Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Survival
3.2. Cox Proportional Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. CMAJ 2020, 192, E199–E205. [Google Scholar] [CrossRef] [Green Version]
- Parise, C.A.; Bauer, K.R.; Brown, M.M.; Caggiano, V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784. [Google Scholar] [CrossRef] [Green Version]
- Female Breast Cancer Subtypes—Cancer Stat Facts [Internet]. SEER. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 28 December 2020).
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann. Oncol. 2017, 28, 16–33. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541. [Google Scholar] [CrossRef]
- Ciruelos, E.; Pascual, T.; Vozmediano, M.L.A.; Blanco, M.; Manso, L.; Parrilla, L.; Muñoz, C.; Vega, E.; Calderón, M.J.; Sancho, B.; et al. The therapeutic role of fulvestrant in the management of patients with hormone receptor positive breast cancer. Breast 2014, 23, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Robertson, J.F.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997. [Google Scholar] [CrossRef]
- Chia, S.; Gradishar, W.; Mauriac, L.; Bines, J.; Amant, F.; Federico, M.; Fein, L.; Romieu, G.; Buzdar, A.; Robertson, J.F.; et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: Results from EFECT. J. Clin. Oncol. 2008, 26, 1664. [Google Scholar] [CrossRef]
- Andrahennadi, S.; Sami, A.; Manna, M.; Pauls, M.; Ahmed, S. Current Landscape of Targeted Therapy in Hormone Receptor-Positive and HER2-Negative Breast Cancer. Curr. Oncol. 2021, 28, 1803–1822. [Google Scholar] [CrossRef]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Final overall survival: Fulvestrant 500 mg vs. 250 mg in the randomized CONFIRM trial. J. Natl. Cancer Inst. 2014, 106, djt337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2010, 28, 4594–4600. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Masuda, N.; Nakayama, T.; Aogi, K.; Anan, K.; Ito, Y.; Ohtani, S.; Sato, N.; Saji, S.; Tokunaga, E.; et al. Outcomes of fulvestrant therapy among japanese women with advanced breast cancer: A retrospective multicenter cohort study (JBCRG-C06; Safari). Breast Cancer Res. Treat. 2017, 163, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Moscetti, L.; Fabbri, M.A.; Natoli, C.; Vici, P.; Gamucci, T.; Sperduti, I.; Iezzi, L.; Iattoni, E.; Pizzuti, L.; Roma, C.; et al. Fulvestrant 500 milligrams as endocrine therapy for endocrine sensitive advanced breast cancer patients in the real world: The Ful500 prospective observational trial. Oncotarget 2017, 8, 54528–54536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, R.; Sottotetti, F.; Quaquarini, E.; Gambaro, A.; Ferzi, A.; Tagliaferri, B.; Teragni, C.; Licata, L.; Serra, F.; Lapidari, P.; et al. Patterns of treatment and outcome with 500-mg fulvestrant in postmenopausal women with hormone receptor-positive/HER2-negative metastatic breast cancer: A real-life multicenter Italian experience. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833864. [Google Scholar]
- Ozawa, H.; Sata, A.; Fukui, R.; Bun, A.; Higuchi, T.; Fujimoto, Y.; Miyagawa, Y.; Imamura, M.; Miyoshi, Y. A Single-centre, Retrospective, Observational Analysis of Fulvestrant for Recurrent/metastatic Breast Cancer According to Metastatic Site. Anticancer Res. 2019, 39, 5653–5662. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Li, H.; Song, G.; Zhang, R.; Ran, R.; Yan, Y.; Di, L.; Jiang, H. Efficacy and Safety of Fulvestrant 500 mg in Hormone-receptor Positive Human Epidermal Receptor 2 Negative Advanced Breast Cancer: A Real-world Study in China. J. Cancer 2020, 11, 6612–6622. [Google Scholar] [CrossRef]
- Skinner, K.E.; Olufade, T.; Walker, M.S.; Schwartzberg, L.S. Real-world effectiveness of fulvestrant monotherapy as first endocrine treatment in patients with metastatic breast cancer. Breast J. 2020, 26, 112–119. [Google Scholar] [CrossRef] [PubMed]
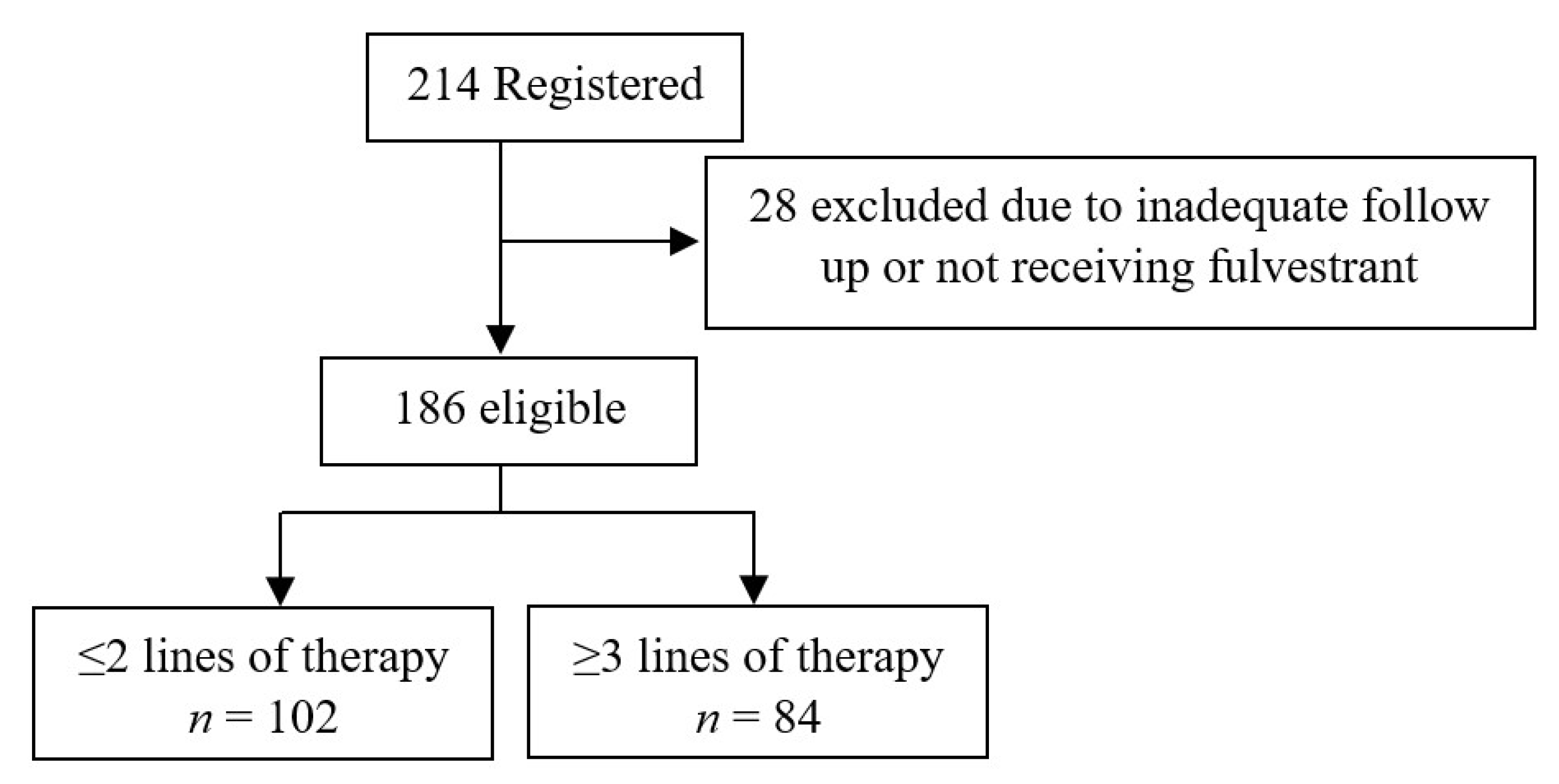

| Variables | Study Cohort n = 186 (%) | ≤2 Lines of Therapy n = 102 (54.8%) (%) | ≥3 Lines of Therapy n = 84 (45.2%) (%) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Median age | 63.5 (IQR: 54.0–74.0) | 67 (56.7–77.3) | 60 (51.0–68.0) | 0.001 |
| >50 years | 157 (84.4) | 93 (91.2) | 64 (76.2) | 0.007 |
| Rural residence | 94 (50.5) | 53 (52) | 41 (48.8) | 0.76 |
| Comorbid illness | 94 (50.5) | 58 (57) | 36 (42.8) | 0.24 |
| Secondary cancer | 22 (11.8) | 14 (13.7) | 8 (9.5) | 0.49 |
| WHO performance status 0–1 | 151 (81.2) | 82 (80.3) | 69 (82.1) | 0.85 |
| Smoking History | 69 (37.1) | 44 (43.1) | 25 (29.8) | 0.21 |
| History of early-stage breast cancer | 150 (80.6) | 85 (83.3) | 65 (77.4) | 0.35 |
| Bilateral breast cancer | 12 (8.0) | 10 (11.7) | 2 (3.0) | 0.12 |
| Surgery for early-stage breast cancer | ||||
| Mastectomy | 94 (62.6) | 53 (62.3) | 41 (63.0) | 0.37 |
| Lumpectomy | 36 (24.0) | 19 (22.3) | 17 (26.1) | 0.70 |
| Bilateral mastectomy | 20 (13.3) | 13 (15.3) | 7 (10.7) | 0.33 |
| Stage of early-breast cancer | ||||
| I | 35 (23.3) | 18 (21.1) | 17 (26.1) | 0.55 |
| II | 71 (47.3) | 40 (47.0) | 31 (47.8) | 1.0 |
| III | 44 (29.3) | 27 (31.7) | 17 (26.1) | 0.47 |
| Received Adjuvant therapy | 134 (89.3) | 75 (88.2) | 59 (90.7) | 0.79 |
| Adjuvant chemotherapy | 81 (54.0) | 44 (51.7) | 37 (56.9) | 0.62 |
| Adjuvant endocrine therapy | 110 (73.3) | 67 (78.8) | 43 (66.2) | 0.09 |
| Adjuvant radiation therapy | 96 (64.0) | 53 (62.4) | 43 (66.2) | 0.73 |
| Diagnosis of metastatic disease | ||||
| Clinical | 39 (21.0) | 30 (29.4) | 9 (10.7) | 0.002 |
| Pathological | 147 (79.0) | 72 (70.6) | 75 (89.3) | 0.004 |
| Receptor status | ||||
| Estrogen receptor positive | 183 (98.4) | 100 (98.0) | 83 (98.8) | 1.0 |
| Progesterone receptor positive | 152 (81.7) | 84 (82.4) | 68 (81.0) | 0.85 |
| HER2 overexpression | 17 (9.1) | 7 (6.9) | 10 (12.3) | 0.30 |
| Visceral metastases | 107 (57.5) | 53 (52.0) | 54 (64.3) | 0.10 |
| Location of Metastases | ||||
| Bone | 143 (76.9) | 75 (73.5) | 68 (81.0) | 0.29 |
| Lung | 78 (40.9) | 39 (38.2) | 39 (46.4) | 0.29 |
| Liver | 58 (31.2) | 27 (26.5) | 31 (36.9) | 0.15 |
| Skin or soft tissue | 47 (25.3) | 22 (21.6) | 25 (29.8) | 0.23 |
| Nodal | 27 (14.5) | 10 (9.8) | 17 (20.2) | 0.06 |
| Brain | 7 (3.8) | 2 (2.0) | 5 (6.0) | 0.24 |
| Peritoneal | 5 (2.7) | 3 (2.9) | 2 (2.4) | 1.0 |
| Variables | Study Cohort n = 186 (%) | ≤2 Lines of Therapy n = 102 (54.8%) (%) | ≥3 Lines of Therapy n = 84 (45.2%) (%) | p Value |
|---|---|---|---|---|
| Median line of therapy | 2 (range 0–8) | 1 (0–2) | 4 (3–8) | <0.001 |
| Four or more lines of therapy | 42 (22.5) | 0 | 56 (71.8) | <0.001 |
| Median time from diagnosis to start of fulvestrant | 34 (IQR: 20.0–63.0) | 24.5 (12.0–44.5) | 44 (28.0–87.0) | 0.002 |
| Received chemotherapy prior to fulvestrant | 80 (43.0) | 18 (17.6) | 62 (73.8) | 0.001 |
| Combination treatment | 34 (18.3) | 21 (20.6) | 13 (15.5) | 0.44 |
| Targeted Therapy | 33 (17.7) | 21 (100) | 12 (92) | 0.32 |
| Endocrine Resistance | 178 (95.7) | 95 (93.1) | 83 (98.8) | 0.07 |
| Primary | 39 (21.9) | 27 (28.4) | 12 (14.4) | 0.04 |
| Reason for discontinuation of fulvestrant | ||||
| Progression | 139 (74.7) | 71 (69.6) | 68 (81.0) | 0.09 |
| Side effects or patient request | 19 (10.2) | 13 (12.7) | 6 (7.2) | 0.23 |
| Others | 3 (1.6) | 2 (1.9) | 1 (1.3) | 1.0 |
| Best Response to fulvestrant | ||||
| Complete response | 2 (1.1) | 2 (1.9) | 0 | 0.50 |
| Partial response | 24 (12.9) | 14 (13.8) | 10 (11.9) | 0.82 |
| Stable disease | 106 (57) | 65 (63.7) | 41 (48.8) | 0.05 |
| Progressive disease | 53 (28.6) | 21 (20.6) | 32 (38.1) | 0.009 |
| Unknown | 1 (0.5) | 0 | 1 (1.2) | 0.45 |
| Received chemotherapy post fulvestrant | 112 (60.2) | 57 (55.9) | 55 (65.5) | 0.22 |
| TTP | OS | |||
|---|---|---|---|---|
| Variables | Months (95% CI) | p Value | Months (95% CI) | p Value |
| Overall | 8 (5.6–10.4) | 21 (16.0–26.0) | ||
| Primary resistance | 7 (2.8–11.2) | 0.098 | 32 (17.2–46.8) | 0.592 |
| Secondary resistance | 9 (6.1–11.9) | 21 (15.6–26.4) | ||
| Chemotherapy before fulvestrant | 6 (4.5–7.5) | 0.039 | 21 (10.7–31.3) | 0.519 |
| No chemotherapy before fulvestrant | 12 (8.7–15.3) | 21 (14.8–27.2) | ||
| Chemotherapy after fulvestrant | - | 34 (30.4–37.6) | <0.001 | |
| No chemotherapy after fulvestrant | - | 8 (4.2–11.8) | ||
| 50 or younger | 10 (4.5–15.5) | 0.829 | 32 (23.3–40.7) | 0.500 |
| 51 or older | 8 (5.9–10.1) | 21 (16.3–25.7) | ||
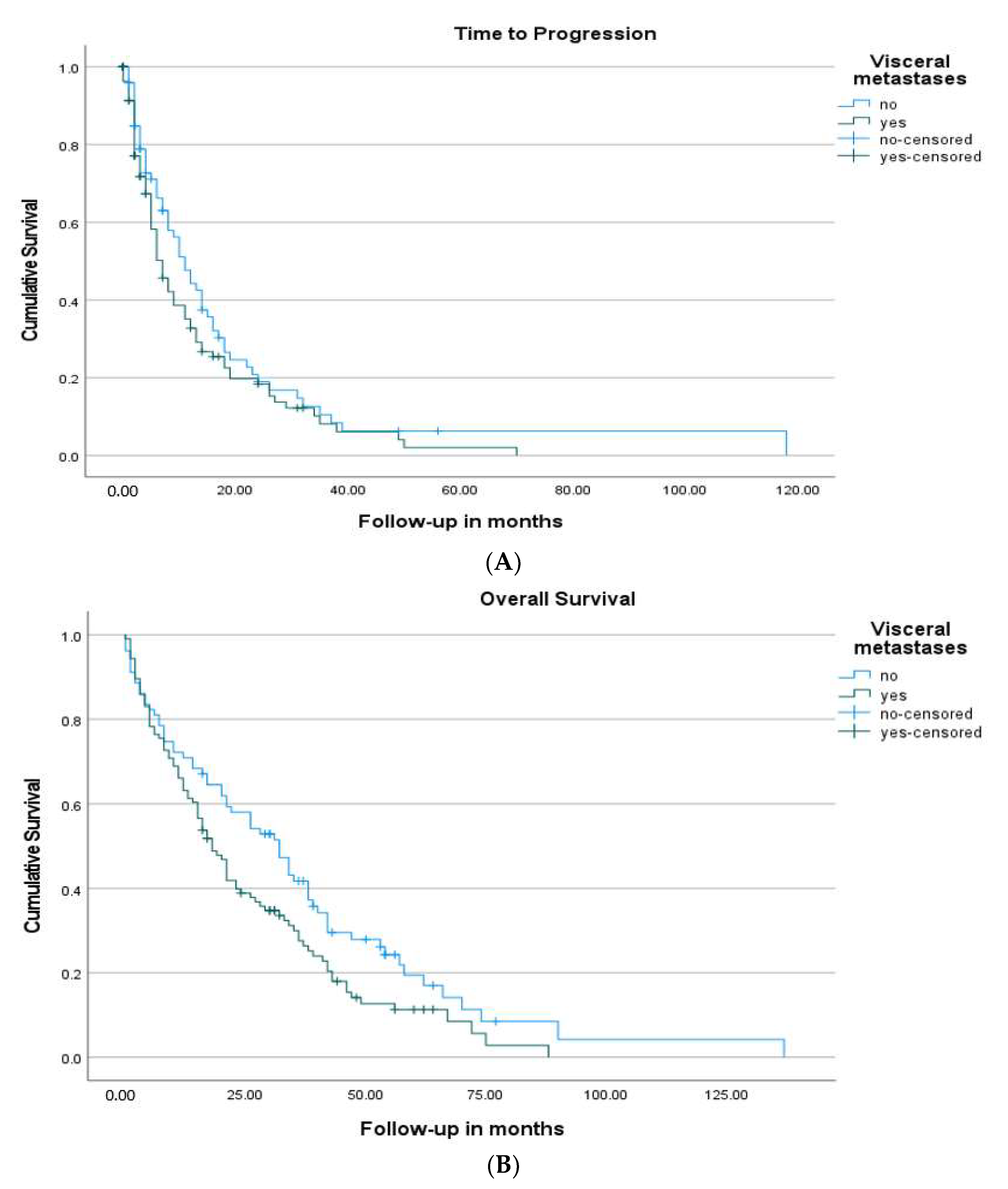
| Visceral Metastasis | 7 (5.1–8.9) | 0.142 | 18 (14.1–21.9) | 0.029 |
| No Visceral Metastasis | 11 (7.9–14.1) | 32 (23.0–41.0) | ||
| ≤2 previous therapies | 12 (9.4–14.6) | 0.015 | 26 (16.0–36.0) | 0.067 |
| ≥3 previous therapies | 6 (5.1–6.9) | 16 (10.5–21.5) | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age < 51 | 0.952 (0.597–1.517) | 0.835 | ||
| WHO PS < 2 | 0.89 (0.53–1.49) | 0.680 | ||
| Comorbid Illness | 0.78 (0.55–1.10) | 0.170 | ||
| History of early-Stage Breast cancer | 0.790 (0. 524–1.191) | 0.260 | ||
| Secondary Endocrine Resistance | 0.728 (0.493–1.077) | 0.112 | 0.717 (0.480–1.071) | 0.104 |
| Lack of chemotherapy prior to Fulvestrant | 0.710 (0.506–0.996) | 0.047 | 0.802 (0.540–1.191) | 0.274 |
| Non-Visceral Metastasis | 0.783 (0.557–1.099) | 0.158 | 0.701 (0.495–0.994) | 0.046 |
| ≤2 previous therapies | 0.672 (0.480–0.940) | 0.020 | 0.775 (0.517–1.161) | 0.216 |
| Combination therapy | 0.714 (0.462–1.104) | 0.130 | 0.745 (0.468–1.187) | 0.216 |
| Time to Fulvestrant | 0.732 (0.523–1.024) | 0.068 | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Rural City | 0.81 (0.59–1.15) | 0.197 | 0.80 (0.58–1.12) | 0.20 |
| Age | 0.857 (0.542–1.353) | 0.507 | ||
| WHO PS < 2 | 0.538 (0.358–0.806) | 0.003 | 0.73 (0.48–1.11) | 0.15 |
| Comorbid Illness | 0.869 (0.633–1.193) | 0.387 | ||
| Secondary Cancer | 0.70 (0.42–1.16) | 0.172 | 0.65 (0.38–1.10) | 0.11 |
| No Smoking | 0.86 (0.62–1.21) | 0.402 | ||
| History of Early-Stage Breast cancer | 0.940 (0.630–1.402) | 0.760 | ||
| Primary Endocrine Resistance | 0.90 (0.60–1.33) | 0.597 | ||
| Chemotherapy prior to Fulvestrant | 0.901 (0.653–1.243) | 0.525 | ||
| Chemotherapy post fulvestrant | 0.425 (0.305–0.593) | <0.001 | 0.32 (0.23–0.47) | <0.0001 |
| Non-Visceral Metastasis | 0.701 (0.506–0.970) | 0.032 | 0.70 (0.50–0.97) | 0.03 |
| ≤2 previous therapies | 0.747 (0.544–1.026) | 0.072 | 0.76 (0.55–1.05) | 0.10 |
| Combination therapy | 0.67 (0.44–1.05) | 0.078 | ||
| Clinical Benefit | 0.574 (0.402–0.819) | 0.002 | 0.44 (0.30–0.65) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrahennadi, S.; Sami, A.; Haider, K.; Chalchal, H.I.; Le, D.; Ahmed, O.; Manna, M.; El-Gayed, A.; Wright, P.; Ahmed, S. Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience. Cancers 2021, 13, 4163. https://doi.org/10.3390/cancers13164163
Andrahennadi S, Sami A, Haider K, Chalchal HI, Le D, Ahmed O, Manna M, El-Gayed A, Wright P, Ahmed S. Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience. Cancers. 2021; 13(16):4163. https://doi.org/10.3390/cancers13164163
Chicago/Turabian StyleAndrahennadi, Samitha, Amer Sami, Kamal Haider, Haji Ibraheem Chalchal, Duc Le, Osama Ahmed, Mita Manna, Ali El-Gayed, Philip Wright, and Shahid Ahmed. 2021. "Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience" Cancers 13, no. 16: 4163. https://doi.org/10.3390/cancers13164163
APA StyleAndrahennadi, S., Sami, A., Haider, K., Chalchal, H. I., Le, D., Ahmed, O., Manna, M., El-Gayed, A., Wright, P., & Ahmed, S. (2021). Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience. Cancers, 13(16), 4163. https://doi.org/10.3390/cancers13164163








