Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnosis of RRMM
3. Indications to Initiate Therapy
4. Choosing the Right Therapy
4.1. Multidisciplinary Decision Making for Therapeutic Options in the Relapsed/Refractory Setting
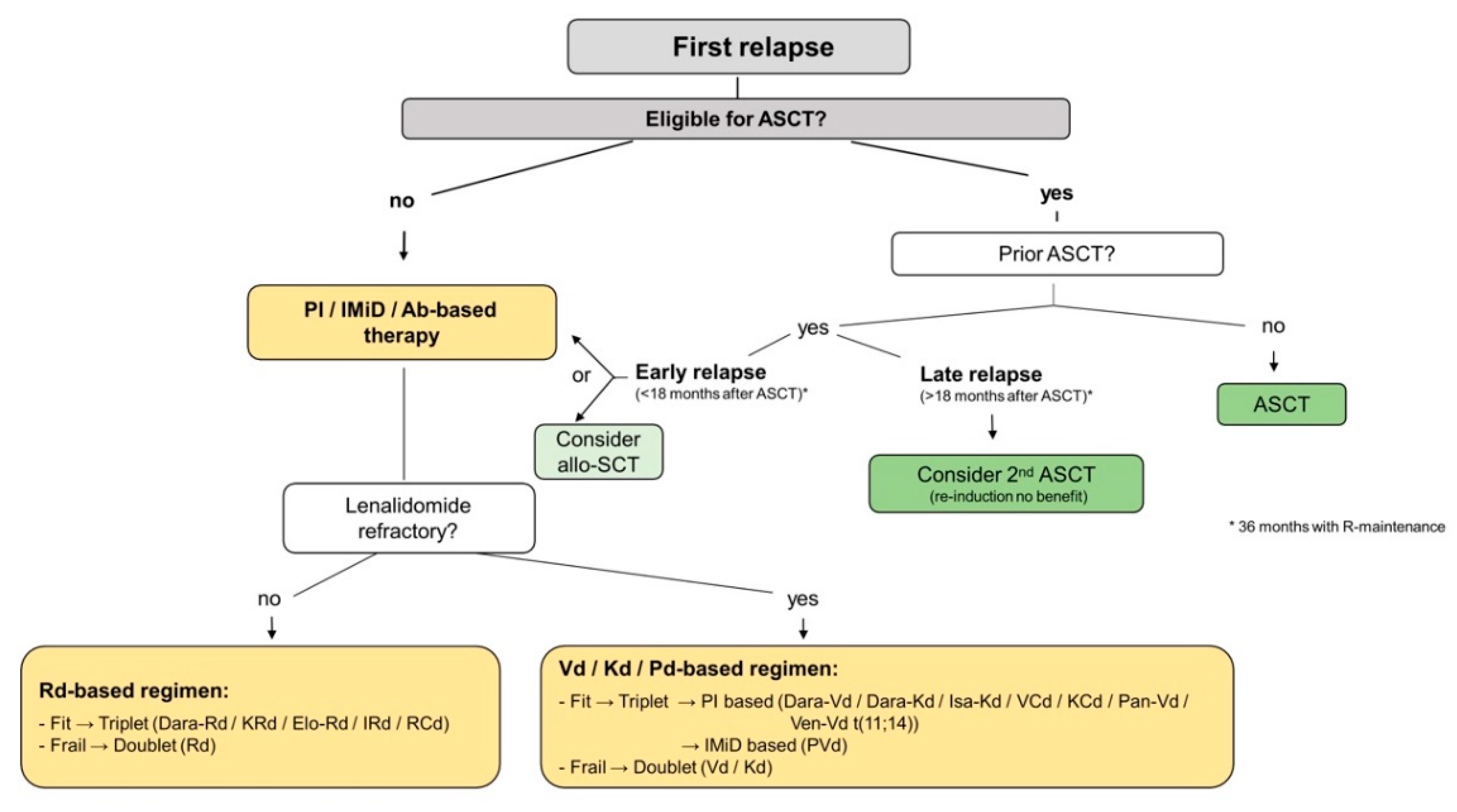
4.2. First Relapse
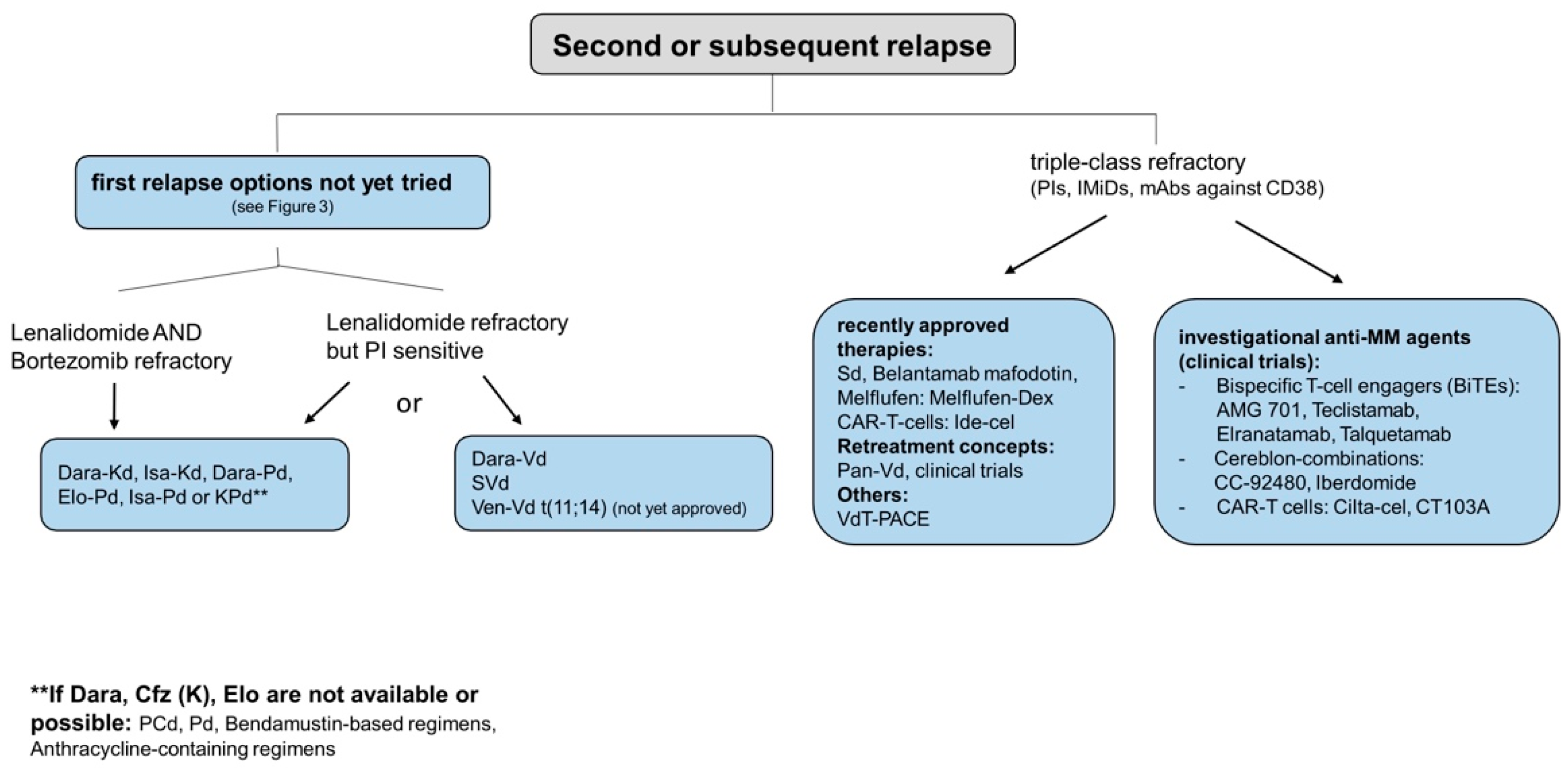
4.3. Second/Subsequent Relapse
4.4. Supportives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Bladé, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [Green Version]
- Gengenbach, L.; Reinhardt, H.; Ihorst, G.; Ajayi, S.; Dold, S.M.; Kohler, M.; Einsele, H.; Duyster, J.; Wäsch, R.; Engelhardt, M. Navigating the changing multiple myeloma treatment landscape: Clinical practice patterns of MM patients treated in- and outside German DSMM study group trials. Leuk. Lymphoma 2018, 59, 2692–2699. [Google Scholar] [CrossRef]
- Engelhardt, M.; Selder, R.; Pandurevic, M.; Möller, M.; Ihorst, G.; Waldschmidt, J.; Herget, G.; Wäsch, R. Multidisciplinary Tumor Boards: Facts and Satisfaction Analysis of an Indispensable Comprehensive Cancer Center Instrument. DMW Dtsch. Med. Wochenschr. 2017, 142, e51–e60. [Google Scholar] [CrossRef]
- Wildes, T.M.; Campagnaro, E. Management of multiple myeloma in older adults: Gaining ground with geriatric assessment. J. Geriatr. Oncol. 2016, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Schinke, M.; Ihorst, G.; Duyster, J.; Wäsch, R.; Schumacher, M.; Engelhardt, M. Risk of disease recurrence and survival in patients with multiple myeloma: A German Study Group analysis using a conditional survival approach with long-term follow-up of 815 patients. Cancer 2020, 126, 3504–3515. [Google Scholar] [CrossRef]
- Rosko, A.E.; Cordoba, R.; Abel, G.; Artz, A.; Loh, K.P.; Klepin, H.D. Advances in Management for Older Adults With Hematologic Malignancies. J. Clin. Oncol. 2021, 39, 2102–2114. [Google Scholar] [CrossRef]
- LaRocca, A.; Dold, S.M.; Zweegman, S.; Terpos, E.; Wäsch, R.; D’Agostino, M.; Scheubeck, S.; Goldschmidt, H.; Gay, F.; Cavo, M.; et al. Patient-centered practice in elderly myeloma patients: An overview and consensus from the European Myeloma Network (EMN). Leukemia 2018, 32, 1697–1712. [Google Scholar] [CrossRef]
- Engelhardt, M.; Dold, S.M.; Ihorst, G.; Zober, A.; Möller, M.; Reinhardt, H.; Hieke, S.; Schumacher, M.; Wäsch, R. Geriatric assessment in multiple myeloma patients: Validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica 2016, 101, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Salazar, A.S.; Recinos, L.M.; Mian, H.S.; Stoll, C.; Simon, L.; Sekhon, S.; Colditz, G.; Wildes, T.M. Geriatric Assessment and Frailty Scores Predict Mortality in Myeloma: Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2019, 19, 488–496.e6. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Kumar, S.K.; Moreau, P.; Lee, J.H.; Lahuerta, J.J.; Morgan, G.; Richardson, P.G.; Crowley, J.; Haessler, J.; Feather, J.; Hoering, A.; et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2011, 26, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Laubach, J.P.; Garderet, L.; Mahindra, A.; Gahrton, G.; Caers, J.; Sezer, O.; Voorhees, P.M.; Leleu, X.; Johnsen, H.E.; Streetly, M.; et al. Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia 2015, 30, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.-J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Naegele, M.; Leppla, L.; Kiote-Schmidt, C.; Ihorst, G.; Rebafka, A.; Koller, A.; May, A.M.; Hasemann, M.; Duyster, J.; Wäsch, R.; et al. Trained clinical nurse specialists proficiently obtain bone marrow aspirates and trephine biopsies in a nearly painless procedure—A prospective evaluation study. Ann. Hematol. 2015, 94, 1577–1584. [Google Scholar] [CrossRef]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef]
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.-L.; Dejoie, T.; Maheo, S.; Stoppa, A.-M.; Pegourie, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018, 132, 2456–2464. [Google Scholar] [CrossRef] [Green Version]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Dold, S.M.; Riebl, V.; Wider, D.; Follo, M.; Pantic, M.; Ihorst, G.; Duyster, J.; Zeiser, R.; Wäsch, R.; Engelhardt, M. Validated single-tube multiparameter flow cytometry approach for the assessment of minimal residual disease in multiple myeloma. Haematologica 2020, 105, e523. [Google Scholar] [CrossRef] [Green Version]
- Caers, J.; Garderet, L.; Kortüm, K.M.; O’Dwyer, M.E.; Van De Donk, N.W.; Binder, M.; Dold, S.M.; Gay, F.; Corre, J.; Beguin, Y.; et al. European Myeloma Network recommendations on tools for the diagnosis and monitoring of multiple myeloma: What to use and when. Haematologica 2018, 103, 1772–1784. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; Garcia-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2018, 93, 1091–1110. [Google Scholar] [CrossRef] [Green Version]
- Sonneveld, P. Management of multiple myeloma in the relapsed/refractory patient. Hematology 2017, 2017, 508–517. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Rosiñol, L.; Beksac, M.; Zamagni, E.; Van de Donk, N.W.C.J.; Anderson, K.C.; Badros, A.; Caers, J.; Cavo, M.; Dimopoulos, M.; Dispenzieri, A.; et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: Definition, disease assessment and treatment considerations. Br. J. Haematol. 2021, 194, 496–507. [Google Scholar] [CrossRef]
- Zhou, X.; Dierks, A.; Kertels, O.; Samnick, S.; Kircher, M.; Buck, A.K.; Haertle, L.; Knorz, S.; Böckle, D.; Scheller, L.; et al. The Link between Cytogenetics/Genomics and Imaging Patterns of Relapse and Progression in Patients with Relapsed/Refractory Multiple Myeloma: A Pilot Study Utilizing 18F-FDG PET/CT. Cancers 2020, 12, 2399. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.-H. Treatment of relapsed and refractory multiple myeloma. Blood Res. 2020, 55, S43–S53. [Google Scholar] [CrossRef]
- Katodritou, E.; Kyrtsonis, M.-C.; Delimpasi, S.; Kyriakou, D.; Symeonidis, A.; Spanoudakis, E.; Vasilopoulos, G.; Anagnostopoulos, A.; Kioumi, A.; Zikos, P.; et al. Real-world data on Len/Dex combination at second-line therapy of multiple myeloma: Treatment at biochemical relapse is a significant prognostic factor for progression-free survival. Ann. Hematol. 2018, 97, 1671–1682. [Google Scholar] [CrossRef] [Green Version]
- Dispenzieri, A.; Stewart, A.K.; Chanan-Khan, A.; Rajkumar, S.V.; Kyle, R.A.; Fonseca, R.; Kapoor, P.; Bergsagel, P.L.; McCurdy, A.; Gertz, M.A.; et al. Smoldering multiple myeloma requiring treatment: Time for a new definition? Blood 2013, 122, 4172–4181. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, M.; Shoumariyeh, K.; Rösner, A.; Ihorst, G.; Biavasco, F.; Meckel, K.; Von Metzler, I.; Treurich, S.; Hebart, H.; Grube, M.; et al. Clinical characteristics and outcome of multiple myeloma patients with concomitant COVID-19 at Comprehensive Cancer Centers in Germany. Haematologica 2020, 105, 2872–2878. [Google Scholar] [CrossRef]
- Chari, A.; Samur, M.; Martinez-Lopez, J.; Cook, G.; Biran, N.; Yong, K.; Hungria, V.; Engelhardt, M.; Gay, F.; Feria, A.G.; et al. Clinical Features Associated with COVID-19 Outcome in multiple meloma: First results from International Myeloma Society COVID-19 Dataset. Blood 2020, 136, 3033–3040. [Google Scholar] [CrossRef]
- Terpos, E.; Engelhardt, M.; Cook, G.; Gay, F.; Mateos, M.-V.; Ntanasis-Stathopoulos, I.; Van De Donk, N.W.C.J.; Avet-Loiseau, H.; Hajek, R.; Vangsted, A.J.; et al. Management of patients with multiple myeloma in the era of COVID-19 pandemic: A consensus paper from the European Myeloma Network (EMN). Leukemia 2020, 34, 2000–2011. [Google Scholar] [CrossRef]
- Shoumariyeh, K.; Biavasco, F.; Ihorst, G.; Rieg, S.; Nieters, A.; Kern, W.V.; Miething, C.; Duyster, J.; Engelhardt, M.; Bertz, H. Covid-19 in patients with hematological and solid cancers at a Comprehensive Cancer Center in Germany. Cancer Med. 2020, 9, 8412–8422. [Google Scholar] [CrossRef]
- Mian, H.; Grant, S.J.; Engelhardt, M.; Pawlyn, C.; Bringhen, S.; Zweegman, S.; Stege, C.A.; Rosko, A.E.; Von Lilienfeld-Toal, M.; Wildes, T.M. Caring for older adults with multiple myeloma during the COVID-19 Pandemic: Perspective from the International Forum for Optimizing Care of Older Adults with Myeloma. J. Geriatr. Oncol. 2020, 11, 764–768. [Google Scholar] [CrossRef]
- Dersch, R.; Wehrum, T.; Fähndrich, S.; Engelhardt, M.; Rauer, S.; Berger, B. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult. Scler. J. 2020, 26, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Ihorst, G.; Caers, J.; Günther, A.; Wäsch, R. Autotransplants in older multiple myeloma patients: Hype or hope in the era of novel agents? Haematologica 2016, 101, 1276–1278. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, M.; Domm, A.-S.-S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Dold, S.M.; Möller, M.-D.; Ihorst, G.; Langer, C.; Pönisch, W.; Mügge, L.-O.; Knop, S.; Jung, J.; Greil, C.; Wäsch, R.; et al. Validation of the revised myeloma comorbidity index and other comorbidity scores in a multicenter German study group multiple myeloma trial. Haematologica 2020, 106, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Ihorst, G.; Duque-Afonso, J.; Wedding, U.; Spät-Schwalbe, E.; Goede, V.; Kolb, G.; Stauder, R.; Wäsch, R. Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age. Haematologica 2020, 105, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, M.; Ihorst, G.; Schumacher, M.; Rassner, M.; Gengenbach, L.; Möller, M.; Shoumariyeh, K.; Neubauer, J.; Farthmann, J.; Herget, G.; et al. Multidisciplinary tumor boards and their analyses: The yin and yang of outcome measures. BMC Cancer 2021, 21, 173. [Google Scholar] [CrossRef]
- Küchlin, S.; Duffner, J.; Scheubeck, S.; Schoeller, K.; Maruschke, L.; Seidl, M.; Waldschmidt, J.M.; Engelhardt, M.; Walz, G.; Prager, E.P.; et al. Kidney embolization induces prompt organ response in a 86-year-old patient with MGRS-related AL-amyloidosis. Hemodial. Int. 2019, 23, E59–E64. [Google Scholar] [CrossRef]
- Greil, C.; Engelhardt, M.; Ihorst, G.; Schoeller, K.; Bertz, H.; Marks, R.; Zeiser, R.; Duyster, J.; Einsele, H.; Finke, J.; et al. Allogeneic transplantation of multiple myeloma patients may allow long-term survival in carefully selected patients with acceptable toxicity and preserved quality of life. Haematologica 2018, 104, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Möller, M.-D.; Ihorst, G.; Pahl, A.; Scheubeck, S.; Barsch, F.; Dold, S.M.; Bertz, H.; Arends, J.; Wäsch, R.; Engelhardt, M. Physical activity is associated with less comorbidity, better treatment tolerance and improved response in patients with multiple myeloma undergoing stem cell transplantation. J. Geriatr. Oncol. 2021, 12, 521–530. [Google Scholar] [CrossRef]
- Engelhardt, M.; Ihorst, G.; Singh, M.; Rieth, A.; Saba, G.; Pellan, M.; Lebioda, A. Real-World Evaluation of Health-Related Quality of Life in Patients With Multiple Myeloma From Germany. Clin. Lymphoma Myeloma Leuk. 2020, 21, e160–e175. [Google Scholar] [CrossRef]
- Scheubeck, S.; Ihorst, G.; Schoeller, K.; Holler, M.; Möller, M.; Reinhardt, H.; Wäsch, R.; Engelhardt, M. Comparison of the prognostic significance of 5 comorbidity scores and 12 functional tests in a prospective multiple myeloma patient cohort. Cancer 2021, 127, 3422–3436. [Google Scholar] [CrossRef]
- Cook, G.; Larocca, A.; Facon, T.; Zweegman, S.; Engelhardt, M. Defining the vulnerable patient with myeloma—a frailty position paper of the European Myeloma Network. Leukemia 2020, 34, 2285–2294. [Google Scholar] [CrossRef]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.-M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef]
- Gross, A.L.; Xue, Q.-L.; Bandeen-Roche, K.; Fried, L.P.; Varadhan, R.; McAdams-DeMarco, M.A.; Walston, J.; Carlson, M.C. Declines and Impairment in Executive Function Predict Onset of Physical Frailty. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Bowcock, S.; Rachet, B. Survival trends in elderly myeloma patients. Eur. J. Haematol. 2020, 106, 126–131. [Google Scholar] [CrossRef]
- Pulte, D.; Redaniel, T.; Lowry, L.; Bird, J.; Jeffreys, M. Age disparities in survival from lymphoma and myeloma: A comparison between US and England. Br. J. Haematol. 2014, 165, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.D.; Newman, A.B.; Hirsch, C.; Gottdiener, J.S.; Seeman, T.; Tracy, R.P.; Kop, W.J.; Burke, G.L.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef]
- Hieke, S.; Kleber, M.; König, C.; Engelhardt, M.; Schumacher, M. Conditional Survival: A Useful Concept to Provide Information on How Prognosis Evolves over Time. Clin. Cancer Res. 2015, 21, 1530–1536. [Google Scholar] [CrossRef] [Green Version]
- Kleber, M.; Ihorst, G.; Terhorst, M.; Koch, B.; Deschler, B.; Wäsch, R.; Engelhardt, M. Comorbidity as a prognostic variable in multiple myeloma: Comparative evaluation of common comorbidity scores and use of a novel MM–comorbidity score. Blood Cancer J. 2011, 1, e35. [Google Scholar] [CrossRef]
- Kleber, M.; Ihorst, G.; Groß, B.; Koch, B.; Reinhardt, H.; Wäsch, R.; Engelhardt, M. Validation of the Freiburg Comorbidity Index in 466 Multiple Myeloma Patients and Combination With the International Staging System Are Highly Predictive for Outcome. Clin. Lymphoma Myeloma Leuk. 2013, 13, 541–551. [Google Scholar] [CrossRef]
- Mian, H.S.; Wildes, T.M.; Fiala, M.A. Development of a Medicare Health Outcomes Survey Deficit-Accumulation Frailty Index and Its Application to Older Patients With Newly Diagnosed Multiple Myeloma. JCO Clin. Cancer Inform. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Dumontier, C.; Loh, K.P.; Bain, P.A.; Silliman, R.A.; Hshieh, T.; Abel, G.A.; Djulbegovic, B.; Driver, J.A.; Dale, W. Defining Undertreatment and Overtreatment in Older Adults With Cancer: A Scoping Literature Review. J. Clin. Oncol. 2020, 38, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Zweegman, S.; Engelhardt, M.; Larocca, A. Elderly patients with multiple myeloma: Towards a frailty approach? Curr. Opin. Oncol. 2017, 29, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bringhen, S.; Mateos, M.V.; Zweegman, S.; Larocca, A.; Falcone, A.P.; Oriol, A.; Rossi, D.; Cavalli, M.; Wijermans, P.; Ria, R.; et al. Age and organ damage correlate with poor survival in myeloma patients: Meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 2013, 98, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Bringhen, S.; Ludwig, H.; Dimopoulos, M.; Bladé, J.; Mateos, M.V.; Rosiñol, L.; Boccadoro, M.; Cavo, M.; Lokhorst, H.; et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood 2011, 118, 4519–4529. [Google Scholar] [CrossRef]
- Moreau, P.; Kumar, S.K.; Miguel, J.S.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- Bobin, A.; Liuu, E.; Moya, N.; Gruchet, C.; Sabirou, F.; Lévy, A.; Gardeney, H.; Nsiala, L.; Cailly, L.; Guidez, S.; et al. Multiple Myeloma: An Overview of the Current and Novel Therapeutic Approaches in 2020. Cancers 2020, 12, 2885. [Google Scholar] [CrossRef]
- Cook, G.; Ashcroft, A.J.; Cairns, D.; Williams, C.D.; Brown, J.M.; Cavenagh, J.D.; Snowden, J.; Parrish, C.; Yong, K.; Cavet, J.; et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): A randomised, open-label, phase 3 trial. Lancet Haematol. 2016, 3, e340–e351. [Google Scholar] [CrossRef] [Green Version]
- Cook, G.; Williams, C.; Cairns, D.; Hockaday, A.; Cavenagh, J.; Snowden, F.J.; Parrish, C.; Yong, M.K.L.; Cavet, F.J.; Hunter, H.; et al. A Salvage Autologous Stem Cell Transplant (ASCT2) Induces Superior Overall Survival Following Bortezomib-Containing Re-Induction Therapy for Relapsed Multiple Myeloma (MM): Results from the Myeloma X (Intensive) Trial. Blood 2015, 126, 394. [Google Scholar] [CrossRef]
- Cook, G.; Williams, C.; Brown, J.M.; Cairns, D.; Cavenagh, J.; A Snowden, J.; Ashcroft, A.J.; Fletcher, M.; Parrish, C.; Yong, K.; et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 874–885. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Moreau, P.; Terpos, E.; Mateos, M.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Rasche, L.; Wäsch, R.; Munder, M.; Goldschmidt, H.; Raab, M.S. Novel immunotherapies in multiple myeloma—Chances and challenges. Haematologica 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Bladé, J.; Bringhen, S.; Ocio, E.M.; Efebera, Y.; Pour, L.; Gay, F.; Sonneveld, P.; Gullbo, J.; Richardson, P.G. Melflufen: A Peptide–Drug Conjugate for the Treatment of Multiple Myeloma. J. Clin. Med. 2020, 9, 3120. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2019, 21, 207–221. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Hu, G.; Wang, W.; Xiao, Y.; Cai, H.; Jiang, L.; Meng, L.; Yang, Y.; Zhou, X.; et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 2890–2901. [Google Scholar] [CrossRef]
- Madduri, D.; Usmani, S.Z.; Jagannath, S.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Banerjee, A.; Goldberg, J.D.; Schecter, J.M.; et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM). Blood 2019, 134, 577. [Google Scholar] [CrossRef]
- Maples, K.T.; Johnson, C.; Lonial, S. Antibody Treatment in Multiple Myeloma. Clin. Adv. Hematol. Oncol. 2021, 19, 166–174. [Google Scholar]
- Zhou, X.; Einsele, H.; Danhof, S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J. Clin. Med. 2020, 9, 2166. [Google Scholar] [CrossRef]
- Caraccio, C.; Krishna, S.; Phillips, D.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Wäsch, R.; Munder, M.; Marks, R. Teaming up for CAR-T cell therapy. Haematologica 2019, 104, 2335–2336. [Google Scholar] [CrossRef] [Green Version]
- Bruno, B.; Wäsch, R.; Engelhardt, M.; Gay, F.; Giaccone, L.; D’Agostino, M.; Rodríguez-Lobato, L.-G.; Danhof, S.; Gagelmann, N.; Kröger, N.; et al. European Myeloma Network perspective on CAR T-Cell therapies for multiple myeloma. Haematologica 2021, 106, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, C.C.; Kang, J.; Amatangelo, M.; Polonskaia, A.; Katz, M.; Chiu, H.; Couto, S.; Wang, M.; Ren, Y.; Ortiz, M.; et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia 2019, 34, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.D.; Correa, M.; Nagy, M.A.; Alexander, M.; Plantevin, V.; Grant, V.; Whitefield, B.; Huang, D.; Kercher, T.; Harris, R.; et al. Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma. J. Med. Chem. 2020, 63, 6648–6676. [Google Scholar] [CrossRef] [PubMed]
- Gormley, N.J.; Pazdur, R. Immunotherapy Combinations in Multiple Myeloma—Known Unknowns. N. Engl. J. Med. 2018, 379, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Greil, C.; Hudecek, M.; Lonial, S.; Raje, N.; Wäsch, R.; Engelhardt, M. Current developments in immunotherapy in the treatment of multiple myeloma. Cancer 2018, 124, 2075–2085. [Google Scholar] [CrossRef] [Green Version]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef]
- Engelhardt, M.; Herget, G.W.; Graziani, G.; Ihorst, G.; Reinhardt, H.; Ajayi, S.; Knop, S.; Wäsch, R. Osteoprotective medication in the era of novel agents: A European perspective on values, risks and future solutions. Haematologica 2018, 103, 755–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herget, G.W.; Kälberer, F.; Ihorst, G.; Graziani, G.; Klein, L.; Rassner, M.; Gehler, C.; Jung, J.; Schmal, H.; Wäsch, R.; et al. Interdisciplinary approach to multiple myeloma—Time to diagnosis and warning signs. Leuk. Lymphoma 2020, 62, 891–898. [Google Scholar] [CrossRef]
- Graziani, G.; Herget, G.W.; Ihorst, G.; Zeissig, M.; Chaidos, A.; Auner, H.; Duyster, J.; Wäsch, R.; Engelhardt, M. Time from first symptom onset to the final diagnosis of multiple myeloma (MM)—Possible risks and future solutions: Retrospective and prospective ‘Deutsche Studiengruppe MM’ (DSMM) and ‘European Myeloma Network’ (EMN) analysis. Leuk. Lymphoma 2019, 61, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Herget, G.W.; Wäsch, R.; Klein, L.; Schmal, H.; Terpos, E.; Engelhardt, M. Prevention of bone disease and early detection of impending fractures in multiple myeloma patients can reduce morbidity and mortality: The necessity of interdisciplinary state-of-the-art treatment. Haematologica 2020, 105, 859–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, S.; Lentzsch, S.; Shen, Y.; Tsai, W.-Y.; Wright, J.D.; Hershman, D.L.; Neugut, A.I. Use and impact of herpes zoster prophylaxis in myeloma patients treated with proteasome inhibitors. Leuk. Lymphoma 2018, 59, 2465–2469. [Google Scholar] [CrossRef]
- Stern, A.; Green, H.; Paul, M.; Vidal, L.; Leibovici, L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst. Rev. 2014, 2014, CD005590. [Google Scholar] [CrossRef] [PubMed]
- Reiser, M.; Borte, M.; Huscher, D.; Baumann, U.; Pittrow, D.; Sommer, C.; Stangel, M.; Fasshauer, M.; Gold, R.; Hensel, M. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: Long-term data of the SIGNS study. Eur. J. Haematol. 2017, 99, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Raanani, P.; Gafter-Gvili, A.; Paul, M.; Ben-Bassat, I.; Leibovici, L.; Shpilberg, O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: Systematic review and meta-analysis. Leuk. Lymphoma 2009, 50, 764–772. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gengenbach, L.; Graziani, G.; Reinhardt, H.; Rösner, A.; Braun, M.; Möller, M.-D.; Greil, C.; Wäsch, R.; Engelhardt, M. Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors. Cancers 2021, 13, 4320. https://doi.org/10.3390/cancers13174320
Gengenbach L, Graziani G, Reinhardt H, Rösner A, Braun M, Möller M-D, Greil C, Wäsch R, Engelhardt M. Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors. Cancers. 2021; 13(17):4320. https://doi.org/10.3390/cancers13174320
Chicago/Turabian StyleGengenbach, Laura, Giulia Graziani, Heike Reinhardt, Amelie Rösner, Magdalena Braun, Mandy-Deborah Möller, Christine Greil, Ralph Wäsch, and Monika Engelhardt. 2021. "Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors" Cancers 13, no. 17: 4320. https://doi.org/10.3390/cancers13174320
APA StyleGengenbach, L., Graziani, G., Reinhardt, H., Rösner, A., Braun, M., Möller, M.-D., Greil, C., Wäsch, R., & Engelhardt, M. (2021). Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors. Cancers, 13(17), 4320. https://doi.org/10.3390/cancers13174320





