Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 177Lutetium-PSMA-617 Radioligand Therapy
2.3. Response Assessment
2.4. Toxicity Assessment
2.5. Data Analysis
3. Results
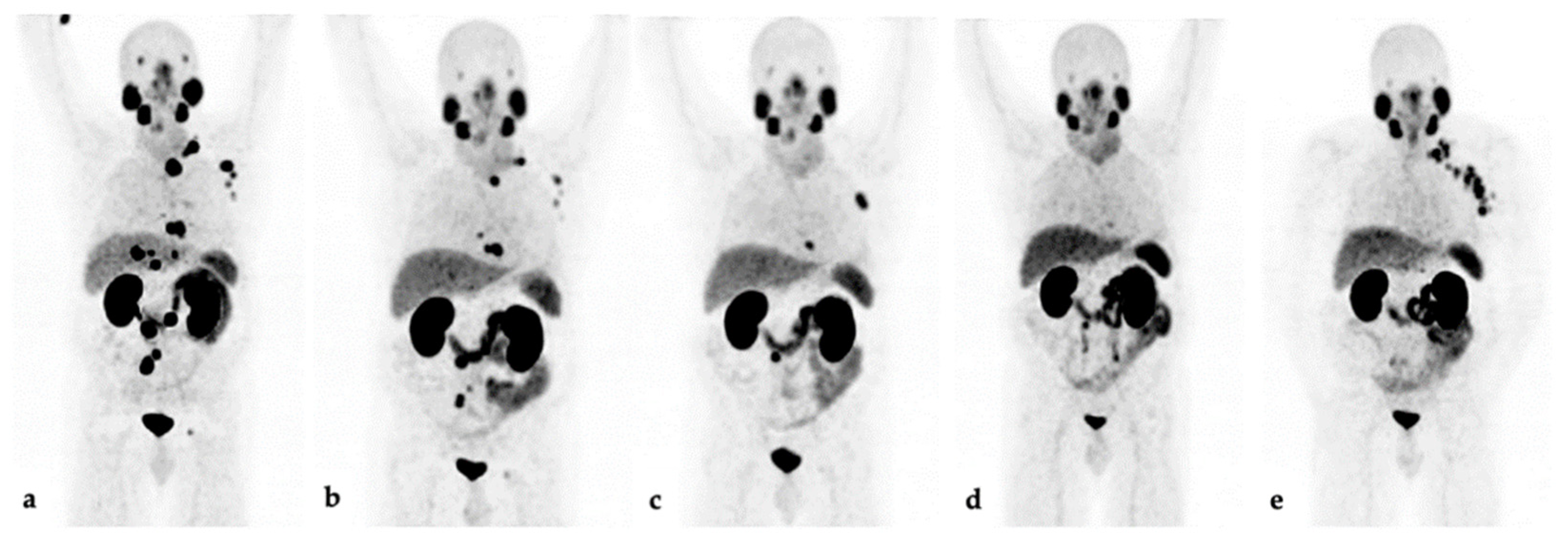
3.1. Response
3.2. Survival
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef]
- Welch, H.G.; Albertsen, P.C. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J. Natl. Cancer Inst. 2009, 101, 1325–1329. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.J.; Fang, S.C.; An, L.; Shao, Y.J. Early-onset prostate cancer is associated with increased risks of disease progression and cancer-specific mortality. Prostate 2021, 81, 118–126. [Google Scholar] [CrossRef]
- Thorstenson, A.; Garmo, H.; Adolfsson, J.; Bratt, O. Cancer Specific Mortality in Men Diagnosed with Prostate Cancer before Age 50 Years: A Nationwide Population Based Study. J. Urol. 2017, 197, 61–66. [Google Scholar] [CrossRef]
- Lin, D.W.; Porter, M.; Montgomery, B. Treatment and survival outcomes in young men diagnosed with prostate cancer: A Population-based Cohort Study. Cancer 2009, 115, 2863–2871. [Google Scholar] [CrossRef]
- Kimura, T.; Onozawa, M.; Miyazaki, J.; Matsuoka, T.; Joraku, A.; Kawai, K.; Nishiyama, H.; Hinotsu, S.; Akaza, H. Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int. J. Urol. 2014, 21, 578–583. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffo, O.; Ortega, C.; Di Lorenzo, G.; Sava, T.; De Giorgi, U.; Cavaliere, C.; Macrini, S.; Spizzo, G.; Aieta, M.; Messina, C.; et al. Clinical outcomes in a contemporary series of “young” patients with castration-resistant prostate cancer who were 60 years and younger. Urol. Oncol. 2015, 33, 265.e15–265.e21. [Google Scholar] [CrossRef]
- Humphreys, M.R.; Fernandes, K.A.; Sridhar, S.S. Impact of Age at Diagnosis on Outcomes in Men with Castrate-Resistant Prostate Cancer (CRPC). J. Cancer 2013, 4, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Rahbar, K.; Kürpig, S.; Bögemann, M.; Claesener, M.; Eppard, E.; Gärtner, F.; Rogenhofer, S.; Schäfers, M.; Essler, M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouviere, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [(177)Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Gafita, A.; Heck, M.M.; Rauscher, I.; Tauber, R.; Cala, L.; Franz, C.; D’Alessandria, C.; Retz, M.; Weber, W.A.; Eiber, M. Early Prostate-Specific Antigen Changes and Clinical Outcome After (177)Lu-PSMA Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Soydal, C.; Araz, M.; Urun, Y.; Nak, D.; Ozkan, E.; Kucuk, N.O. Prognostic importance of PSA response in patients who received Lutetium-177 PSMA treatment for castration resistant prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Nie, L.; Qin, K.; Zhang, H.; Shi, H. The meta-analysis of the effect of 68Ga-PSMA-PET/CT diagnosis of prostatic cancer compared with bone scan. Medicine 2021, 100, e25417. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical Outcomes of (177)Lu-PSMA Radioligand Therapy in Earlier and Later Phases of Metastatic Castration-Resistant Prostate Cancer Grouped by Previous Taxane Chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.-J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Calais, J.; Gafita, A.; Eiber, M.R.; Armstrong, W.R.; Gartmann, J.; Thin, P.; Nguyen, K.; Lok, V.; Gosa, L.; Grogan, T.; et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with 177Lu-PSMA-617 for metastatic Castration-reSISTant Prostate Cancer (RESIST-PC): Efficacy results of the UCLA cohort. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Rathke, H.; Holland-Letz, T.; Mier, W.; Flechsig, P.; Mavriopoulou, E.; Röhrich, M.; Kopka, K.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; et al. Response Prediction of (177)Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J. Nucl. Med. 2020, 61, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Linden, P.; Hauser, S.; Feldmann, G.; Brossart, P.; Fimmers, R.; Essler, M.; Holdenrieder, S.; Ahmadzadehfar, H. The value of tumor markers in men with metastatic prostate cancer undergoing [(177) Lu]Lu-PSMA therapy. Prostate 2020, 80, 17–27. [Google Scholar] [CrossRef]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; Spreafico, F.; Barr, R. Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer 2020, 126, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhauser, C.; Favero, F.; Risch, T.; Simon, R.; Feuerbach, L.; Assenov, Y.; Heckmann, D.; Sidiropoulos, N.; Waszak, S.M.; Hubschmann, D.; et al. Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell 2018, 34, 996–1011.e8. [Google Scholar] [CrossRef] [Green Version]
- Weischenfeldt, J.; Korbel, J.O. Genomes of early onset prostate cancer. Curr. Opin. Urol. 2017, 27, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Burns, M.C.; Ebot, E.M.; Frampton, G.M.; Ross, J.S.; Hussain, M.H.A.; Abdulkadir, S.A. Early-onset metastatic and clinically advanced prostate cancer is a distinct clinical and molecular entity characterized by increased TMPRSS2-ERG fusions. Prostate Cancer Prostatic Dis. 2021, 24, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Satturwar, S.; Van der Kwast, T. Young-age prostate cancer. J. Clin. Pathol. 2015, 68, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S.M.; Kote-Jarai, Z.; Meitz, J.; Hamoudi, R.; Hope, Q.; Osin, P.; Jackson, R.; Southgate, C.; Singh, R.; Falconer, A.; et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am. J. Hum. Genet. 2003, 72, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Nientiedt, C.; Heller, M.; Endris, V.; Volckmar, A.L.; Zschabitz, S.; Tapia-Laliena, M.A.; Duensing, A.; Jager, D.; Schirmacher, P.; Sultmann, H.; et al. Mutations in BRCA2 and taxane resistance in prostate cancer. Sci. Rep. 2017, 7, 4574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yordanova, A.; Becker, A.; Eppard, E.; Kürpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Kochems, N.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Khreish, F.; Ezziddin, S. Renal Safety of [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Compromised Baseline Kidney Function. Cancers 2021, 13, 3095. [Google Scholar] [CrossRef]



| Variable | All Patients (n = 25) |
|---|---|
| Age at diagnosis, years | 54 (51–55) |
| Age at LuPSMA-RLT initiation, years | 62 (57–71) |
| Time to CR, years | 4.5 (1.3–13.8) |
| Time to LuPSMA-RLT initiation after diagnosis, years | 8.3 (4–13.9) |
| Gleason score *: | |
| <8 | 8 (38) |
| ≥8 | 13 (62) |
| PSA at LuPSMA-RLT initiation (ng/mL) | 179 (69.5–1142) |
| PSA Doubling Time, months | 2 (1.2–3.4) |
| Hemoglobin (g/dL) | 11 (9.5–11.9) |
| White blood cells (109/L) | 6.2 (5.1–7.2) |
| Platelets (109/L) | 276 (217–335) |
| eGFR (mL/min/1.73 m2) | 95.3 (72.5–103.8) |
| Alkaline phosphatase (U/L) | 235 (124–568.5) |
| Lactate dehydrogenase (U/L) | 315 (268–509.5) |
| Disease involvement: | |
| Bone metastases | 24 (96) |
| Lymph node metastases | 20 (80) |
| Visceral metastases | 11 (44) |
| hepatic | 7 (28) |
| pulmonary | 4 (16) |
| Primary | 6 (24) |
| VAS: | |
| <4 | 11 (44) |
| ≥4 | 14 (56) |
| ECOG performance status: | |
| <2 | 8 (32) |
| ≥2 | 17 (68) |
| Previous treatment: | |
| Abiraterone | 24 (96) |
| Enzalutamide | 18 (72) |
| 223Radium dichloride | 5 (20) |
| Docetaxel | 25 (100) |
| Cabazitaxel | 15 (60) |
| Nontaxane chemotherapy ** | 4 (16) |
| Toxicity | Baseline, n (%) | Post-Therapeutic, n (%) | ||
|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | |
| Anemia | 20 (80) | 3 (12) | 20 (80) | 5 (20) |
| Leukopenia | 1 (4) | 0 (0) | 12 (48) | 1 (4) |
| Thrombocytopenia | 5 (20) | 0 (0) | 7 (28) | 3 (12) |
| eGFR * | 4 (16) | 0 (0) | 7 (28) | 1 (4) |
| Xerostomia | 0 (0) | 0 (0) | 6 (24) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mader, N.; Groener, D.; Tselis, N.; Banek, S.; Nagarajah, J.; Grünwald, F.; Sabet, A. Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer. Cancers 2021, 13, 4193. https://doi.org/10.3390/cancers13164193
Mader N, Groener D, Tselis N, Banek S, Nagarajah J, Grünwald F, Sabet A. Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer. Cancers. 2021; 13(16):4193. https://doi.org/10.3390/cancers13164193
Chicago/Turabian StyleMader, Nicolai, Daniel Groener, Nikolaos Tselis, Séverine Banek, James Nagarajah, Frank Grünwald, and Amir Sabet. 2021. "Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer" Cancers 13, no. 16: 4193. https://doi.org/10.3390/cancers13164193
APA StyleMader, N., Groener, D., Tselis, N., Banek, S., Nagarajah, J., Grünwald, F., & Sabet, A. (2021). Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer. Cancers, 13(16), 4193. https://doi.org/10.3390/cancers13164193






