Simple Summary
Interstitial lung disease (ILD) is a risk factor for lung cancer, but the treatment options are often limited because of concerns that ILD may worsen with treatment. In this study, we analyzed whether the presence or absence of ILD affects the outcome of carbon-ion radiotherapy (CIRT) for clinical stage I non-small cell lung cancer (NSCLC). For all cases, CT and clinical data were reviewed by a respiratory physician to determine the presence of ILD. Overall survival and disease-specific survival were lower in patients with ILD than in patients without ILD. There was no significant difference between the ILD group and the non-ILD group with respect to safety. CIRT was not associated with significantly more side-effects in patients with ILD than in patients without ILD. Coexisting ILD was a poor prognostic factor with respect to CIRT for clinical stage I lung cancer, as reported for other treatment methods.
Abstract
Interstitial lung disease (ILD) is a risk factor both for the development and treatment failure of lung cancer. In this retrospective study, we analyzed the outcome of carbon-ion radiotherapy (CIRT) in 124 patients with clinical stage I non-small cell lung cancer (NSCLC), of whom 26 (21%) had radiological signs of pre-existing ILD. ILD was diagnosed retrospectively by a pulmonologist based on critical review of CT-scans. Ninety-eight patients were assigned to the non-ILD group and 26 patients (21.0%) to the ILD group. There were significant differences in pre-treatment KL-6 values between the two groups. The three year overall survival and cause-specific survival rates were 83.2% and 90.7%, respectively, in the non-ILD group, and 59.7% and 59.7%, respectively, in the ILD group (between-group differences, p = 0.002 and p < 0.001). Radiation pneumonitis worse than Grade 2 was observed in three patients (3.0%) in the non-ILD group and two patients (7.6%) in the ILD group (p = 0.29). There were no cases of acute exacerbation in the ILD group. CIRT for stage I NSCLC was as safe in the ILD group as in the non-ILD group. Coexisting ILD was a poor prognostic factor in CIRT for clinical stage I lung cancer.
1. Introduction
Lung cancer occurs in 7–40% of patients with interstitial lung disease (ILD); the causes are similar to those in other groups and include smoking and old age; however, fibrosis itself can be a risk factor for ILD patients [1,2,3,4].
However, anti-cancer treatments for lung cancer patients with ILD are challenging because of the risk that cancer treatments such as surgery, chemotherapy, immunotherapy, and radiotherapy can cause acute exacerbation (AE) of ILD [5,6,7,8,9,10,11,12,13,14,15,16].
Although stereotactic body radiotherapy (SBRT) using X-rays for stage I non-small cell lung cancer (NSCLC) has been reported to be safe with good outcomes [17,18,19], many prospective studies exclude cases with ILD. This is because patients with concomitant ILD have a higher risk of developing both radiation pneumonia and AE of ILD, which can be fatal [20,21,22,23,24].
At our hospital, carbon-ion radiotherapy (CIRT) has been used for clinical stage I NSCLC since 2010. Compared with X-rays, carbon ions have higher linear energy transfer and larger relative biological effectiveness (RBE) [25]. The physical characteristics of carbon ions, such as the Bragg peak and small lateral scattering, are theoretically superior to those of X-rays, allowing carbon ions to provide a more localized delivery of the radiation dose. With this benefit, CIRT for clinical stage I NSCLC has demonstrated favorable local control while minimizing damage to lung tissues [26,27,28]. However, there is little information on how the safety and efficacy of CIRT compares between patients with and without ILDs.
In the present study, we evaluated whether the presence or absence of ILD affects the clinical outcomes of CIRT for clinical stage I NSCLC.
2. Materials and Methods
2.1. Patients
This single-institution retrospective study was approved by the institutional review board of Gunma University Hospital (trial registration number: HS2019-233). Eligibility criteria for CIRT are clinically or histologically diagnosed stage I primary lung cancer, inoperable or refused surgery, and an Eastern Cooperative Oncology Group’s scale performance status between 0 and 2. Exclusion criteria include a previous history of radiotherapy near the target, an intractable infectious disease, a second active malignancy, and inability to obtain patient consent. Patients who underwent CIRT at Gunma University heavy ion medical center between June 2010 and December 2019 were included in the study. All patients were histologically or clinically diagnosed with stage I NSCLC and were confirmed as eligible for CIRT by the multidisciplinary tumor board of Gunma University Hospital. The tumor board consists of radiologists, respiratory physicians, respiratory surgeons, and radiation oncologists, and who discuss the clinical diagnosis and treatment plan. The indication of surgery was judged by a respiratory surgeon based on the respiratory function, history and complications. All patients underwent the following evaluations: whole blood cell count, differential count, routine chemistry measurements, electrocardiogram, pulmonary function tests, chest radiography, chest computed tomography (CT), abdominal CT, whole-brain magnetic resonance imaging or CT, and 18F-fluorodeoxyglucose positron emission tomography. In cases where either bronchoscopy or CT-guided biopsy was possible, a biopsy was performed for definitive diagnosis. If there were enlarged mediastinal lymph nodes that were difficult to distinguish from lymph node metastases on imaging, and examination was possible based on the patient’s general condition and the location of the lymph nodes, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed. To identify those patients with complications due to ILD, the pre-treatment CT images and medical history of all patients were reviewed. In this study, the primary endpoint was a difference in the incidence of adverse events according to the presence or absence of ILD. The secondary endpoints were overall survival (OS), cause-specific survival (CSS), and local control (LC).
2.2. Carbon-Ion Radiotherapy
CIRT was performed as described previously [27]. Patients were positioned in customized cradles (Moldcare; Alcare, Tokyo, Japan) and immobilized using thermoplastic shells (Shellfitter; Kuraray, Osaka, Japan). CT images were acquired at 2 mm slice thickness and used for the treatment planning. Contouring of the gross tumor volume (GTV) was performed on the planning CT using a lung window. The clinical target volume (CTV) was defined as the GTV plus a margin of 5 to 8 mm. The planning target volume (PTV) margin included the setup and internal margins, which were determined by assessing tumor motion on four-dimensional CT images. If the patient’s breathing rhythm was stable, respiratory gated irradiation was performed (Anzai Medical Co., Ltd., Tokyo, Japan).
Carbon-ion doses were prescribed using units of Gy (RBE), calculated by multiplying the physical dose (Gy) by the RBE values for carbon ions. The RBE values for carbon ions were obtained from a biological linear-quadratic model constructed by the Japanese National Institute for Radiological Science [29,30].
The clinical dose distribution was calculated with XiO-N treatment planning software (Elekta/Mitsubishi Electric) and was used to calculate the passive scattering carbon-ion dose distribution. From 2010 to 2015, the prescribed doses were 52.8 Gy (RBE) for T1 tumors and 60.0 Gy(RBE) for T2 tumors, delivered in four fractions, whereas since 2016, all cases were treated with 60.0 Gy(RBE) in four fractions.
2.3. Assessment of Interstitial Lung Disease
Two respiratory physicians (K.Y. and S.K.) reviewed the pre-treatment CT images of all patients to determine the presence of ILD. High-resolution CT (HRCT) imaging was performed using a multidetector CT scanner (Aquilion 64; Toshiba Medical Systems, Tochigi, Japan).
The ground-glass opacity (GGO) and fibrosis scores of ILD were determined according to previous reports [31,32,33]. Briefly, the percentages of GGO and fibrosis in each of the five lung lobes under three limited CT levels (the mid-aortic arch, left tracheal bifurcation, and 1 cm above the diaphragm) were evaluated, with scores of 0 to 5 given for each lobe, and the five scores were summed. The GGO score for each lobe was evaluated as follows: 0, none; 1, ≤5%; 2, 5% to <25%; 3, 25–49%; 4, 50–75%; and 5, >75% of the lobe. The fibrosis score was evaluated as follows: 0, no fibrosis; 1, interlobular septal thickening without honeycombing; 2, honeycombing <25%; 3, 25–49%; 4, 50–75%; and 5, >75% of the lobe.
CT findings of GGO, consolidation, reticulations, honeycombing, bronchiectasis, and cysts were also evaluated according to the Fleischner Society guidelines [34].
A diagnosis of ILD was made if any of the above findings were seen on HRCT. Although a histological diagnosis is normally required, the purpose of this study was not to treat ILD; therefore, a histological diagnosis was not required. Thus, the presence or absence of ILD was based on clinical judgment of imaging findings and patient symptoms.
2.4. Dose-Volume Histogram Evaluation
Irradiated lung volumes were evaluated as the total lung without the GTV volume. Vn describes the fraction of the volume irradiated above n Gy or n Gy (RBE). The mean lung dose (MLD) was defined as the average dose to the whole lung. The V5, V20, V30, and MLD, which have been indicated to have an effect on the lungs, were investigated. Target coverage was evaluated as the percentage of the prescribed dose covering 95% of the CTV.
2.5. Follow-Up
The patients had a physical examination, toxicity assessments, and X-ray of the chest every month until 6 months after CIRT, while a thoracic CT scan and blood analyses were obtained every 3 months. After 6 months, the frequency was extended to once every 6 months, and the examination and consultation were continued until 5 years after the end of radiotherapy. In these follow-up consultations, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) was performed once a year instead of CT. Additional examinations were performed when recurrence was suspected, and the follow-up schedule was adjusted according to the treatment schedule and the patient’s general condition. Treatment toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [35].
2.6. Statistical Analysis
OS was defined as the time between the day of the first CIRT fraction and the day of the last follow-up or death. CSS was measured as the time from the date of initial CIRT until the date of death from the primary lung cancer or the last follow-up. Any death after disease progression was defined as death from the primary lung cancer. LC was measured from the date of initial CIRT to the date of the first local progression in the irradiated area or the date of the last follow-up. The OS, CSS, and LC rates were calculated using the Kaplan–Meier method. Differences in the OS, CSS, and LC rates between non-ILD and ILD groups were compared using the log-rank test. Differences in the numerical variables between the two groups were examined using Fisher’s exact test and the chi-square test. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 26; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient Characteristics
Table 1 summarizes the patient characteristics. Among the 124 eligible patients, 26 (21%) had ILD. The pre-treatment sialylated carbohydrate antigen KL-6 (KL-6) level in the ILD group was significantly higher than that in the non-ILD group (p = 0.003), although there were no significant between-group differences in age, proportion of T-factor, prescribed dose, and pre-treatment respiratory functions including %VC and forced expiratory volume in one second.

Table 1.
Patient characteristics.
3.2. CT Findings Associated with ILD
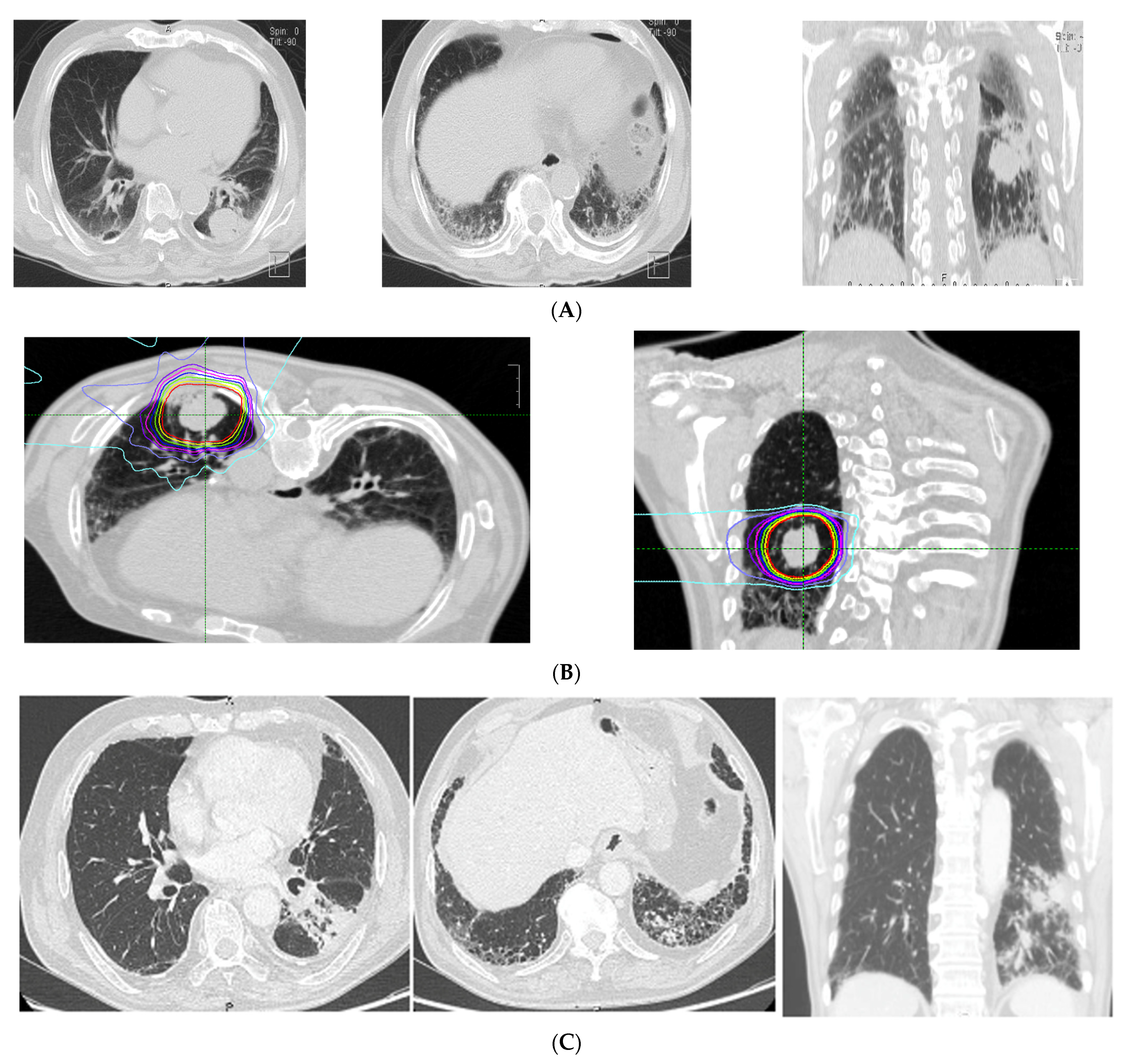
The CT findings of the 26 patients with ILD showed a median total GGO score of 0 (range, 0–4), and a median total fibrosis score of 2 (range, 0–10). Specific CT findings included GGO in 16 patients, reticulation in 22 patients, contraction bronchiectasis in seven patients, honeycombing in three patients, and emphysema in 15 patients. No patient showed consolidation or cysts on CT images. In the ILD group, 18 cases (66.7%) had a tumor inside the stromal shadow. CT imaging and the dose distribution in a representative case with ILD are shown in Figure 1.
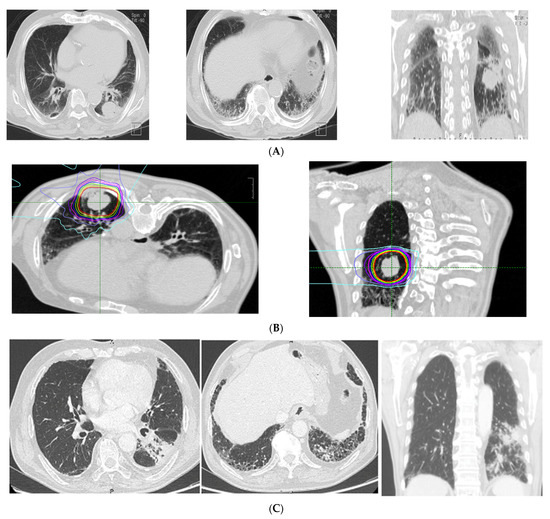
Figure 1.
Representative case of imaging findings and dose distributions in the interstitial lung disease (ILD) group. (A) pre-treatment CT (diagnosis: usual interstitial pneumonitis (UIP); fibrosis score: 5, GGO score: 0, CT findings: honeycomb, GGO, reticulation, traction bronchitis, emphysema). (B) Dose distribution. (C) Post-treatment CT (6 months after CIRT).
3.3. Dose-Volume Histogram Parameters
The dose-volume histogram parameters in the non-ILD and ILD groups are shown in Table 2. The irradiated lung volumes in the non-ILD and ILD groups evaluated as the total lung without the GTV volume were 10.4% and 9.94% in V5; 5.58% and 5.6% in V20; and 3.88% and 4.09% in V30, respectively. Target coverage, evaluated as the percentage of the prescribed dose covering 95% of the CTV, was 99.2% in the non-ILD group and 99.4% in the ILD group. There were no significant differences in V5, V20, V30, MLD, and CTV coverage between the two groups.

Table 2.
Treatment parameters.
3.4. Tumor Control and Survival
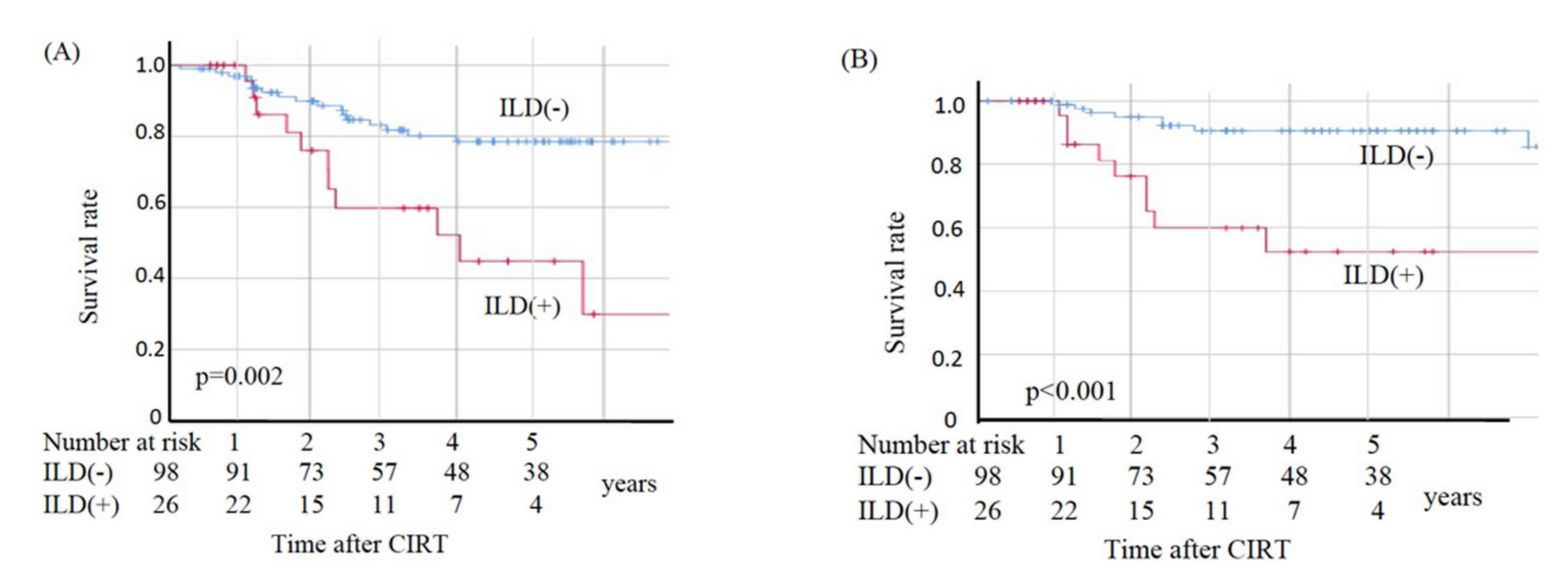
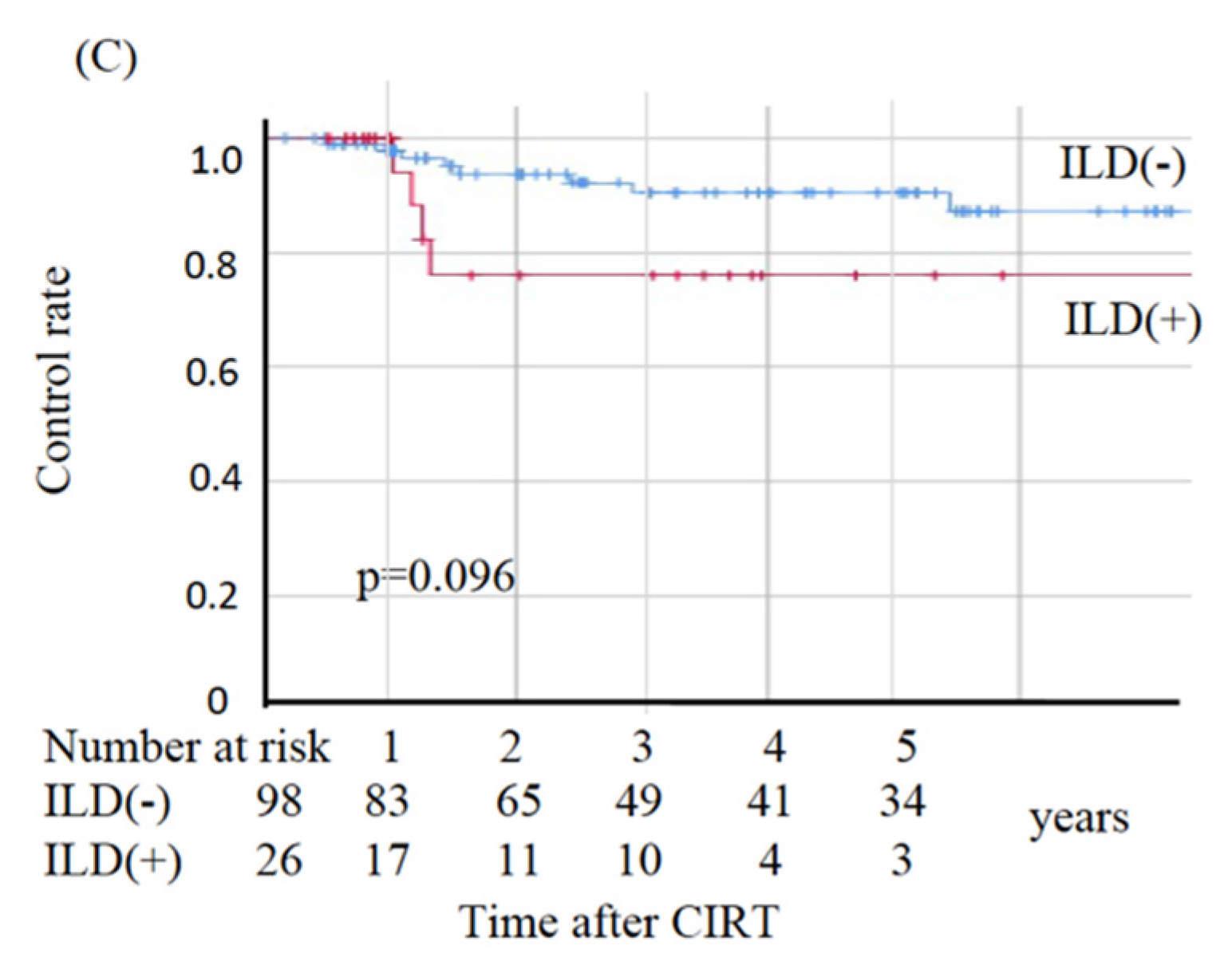
In the non-ILD group, eight patients died from primary disease and ten died from other diseases, whereas in the ILD group nine died from primary disease and two died from other diseases. The two deaths from other diseases in the ILD group were observed at 49.2 and 69.6 months post-CIRT. The OS, CSS, and LC values are shown in Figure 2. The sites of recurrence in patients who died of primary disease were as follows: in the non-ILD group, the recurrence cites were local (n = 3), brain (n = 2), mediastinal lymph node (n = 1), bone (n = 1), and pleural effusion (n = 1); in the ILD group, the recurrence sites were local (n = 4), mediastinal lymph nodes (n = 4), lung (n = 2), liver (n = 2), and pleural effusion (n = 1). The OS rates at three and five years were 83.2% and 78.5%, respectively, in the non-ILD group, and 59.7% and 44.8%, respectively, in the ILD group. The non-ILD group showed significantly better OS than the ILD group (p = 0.002). The CSS rates at three and five years were 90.7% and 90.7%, respectively, in the non-ILD group, and 59.7% and 52.2%, respectively, in the ILD group. The non-ILD group showed significantly better CSS than the ILD group (p < 0.001). The LC rates at three and five years were 90.4% and 90.4%, respectively, in the non-ILD group, and 76.0% and 76.0%, respectively, in the ILD group. There was no significant difference in LC between the two groups (p = 0.096).
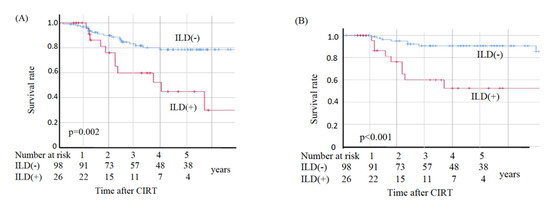
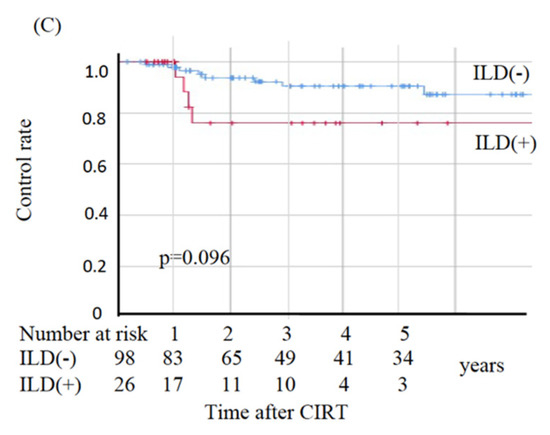
Figure 2.
Kaplan–Meier estimates for (A) overall survival rates, (B) cause-specific survival rates, and (C) local control rates. Blue lines: non-ILD group (n = 98), red lines: ILD group (n = 26).
3.5. Adverse Events
Adverse events are listed in Table 3. There were no adverse events of ILD related to the CIRT. No patient developed Grade 4 or 5 radiation pneumonitis in either the ILD group or the non-ILD group. The incidence of radiation pneumonitis (Grade 2 or worse) was 4.0% (5/124) overall, and 3.0% (3/98) in the non-ILD group and 7.6% (2/26) in the ILD group.

Table 3.
Adverse events (CTCAE ver.4.0).
4. Discussion
To the best of our knowledge, the present study is the first to compare the safety and efficacy of CIRT for NSCLC between non-ILD and ILD groups. The OS and CSS rates in the ILD group were significantly worse than those in the non-ILD group, whereas the LC rate was not significantly different. Regarding lung toxicity, no patients developed AE or fatal radiation pneumonitis, and the incidence of radiation pneumonitis of Grade 2 or 3 was not significantly different between the non-ILD and ILD groups. In addition, there was no significant difference in dose-volume histogram parameters between the two groups.
Patients with NSCLC and coexisting ILD are at an increased risk of severe adverse events after SBRT. According to retrospective studies on SBRT for lung cancer, patients with ILD have a significantly higher risk of radiation pneumonitis than patients without ILD [37]. As shown in Table 4, previous incidences of radiation pneumonitis of Grade 2 or worse and Grade 3 or worse after SBRT ranged from 19.0–50.0% and 10.0–38.9%, respectively, in the ILD group, and from 1.7–14.8% and 1.0–2.6% in the non-ILD group [21,22,38,39,40,41]. For SBRT, there were clear increases in Grade 2 or worse and Grade 3 or worse pneumonia in the ILD group. The corresponding values in our study were 7.6% and 3.8%, respectively, in the ILD group, and 3.0% and 1.0%, respectively, in the non-ILD group. Therefore, our findings showed a relatively lower incidence of radiation pneumonitis in CIRT-treated NSCLC patients with ILD than in previously reported SBRT-treated NSCLC patients with ILD.

Table 4.
Incidence of radiation pneumonitis (Grade 2 or worse) in patients with or without interstitial pneumonitis.
A possible reason for the lower incidence of radiation pneumonitis after CIRT was the better dose distribution. Characteristics of carbon-ion beams include the distal fall-off at the Bragg peak and less lateral scatter than X-rays, realizing a more-conformal target dose and reducing the lung tissue dose. A previous dosimetric study comparing dose-volume histogram parameters demonstrated that the values of MLD, V5, V20, and V30 were significantly lower in CIRT than in SBRT [42]. Furthermore, Chen et al. reported that the proportions of treatment-related mortality and ILD-specific toxicity were 15.6% and 25%, respectively, for SBRT, and 4.3% and 18.2%, respectively, for particle therapy including CIRT, indicating that CIRT has the potential to deliver a safer dose to organs at risk compared with SBRT [20].
Previous studies have reported inferior survival in patients with ILD after cancer treatment compared with patients without ILD and listed Table 5. In addition to primary disease progression, the occurrence of fatal treatment-related pneumonitis and AE can decrease survival. Regarding SBRT, Ueki et al. reported three year OS and LC rates of 70.8% and 77.7%, respectively, in a non-ILD group, and 53.8% and 71.4%, respectively, in an ILD group. [23] Other studies on SBRT reported 3 year OS ranging from 54–80% in non-ILD groups and 0–50% in ILD groups [38,43,44]. A similar trend was demonstrated in a surgical series, with Sekihara et al. reporting that in patients with stage I lung cancer, five year OS was significantly higher in a non-ILD group (84.6%) than in an ILD group (44.0%) [45]. The authors also reported that death from primary disease was significantly higher in the ILD group, and that CSS rates were 89.8% in the non-ILD group and 56.6% in the ILD group. In the current study, in CIRT, OS in the ILD group was worse than in the non-ILD group (three year OS, 59.7% vs. 83.2%, respectively), with most of the deaths being due to the primary cancer, which is consistent with previous reports of OS in SBRT or surgery.

Table 5.
Overall survival and local control in patients with or without interstitial pneumonitis.
The limitations to our study should be mentioned. First, making a diagnosis of ILD is sometimes difficult; indeed, there are no standardized criteria. However, to minimize diagnostic variations, the respiratory physicians re-evaluated CT images according to current guidelines [34]. Second, the study was a retrospective single-institution study that included a relatively small number of patients. In particular, it was difficult to obtain sufficient data to make a statistical analysis of adverse events. A multicenter validation of the present results, with a larger patient cohort, is warranted in the future. Third, regarding DLCO, many patients had poor respiratory function and so, in many cases, DLCO was not measured.
5. Conclusions
Following CIRT for NSCLC, the incidence of radiation pneumonitis in patients with ILD was not significantly different from that in patients without ILD. Coexisting ILD was a poor prognostic factor for CIRT for clinical stage I lung cancer. However, it is necessary to validate our results through prospective multicenter trials and to explore the causes of the lower lung cancer survival rate in the ILD group compared with the non-ILD group.
Author Contributions
Conceptualization, N.O., T.M. (Tatsuji Mizukami) and Y.M.; formal analysis, N.O.; data curation, N.O., K.Y. and S.K.; writing—original draft preparation, N.O., N.K. and K.Y.; writing—review and editing, N.O., N.K., K.Y., S.K., T.M. (Tatsuji Mizukami), K.S., J.-i.S., T.E., H.K. and T.M. (Toshitaka Maeno); Supervision, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study was designed and performed according to the principles outlined in the Declaration of Helsinki and the Guidelines of Good Clinical Practices. The institutional ethics review board of Gunma University approved our study (ID: HS2019-233).
Informed Consent Statement
The need for written informed consent was waived due to the study’s retrospective observational nature, but all the participants or their relatives had the opportunity to opt-out.
Data Availability Statement
The supporting data are not publicly available because they contain information that could compromise the privacy of the research participants.
Acknowledgments
The authors are grateful to Bioedit (https://www.bioedit.jp/ accessed on 2 August 2021) for reviewing English language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raghu, G.; Nyberg, F.; Morgan, G. The epidemiology of interstitial lung disease and its association with lung cancer. Br. J. Cancer 2004, 91 (Suppl. 2), S3–S10. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Tanaka, S.; Saiki, Y.; Hara, M.; Nakata, K.; Tanimura, S.; Banba, J. Lung cancer associated with usual interstitial pneumonia. Pathol Int. 1995, 45, 925–932. [Google Scholar] [CrossRef]
- Ozawa, Y.; Suda, T.; Naito, T.; Enomoto, N.; Hashimoto, D.; Fujisawa, T.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009, 14, 723–728. [Google Scholar] [CrossRef]
- Ando, M.; Okamoto, I.; Yamamoto, N.; Takeda, K.; Tamura, K.; Seto, T.; Ariyoshi, Y.; Fukuoka, M. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 2006, 24, 2549–2556. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef] [Green Version]
- Chiyo, M.; Sekine, Y.; Iwata, T.; Tatsumi, K.; Yasufuku, K.; Iyoda, A.; Otsuji, M.; Yoshida, S.; Shibuya, K.; Iizasa, T.; et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: Analyses of short-term and long-term outcomes. J. Thorac. Cardiovasc. Surg. 2003, 126, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, H.; Takeda, A.; Hoshi, T.; Kawabata, Y.; Sayama, K.; Jinzaki, M.; Kuribayashi, S. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann. Thorac. Surg. 2012, 93, 937–943. [Google Scholar] [CrossRef]
- Voltolini, L.; Bongiolatti, S.; Luzzi, L.; Bargagli, E.; Fossi, A.; Ghiribelli, C.; Rottoli, P.; Gotti, G. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: Analysis of risk factors. Eur. J. Cardiothorac. Surg. 2013, 43, e17–e23. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Teramukai, S.; Kondo, H.; Watanabe, A.; Ebina, M.; Kishi, K.; Fujii, Y.; Mitsudomi, T.; Yoshimura, M.; Maniwa, T.; et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg 2014, 147, 1604–1611.e1603. [Google Scholar] [CrossRef] [Green Version]
- Kenmotsu, H.; Naito, T.; Kimura, M.; Ono, A.; Shukuya, T.; Nakamura, Y.; Tsuya, A.; Kaira, K.; Murakami, H.; Takahashi, T.; et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J. Thorac. Oncol. 2011, 6, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minegishi, Y.; Takenaka, K.; Mizutani, H.; Sudoh, J.; Noro, R.; Okano, T.; Azuma, A.; Yoshimura, A.; Ando, M.; Tsuboi, E.; et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern. Med. 2009, 48, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Tomassetti, S.; Gurioli, C.; Ryu, J.H.; Decker, P.A.; Ravaglia, C.; Tantalocco, P.; Buccioli, M.; Piciucchi, S.; Sverzellati, N.; Dubini, A.; et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015, 147, 157–164. [Google Scholar] [CrossRef]
- Tsujino, K.; Hashimoto, T.; Shimada, T.; Yoden, E.; Fujii, O.; Ota, Y.; Satouchi, M.; Negoro, S.; Adachi, S.; Soejima, T. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, Y.; Abe, T.; Omae, M.; Matsui, T.; Kato, M.; Hasegawa, H.; Enomoto, Y.; Ishihara, T.; Inui, N.; Yamada, K.; et al. Impact of Preexisting Interstitial Lung Disease on Acute, Extensive Radiation Pneumonitis: Retrospective Analysis of Patients with Lung Cancer. PLoS ONE 2015, 10, e0140437. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Ricardi, U.; Filippi, A.R.; Guarneri, A.; Giglioli, F.R.; Ciammella, P.; Franco, P.; Mantovani, C.; Borasio, P.; Scagliotti, G.V.; Ragona, R. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung Cancer 2010, 68, 72–77. [Google Scholar] [CrossRef]
- Nagata, Y.; Hiraoka, M.; Shibata, T.; Onishi, H.; Kokubo, M.; Karasawa, K.; Shioyama, Y.; Onimaru, R.; Kozuka, T.; Kunieda, E.; et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 989–996. [Google Scholar] [CrossRef]
- Chen, H.; Senan, S.; Nossent, E.J.; Boldt, R.G.; Warner, A.; Palma, D.A.; Louie, A.V. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 622–631. [Google Scholar] [CrossRef]
- Glick, D.; Lyen, S.; Kandel, S.; Shapera, S.; Le, L.W.; Lindsay, P.; Wong, O.; Bezjak, A.; Brade, A.; Cho, B.C.J.; et al. Impact of Pretreatment Interstitial Lung Disease on Radiation Pneumonitis and Survival in Patients Treated With Lung Stereotactic Body Radiation Therapy (SBRT). Clin. Lung Cancer 2018, 19, e219–e226. [Google Scholar] [CrossRef]
- Bahig, H.; Filion, E.; Vu, T.; Chalaoui, J.; Lambert, L.; Roberge, D.; Gagnon, M.; Fortin, B.; Béliveau-Nadeau, D.; Mathieu, D.; et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract. Radiat. Oncol. 2016, 6, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ueki, N.; Matsuo, Y.; Togashi, Y.; Kubo, T.; Shibuya, K.; Iizuka, Y.; Mizowaki, T.; Togashi, K.; Mishima, M.; Hiraoka, M. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 2015, 10, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Kobayashi-Shibata, S.; Terahara, A.; Okuma, K.; Haga, A.; Wakui, R.; Ohtomo, K.; Nakagawa, K. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat. Oncol. 2010, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, T.; Furusawa, Y.; Fukutsu, K.; Itsukaichi, H.; Eguchi-Kasai, K.; Ohara, H. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat. Res. 1997, 147, 78–85. [Google Scholar] [CrossRef]
- Miyamoto, T.; Baba, M.; Sugane, T.; Nakajima, M.; Yashiro, T.; Kagei, K.; Hirasawa, N.; Sugawara, T.; Yamamoto, N.; Koto, M.; et al. Working Group for Lung Cancer. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J. Thorac. Oncol. 2007, 2, 916–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, N.; Miyamoto, T.; Nakajima, M.; Karube, M.; Hayashi, K.; Tsuji, H.; Tsujii, H.; Kamada, T.; Fujisawa, T. A Dose Escalation Clinical Trial of Single-Fraction Carbon Ion Radiotherapy for Peripheral Stage I Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, J.I.; Shirai, K.; Mizukami, T.; Abe, T.; Ebara, T.; Ohno, T.; Minato, K.; Saito, R.; Yamada, M.; Nakano, T. Hypofractionated carbon-ion radiotherapy for stage I peripheral nonsmall cell lung cancer (GUNMA0701): Prospective phase II study. Cancer Med. 2019, 8, 6644–6650. [Google Scholar] [CrossRef]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Ohno, T.; Kanai, T.; Yamada, S.; Yusa, K.; Tashiro, M.; Shimada, H.; Torikai, K.; Yoshida, Y.; Kitada, Y.; Katoh, H.; et al. Carbon Ion Radiotherapy at the Gunma University Heavy Ion Medical Center: New Facility Set-up. Cancers 2011, 3, 4046–4060. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Martinez, F.J.; Flint, A.; Jamadar, D.A.; Gross, B.H.; Spizarny, D.L.; Cascade, P.N.; Whyte, R.I.; Lynch, J.P., 3rd; Toews, G. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: Correlation with pathologic scoring. AJR Am. J. Roentgenol. 1997, 169, 977–983. [Google Scholar] [CrossRef]
- Fujiki, Y.; Kotani, T.; Isoda, K.; Ishida, T.; Shoda, T.; Yoshida, S.; Takeuchi, T.; Makino, S. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod. Rheumatol. 2018, 28, 133–140. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 10 May 2019).
- Brierley, J.; Gospodarowicz, M.; Wittekind, C. UICC TNM Classification of Malignant Tumours, 8th ed.; Wiley: Chichester, UK, 2017. [Google Scholar]
- Doi, H.; Nakamatsu, K.; Nishimura, Y. Stereotactic body radiotherapy in patients with chronic obstructive pulmonary disease and interstitial pneumonia: A review. Int. J. Clin. Oncol. 2019, 24, 899–909. [Google Scholar] [CrossRef]
- Yoshitake, T.; Shioyama, Y.; Asai, K.; Nakamura, K.; Sasaki, T.; Ohga, S.; Kamitani, T.; Yamaguchi, T.; Ohshima, K.; Matsumoto, K.; et al. Impact of Interstitial Changes on Radiation Pneumonitis After Stereotactic Body Radiation Therapy for Lung Cancer. Anticancer Res. 2015, 35, 4909–4913. [Google Scholar] [PubMed]
- Nakamura, M.; Nishimura, H.; Nakayama, M.; Mayahara, H.; Uezono, H.; Harada, A.; Hashimoto, N.; Ejima, Y.; Ishihara, T.; Sasaki, R. Dosimetric factors predicting radiation pneumonitis after CyberKnife stereotactic body radiotherapy for peripheral lung cancer. Br. J. Radiol. 2016, 89, 20160560. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Itonaga, T.; Saito, T.; Shiraishi, S.; Mikami, R.; Nakayama, H.; Sakurada, A.; Sugahara, S.; Koizumi, K.; Tokuuye, K. Predicting risk factors for radiation pneumonitis after stereotactic body radiation therapy for primary or metastatic lung tumours. Br. J. Radiol. 2017, 90, 20160508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurugai, Y.; Takeda, A.; Sanuki, N.; Enomoto, T.; Kaneko, T.; Hara, Y.; Mizuno, T.; Saeki, N.; Aoki, Y.; Oku, Y.; et al. Stereotactic body radiotherapy for lung cancer patients with idiopathic interstitial pneumonias. Radiother. Oncol. 2017, 125, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ebara, T.; Shimada, H.; Kawamura, H.; Shirai, K.; Saito, J.; Kawashima, M.; Tashiro, M.; Ohno, T.; Kanai, T.; Nakano, T. Dosimetric analysis between carbon ion radiotherapy and stereotactic body radiotherapy in stage I lung cancer. Anticancer Res. 2014, 34, 5099–5104. [Google Scholar]
- Yamaguchi, S.; Ohguri, T.; Ide, S.; Aoki, T.; Imada, H.; Yahara, K.; Narisada, H.; Korogi, Y. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer 2013, 82, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Takeda, A.; Eriguchi, T.; Sanuki, N.; Aoki, Y.; Nishimura, S.; Enomoto, T.; Shinkai, M.; Kawana, A.; Kaneko, T. Stereotactic body radiotherapy for chronic obstructive pulmonary disease patients undergoing or eligible for long-term domiciliary oxygen therapy. J. Radiat. Res. 2016, 57, 62–67. [Google Scholar] [CrossRef]
- Sekihara, K.; Aokage, K.; Oki, T.; Omori, T.; Katsumata, S.; Ueda, T.; Miyoshi, T.; Goto, M.; Nakasone, S.; Ichikawa, T.; et al. Long-term survival after complete resection of non-small-cell lung cancer in patients with interstitial lung disease. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 638–643. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).