular, current literature lacks studies that reported on the effect of MDTMs on patient outcomes and process outcomes for prostate cancer. Overall, high-quality research is required for all cancer types to confirm the potential benefit of MDTMs, preferably in a multicenter study with appropriate statistical analysis (e.g., a power calculation).
Another valuable focus for future research might be to investigate whether all patients should be discussed at MDTMs. Most studies showed that the majority of management plans did not change after MDTM discussion. Several of the included studies showed that MDTMs only had a significant effect on a specific subset of cancer patients, typically advanced cases. MDTMs are considered time-consuming and expensive, however these statements are mostly based on physicians/clinical experience. The increasing incidence of colorectal, lung, prostate and breast cancer might further pressure effort, time and financial resources. In this context, it might be important to re-evaluate the recommendations to discuss every patient in MDTMs and to focus on the cost-effectiveness of MDTMs. Cost-effectiveness studies on all four cancer types could be beneficial. In order to better understand the impact of MDTMs in addition to the clinical outcomes, cost-effectiveness studies would be essential, allowing for a critical evaluation of the effectiveness of MDTMs, based on clinical and process outcomes.
5. Conclusions
The number of studies that evaluated the effect of MDTMs is sparse, especially for lung, prostate and breast cancer compared to colorectal cancer. The reported evidence suggests that the implementation of MDTMs can have a significant impact on treatment decisions for colorectal, lung, prostate and breast cancer. In colorectal cancer, there is weak evidence that MDTMs result in more accurate staging. There is weak evidence that MDTMs improve patient outcomes (e.g., survival) for colorectal, lung and breast cancer patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13164159/s1, Table S1: Database search string; Table S2: Search results per database; Figure S1: Risk of bias and quality of care.
Author Contributions
L.K.: Conceptualization, Methodology, Investigation, Writing—Original Draft, Project administration; H.H.A.W.: Conceptualization, Methodology, Investigation, Writing—Original Draft, Project administration; D.M.J.L.: Writing—Review and Editing; J.P.M.S.: Writing—Review and Editing; M.P.: Writing—Review and Editing; J.J.F.: Writing—Review and Editing, Conceptualization, Supervision; R.M.M.: Writing—Review and Editing, Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by Siemens Healthineers, Erlangen, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
The authors would like to thank all experts who provided input for our systematic review. Special thanks to OnYing Chan for her support in the development of our search strategy.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lamb, B.W.; Brown, K.F.; Nagpal, K.; Vincent, C.; Green, J.S.; Sevdalis, N. Quality of care management decisions by multidisciplinary cancer teams: A systematic review. Ann. Surg. Oncol. 2011, 18, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- El Saghir, N.S.; Keating, N.L.; Carlson, R.W.; Khoury, K.E.; Fallowfield, L. Tumor boards: Optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e461–e466. [Google Scholar] [CrossRef]
- Calman, K.; Hine, D. Report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales. In A Policy Framework for Comissioning Cancer Services (The Calman-Hine Report); Department of Health: London, UK, 1995. [Google Scholar]
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Globocan. Cancer Today—International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysispie?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0&population_group_globocan_id= (accessed on 7 December 2020).
- Basta, Y.L.; Bolle, S.; Fockens, P.; Tytgat, K.M. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: A systematic review. Ann. Surg. Oncol. 2017, 24, 2669–2678. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.; Remue, E.; Van Hoof, E.; Borras, J.M. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy 2015, 119, 464–474. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Koco, L.; Weekenstroo, H. The effects of implementing multidisciplinary team meetings in breast, prostate and lung cancer pathways: A systematic review. 2019. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=127476 (accessed on 7 December 2020).
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. JMLA 2016, 104, 240. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P. Assessing risk of bias in a non-randomized study. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 621–641. [Google Scholar]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Anania, G.; Resta, G.; Marino, S.; Fabbri, N.; Scagliarini, L.; Marchitelli, I.; Fiorica, F.; Cavallesco, G. Treatment of colorectal cancer: A multidisciplinary approach. J. Gastrointest. Cancer 2019, 50, 458–468. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsieh, M.C.; Lao, W.T.; Lin, E.K.; Lu, Y.J.; Wu, S.Y. Multidisciplinary team intervention associated with improved survival for patients with colorectal adenocarcinoma with liver or lung metastasis. Am. J. Cancer Res. 2018, 8, 1887. [Google Scholar]
- Chinai, N.; Bintcliffe, F.; Armstrong, E.; Teape, J.; Jones, B.; Hosie, K. Does every patient need to be discussed at a multidisciplinary team meeting? Clin. Radiol. 2013, 68, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Fernando, C.; Frizelle, F.; Wakeman, C.; Frampton, C.; Robinson, B. Colorectal multidisciplinary meeting audit to determine patient benefit. ANZ J. Surg. 2017, 87, E173–E177. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Hong, Y.S.; Kim, T.W.; Park, J.H.; Kim, J.H.; Park, S.H.; Kim, A.Y.; Lim, S.B.; Lee, Y.J.; Yu, C.S. Impact of a multidisciplinary team approach for managing advanced and recurrent colorectal cancer. World J. Surg. 2018, 42, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Karagkounis, G.; Stocchi, L.; Lavery, I.C.; Liska, D.; Gorgun, E.; Veniero, J.; Plesec, T.; Amarnath, S.; Khorana, A.A.; Kalady, M.F. Multidisciplinary conference and clinical management of rectal cancer. J. Am. Coll. Surg. 2018, 226, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.T.; Jiang, J.K.; Chang, S.C.; Yang, S.H.; Lin, C.C.; Lin, H.H.; Wang, H.S.; Chen, W.S.; Lin, T.C.; Lin, J.K. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int. J. Colorectal Dis. 2016, 31, 403–411. [Google Scholar] [CrossRef]
- MacDermid, E.; Hooton, G.; MacDonald, M.; McKay, G.; Grose, D.; Mohammed, N.; Porteous, C. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009, 11, 291–295. [Google Scholar] [CrossRef]
- Munro, A.; Brown, M.; Niblock, P.; Steele, R.; Carey, F. Do Multidisciplinary Team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience. BMC Cancer 2015, 15, 686. [Google Scholar] [CrossRef]
- Nikolovski, Z.; Watters, D.A.; Stupart, D.; Guest, G.D. Colorectal multidisciplinary meetings: How do they affect the timeliness of treatment? ANZ J. Surg. 2017, 87, E112–E115. [Google Scholar] [CrossRef]
- Palmer, G.; Martling, A.; Cedermark, B.; Holm, T. Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Colorectal Dis. 2011, 13, 1361–1369. [Google Scholar] [CrossRef]
- Richardson, B.; Preskitt, J.; Lichliter, W.; Peschka, S.; Carmack, S.; de Prisco, G.; Fleshman, J. The effect of multidisciplinary teams for rectal cancer on delivery of care and patient outcome: Has the use of multidisciplinary teams for rectal cancer affected the utilization of available resources, proportion of patients meeting the standard of care, and does this translate into changes in patient outcome? Am. J. Surg. 2016, 211, 46–52. [Google Scholar] [CrossRef]
- Ryan, J.; Faragher, I. Not all patients need to be discussed in a colorectal cancer MDT meeting. Colorectal Dis. 2014, 16, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, R.C.; Subendran, J.; Jhaveri, K.; Thipphavong, S.; Cummings, B.; Brierley, J.; Kirsch, R.; Kennedy, E.D. Effect of multidisciplinary cancer conference on treatment plan for patients with primary rectal cancer. Dis. Colon Rectum 2015, 58, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Swellengrebel, H.; Peters, E.; Cats, A.; Visser, O.; Blaauwgeers, H.; Verwaal, V.; van Velthuysen, M.; Cense, H.; Bruin, S.; Marijnen, C. Multidisciplinary discussion and management of rectal cancer: A population-based study. World J. Surg. 2011, 35, 2125–2133. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.; Wheeler, J.; Borley, N. The impact of a dedicated multidisciplinary team on the management of early rectal cancer. Colorectal Dis. 2015, 17, 704–709. [Google Scholar] [CrossRef]
- Wanis, K.N.; Pineda-Solis, K.; Tun-Abraham, M.E.; Yeoman, J.; Welch, S.; Vogt, K.; Van Koughnett, J.A.M.; Ott, M.; Hernandez-Alejandro, R. Management of colorectal cancer with synchronous liver metastases: Impact of multidisciplinary case conference review. Hepatobiliary Surg. Nutr. 2017, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Wille-Jørgensen, P.; Sparre, P.; Glenthøj, A.; Holck, S.; Nørgaard Petersen, L.; Harling, H.; Stub Højen, H.; Bülow, S. Result of the implementation of multidisciplinary teams in rectal cancer. Colorectal Dis. 2013, 15, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.J.; Shen, Z.L.; Sun, X.T.; Wang, Z.F.; Shen, D.H.; Liu, H.J.; Zhang, W.L.; Chen, Y.L.; Jing, Z.; Poston, J. Impact of multidisciplinary team working on the management of colorectal cancer. Chin. Med. J. 2012, 125, 172–177. [Google Scholar] [CrossRef]
- Maurizi, A.C.R. Improved Utilization of Resources as an Improvement of Outcome: The Effect of Multidisciplinary Team for Rectal Cancer in a District Hospital. Clin. Oncol. 2017, 2, 1267. [Google Scholar]
- Foucan, A.S.; Grosclaude, P.; Bousser, V.; Bauvin, E.; Smith, D.; Andre-Fardeau, C.; Daubisse-Marliac, L.; Mathoulin-Pelissier, S.; Amadeo, B.; Coureau, G. Management of colon cancer patients: A comprehensive analysis of the absence of multidisciplinary team meetings in two French departments. Clin. Res. Hepatol. Gastroenterol. 2020, 45, 101413. [Google Scholar] [CrossRef]
- Boxer, M.M.; Vinod, S.K.; Shafiq, J.; Duggan, K.J. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer 2011, 117, 5112–5120. [Google Scholar] [CrossRef]
- Bydder, S.; Nowak, A.; Marion, K.; Phillips, M.; Atun, R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern. Med. J. 2009, 39, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.K.; Ascioti, A.J.; Dake, M.; Mahidhara, R.S. The effects of a multidisciplinary care conference on the quality and cost of care for lung cancer patients. Ann. Thorac. Surg. 2015, 100, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Kung, P.T.; Wang, Y.H.; Chang, Y.C.; Wang, S.T.; Tsai, W.C. Effects of multidisciplinary team care on the survival of patients with different stages of non-small cell lung cancer: A national cohort study. PLoS ONE 2015, 10, e0126547. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.M.; Roberts, J.M.; Bodnar, A.M.; Kunz, S.; Kirtland, S.H.; Koehler, R.P.; Hubka, M.; Low, D.E. Thoracic multidisciplinary tumor board routinely impacts therapeutic plans in patients with lung and esophageal cancer: A prospective cohort study. Ann. Thorac. Surg. 2015, 99, 1719–1724. [Google Scholar] [CrossRef]
- Stone, E.; Rankin, N.; Kerr, S.; Fong, K.; Currow, D.C.; Phillips, J.; Connon, T.; Zhang, L.; Shaw, T. Does presentation at multidisciplinary team meetings improve lung cancer survival? Findings from a consecutive cohort study. Lung Cancer 2018, 124, 199–204. [Google Scholar] [CrossRef]
- Tamburini, N.; Maniscalco, P.; Mazzara, S.; Maietti, E.; Santini, A.; Calia, N.; Stefanelli, A.; Frassoldati, A.; Santi, I.; Rinaldi, R. Multidisciplinary management improves survival at 1 year after surgical treatment for non-small-cell lung cancer: A propensity score-matched study. Eur. J. Cardio Thorac. Surg. 2018, 53, 1199–1204. [Google Scholar] [CrossRef]
- Ung, K.A.; Campbell, B.A.; Duplan, D.; Ball, D.; David, S. Impact of the lung oncology multidisciplinary team meetings on the management of patients with cancer. Asia Pac. J. Clin. Oncol. 2016, 12, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Tseng, Y.H.; Chao, H.S.; Chiu, C.H.; Hsu, W.H.; Hsu, H.S.; Wu, Y.C.; Chou, T.Y.; Chen, C.K.; Lan, K.L. Multidisciplinary team discussion results in survival benefit for patients with stage III non-small-cell lung cancer. PLoS ONE 2020, 15, e0236503. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, P.T.; Ratnam, M.; Nguyen, M.T.; Le, M.; Gunzler, D.; Bruno, D.; Infeld, M. Pre-diagnosis Multidisciplinary Tumor Board and Time to Staging in Lung Cancer: The Case Western MetroHealth Experience. Cureus 2020, 12, e6595. [Google Scholar] [CrossRef] [PubMed]
- Acher, P.L.; Young, A.J.; Etherington-Foy, R.; McCahy, P.J.; Deane, A.M. Improving outcomes in urological cancers: The impact of “multidisciplinary team meetings”. Int. J. Surg. 2005, 3, 121–123. [Google Scholar] [CrossRef][Green Version]
- De Luca, S.; Fiori, C.; Tucci, M.; Poggio, M.; Allis, S.; Bollito, E.; Solitro, F.; Passera, R.; Buttigliero, C.; Porpiglia, F. Prostate cancer management at an Italian tertiary referral center: Does multidisciplinary team meeting influence diagnostic and therapeutic decision-making process? A snapshot of the everyday clinical practice. Ital. J. Urol. Nephrol. 2019, 71, 576–582. [Google Scholar] [CrossRef]
- El Khoury, R.; Chahrouri, M.; Hachem, C.; Abi Zeid, J.; El Alam, P.; Abdessater, M. Evaluation of Multidisciplinary Team Meetings in Uro-Oncology. Leban. Med. J. 2016, 64, 84–90. [Google Scholar] [CrossRef]
- Kurpad, R.; Kim, W.; Rathmell, W.K.; Godley, P.; Whang, Y.; Fielding, J.; Smith, L.; Pettiford, A.; Schultz, H.; Nielsen, M. A multidisciplinary approach to the management of urologic malignancies: Does it influence diagnostic and treatment decisions? Urol. Oncol. Semin. Orig. Investig. 2011, 29, 378–382. [Google Scholar] [CrossRef]
- Rao, K.; Manya, K.; Azad, A.; Lawrentschuk, N.; Bolton, D.; Davis, I.D.; Sengupta, S. Uro-oncology multidisciplinary meetings at an Australian tertiary referral centre–impact on clinical decision-making and implications for patient inclusion. BJU Int. 2014, 114, 50–54. [Google Scholar] [CrossRef]
- Scarberry, K.; Ponsky, L.; Cherullo, E.; Larchian, W.; Bodner, D.; Cooney, M.; Ellis, R.; MacLennan, G.; Johnson, B.; Tabayoyong, W. Evaluating the impact of the genitourinary multidisciplinary tumour board: Should every cancer patient be discussed as standard of care? Can. Urol. Assoc. J. 2018, 12, E403. [Google Scholar] [CrossRef]
- Murthy, V.; Nobre, S.; Sparber, L.; Schaefer, S.; Santoro, E.; McDermott, J.; Chamberlain, R.; Blackwood, M. Multidisciplinary breast conference improves patient management and treatment. Surg. Sci. 2014, 5, 314–319. [Google Scholar] [CrossRef]
- Brandão, M.; Guisseve, A.; Bata, G.; Firmino-Machado, J.; Alberto, M.; Ferro, J.; Garcia, C.; Zaqueu, C.; Jamisse, A.; Lorenzoni, C. Survival impact and cost-effectiveness of a multidisciplinary tumor board for breast cancer in Mozambique, Sub-Saharan Africa. Oncologist 2020, 26, e996–e1008. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hsieh, H.F.; Lai, T.W.; Kung, P.T.; Kuo, W.Y.; Tsai, W.C. Effect of multidisciplinary team care on the risk of recurrence in breast cancer patients: A national matched cohort study. Breast 2020, 53, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, J.; Zhu, X.; Shen, K.; Zhu, J.; Chen, X. Compliance with multidisciplinary team recommendations and disease outcomes in early breast cancer patients: An analysis of 4501 consecutive patients. Breast 2020, 52, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.M.; Blazeby, J.M.; Strong, S.; Carroll, F.E.; Ness, A.R.; Hollingworth, W. Are multidisciplinary teams in secondary care cost-effective? A systematic review of the literature. Cost Eff. Resour. Alloc. 2013, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.M.; Silverstein, S.C.; Quinn, M.; Waterston, L.B.; Thomas, C.A.; Benneyan, J.C.; Han, P.K.J. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017, 112, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Coory, M.; Gkolia, P.; Yang, I.A.; Bowman, R.V.; Fong, K.M. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer 2008, 60, 14–21. [Google Scholar] [CrossRef]
- Hong, N.J.; Wright, F.C.; Gagliardi, A.R.; Paszat, L.F. Examining the potential relationship between multidisciplinary cancer care and patient survival: An international literature review. J. Surg. Oncol. 2010, 102, 125–134. [Google Scholar] [CrossRef]
- Ioannidis, A.; Konstantinidis, M.; Apostolakis, S.; Koutserimpas, C.; Machairas, N.; Konstantinidis, K.M. Impact of multidisciplinary tumor boards on patients with rectal cancer. Mol. Clin. Oncol. 2018, 9, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Kelly, B.D.; Perera, M.; Eapen, R.S.; Bolton, D.M.; Lawrentschuk, N. A systematic scoping review of multidisciplinary cancer team and decision-making in the management of men with advanced prostate cancer. World J. Urol. 2020, 39, 297–306. [Google Scholar] [CrossRef]
- Blackwood, O.; Deb, R. Multidisciplinary team approach in breast cancer care: Benefits and challenges. Indian J. Pathol. Microbiol. 2020, 63, 105–112. [Google Scholar] [CrossRef]
- Shao, J.; Rodrigues, M.; Corter, A.L.; Baxter, N.N. Multidisciplinary care of breast cancer patients: A scoping review of multidisciplinary styles, processes, and outcomes. Curr. Oncol. 2019, 26, e385–e397. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).