68Ga-DOTATATE PET/CT and MRI with Diffusion-Weighted Imaging (DWI) in Short- and Long-Term Assessment of Tumor Response of Neuroendocrine Liver Metastases (NELM) Following Transarterial Radioembolization (TARE)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. TARE
2.3. MR Imaging
2.4. PET/CT
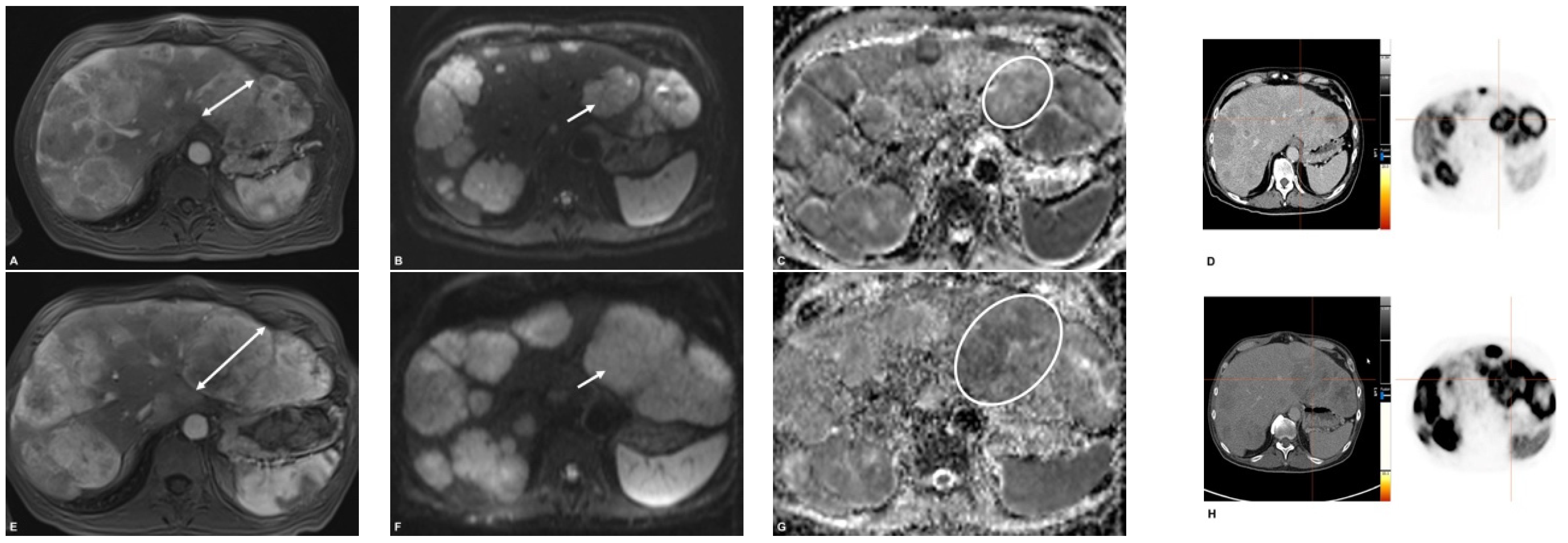
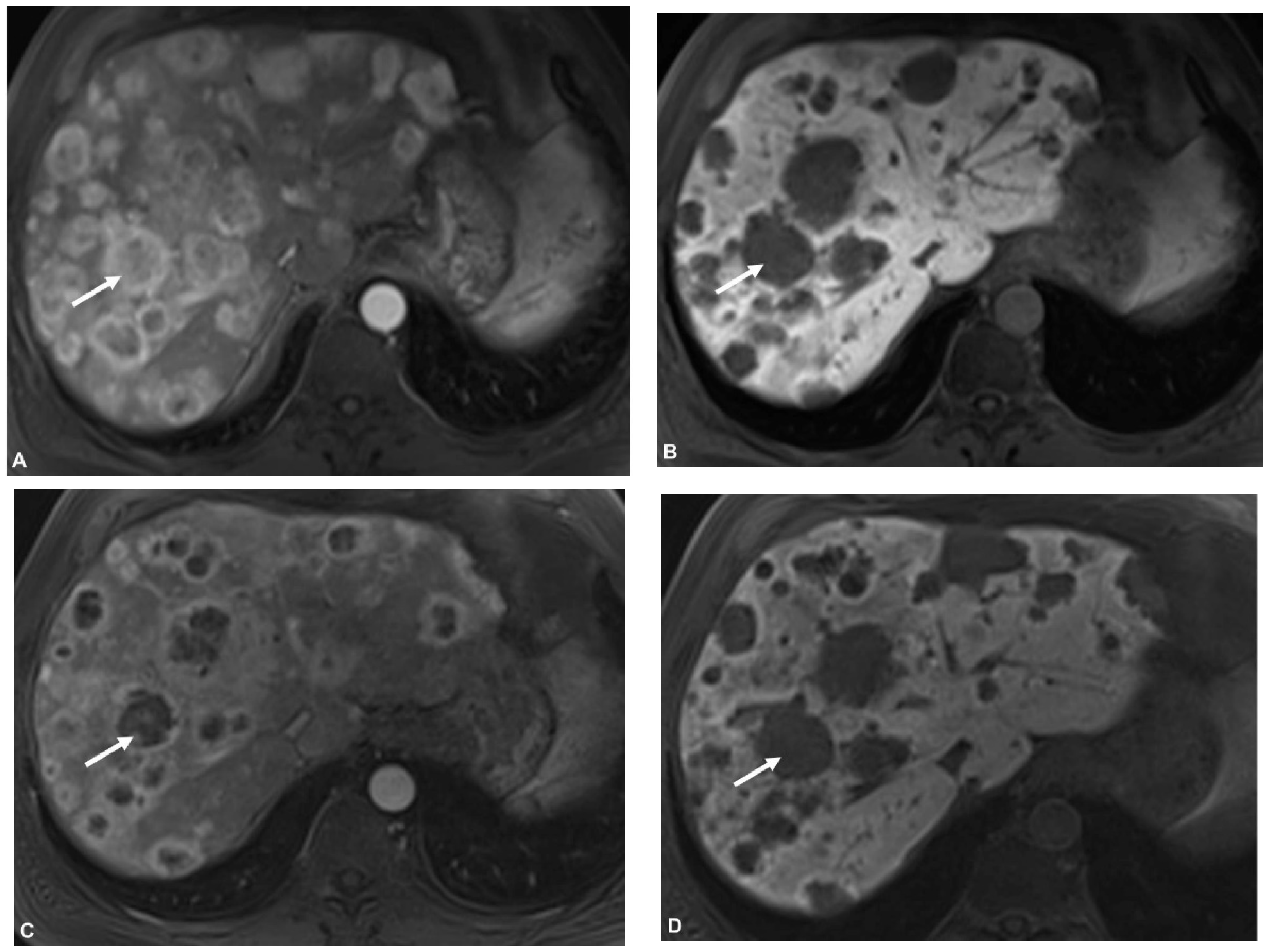
2.5. Image Analysis
2.6. Standard of Reference and Response to Treatment
2.7. Statistical Analysis
3. Results
3.1. Patients’ Cohort and TARE
3.2. Pre- and Postinterventional Measurements
3.3. Response Assessment on First Follow-Up
3.4. Response According to HPFS > 6 Months
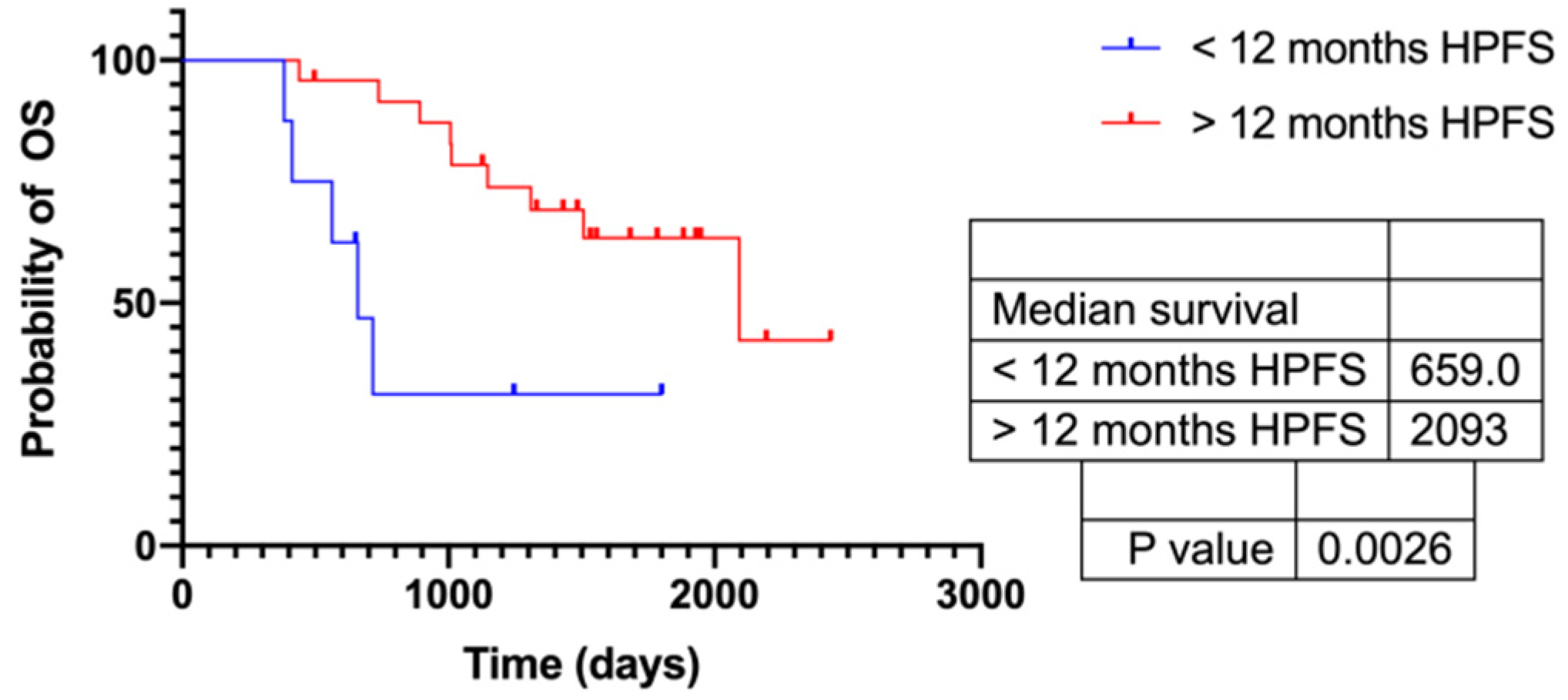
3.5. Response According to HPFS > 12 Months
3.6. Response According to HPFS > Median (720 d)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB 2015, 17, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Gophinatan, A. Yttrium-90 radioembolization of liver tumors: What do the images tell us? Cancer Imaging 2014, 13, 645–657. [Google Scholar] [CrossRef]
- Singh, S.; Poon, R.; Wong, R.; Metser, U. 68Ga PET Imaging in Patients with Neuroendocrine Tumors: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2018, 43, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Burggraaff, C.; De Jong, A.; Hoekstra, O.S.; Hoetjes, N.J.; Nievelstein, R.A.J.; Jansma, E.; Heymans, M.; De Vet, H.C.W.; Zijlstra, J.M. Predictive value of interim positron emission tomography in diffuse large B-cell lymphoma: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Eschmann, S.M.; Friedel, G.; Paulsen, F.; Reimold, M.; Hehr, T.; Budach, W.; Langen, H.-J.; Bares, R. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pinker, K.; Riedl, C.; Weber, W.A. Evaluating tumor response with FDG PET: Updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 55–66. [Google Scholar] [CrossRef]
- Koch, W.; Auernhammer, C.J.; Geisler, J.; Spitzweg, C.; Cyran, C.C.; Ilhan, H.; Bartenstein, P.; Haug, A. Treatment with Octreotide in Patients with Well-Differentiated Neuroendocrine Tumors of the Ileum: Prognostic Stratification with Ga-68-DOTA-TATE Positron Emission Tomography. Mol. Imaging 2014, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.R.; Auernhammer, C.J.; Wängler, B.; Schmidt, G.P.; Uebleis, C.; Göke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68Ga-DOTATATE PET/CT for the Early Prediction of Response to Somatostatin Receptor–Mediated Radionuclide Therapy in Patients with Well-Differentiated Neuroendocrine Tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Filippi, L.; Scopinaro, F.; Pelle, G.; Cianni, R.; Salvatori, R.; Schillaci, O.; Bagni, O. Molecular response assessed by 68Ga-DOTANOC and survival after 90Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 432–440. [Google Scholar] [CrossRef]
- Galea, N.; Cantisani, V.; Taouli, B. Liver lesion detection and characterization: Role of diffusion-weighted imaging. J. Magn. Reson. Imaging 2013, 37, 1260–1276. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1: ADC(mean). Oncotarget 2017, 8, 75434–75444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padhani, A.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Takashima, S.; Takayama, F.; Kawakami, S.; Saito, A.; Matsushita, T.; Momose, M.; Ishiyama, T. Head and Neck Lesions: Characterization with Diffusion-weighted Echoplanar MR Imaging. Radiology 2001, 220, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kamel, I.R.; Liapi, E.; Reyes, D.K.; Zahurak, M.; Bluemke, D.; Geschwind, J.-F.H. Unresectable Hepatocellular Carcinoma: Serial Early Vascular and Cellular Changes after Transarterial Chemoembolization as Detected with MR Imaging. Radiology 2009, 250, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Besa, C.; Ward, S.; Cui, Y.; Jajamovich, G.; Kim, M.; Taouli, B. Neuroendocrine liver metastases: Value of apparent diffusion coefficient and enhancement ratios for characterization of histopathologic grade. J. Magn. Reson. Imaging 2016, 44, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Caysa, H.; Wienke, A.; Spielmann, R.P.; Fiedler, E. Correlation Between Different ADC Fractions, Cell Count, Ki-67, Total Nucleic Areas and Average Nucleic Areas in Meningothelial Meningiomas. Anticancer. Res. 2015, 35, 6841–6846. [Google Scholar]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef] [Green Version]
- Halappa, V.G.; Corona-Villalobos, C.P.; Bonekamp, S.; Li, Z.; Reyes, D.; Cosgrove, D.; Pawlik, T.M.; Diaz, L.A.; Bhagat, N.; Eng, J.; et al. Neuroendocrine liver metastasis treated by using intraarterial therapy: Volumetric functional imaging biomarkers of early tumor response and survival. Radiology 2013, 266, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Barabasch, A.; Heinzel, A.; Bruners, P.; Kraemer, N.; Kuhl, C.K. Diffusion-weighted MRI Is Superior to PET/CT in Predicting Survival of Patients Undergoing 90Y Radioembolization of Hepatic Metastases. Radiology 2018, 288, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.; Nag, S.; Salem, R.; Murthy, R.; McEwan, A.J.; Nutting, C.; Benson, A.; Espat, J.; Bilbao, J.I.; Sharma, R.A.; et al. Recommendations for Radioembolization of Hepatic Malignancies Using Yttrium-90 Microsphere Brachytherapy: A Consensus Panel Report from the Radioembolization Brachytherapy Oncology Consortium. Int. J. Radiat. Oncol. 2007, 68, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.; de Jong, M.; de Blois, E.; Bernard, B.F.; Konijnenberg, M.; Krenning, E.P. Radiolabelling DOTA-peptides with 68Ga. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Pech, M.; Omari, J.; Fischbach, F.; Damm, R.; Fischbach, K.; Powerski, M.; Relja, B.; Wienke, A. Diffusion-Weighted Imaging Reflects Tumor Grading and Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2021, 10, 10–24. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, S.-M.; Ro, J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017, 29, 11–16. [Google Scholar] [CrossRef]
- Rossi, R.E.; Ciafardini, C.; Sciola, V.; Conte, D.; Massironi, S. Chromogranin A in the Follow-up of Gastroenteropancreatic Neuroendocrine Neoplasms: Is It Really Game Over? A Systematic Review and Meta-analysis. Pancreas 2018, 47, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.F.; Brennan, V.K.; Peters, R.; Paprottka, P.M. Association between objective response rate and overall survival in metastatic neuroendocrine tumors treated with radioembolization: A systematic literature review and regression analysis. Expert Rev. Anticancer. Ther. 2020, 20, 997–1009. [Google Scholar] [CrossRef]
- Imaoka, H.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Ohno, I.; Mitsunaga, S.; Watanabe, K.; Umemoto, K.; Kimura, G.; Suzuki, Y.; et al. Progression-free survival as a surrogate endpoint in advanced neuroendocrine neoplasms. Endocr. Relat. Cancer 2017, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.J.A.T.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.; Frilling, A.; Deroose, C.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with 90Y Resin Microspheres of Neuroendocrine Liver Metastases: International Multicenter Study on Efficacy and Toxicity. Cardiovasc. Interv. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Huizing, D.M.V.; Aalbersberg, E.A.; Versleijen, M.W.J.; Tesselaar, M.E.T.; Walraven, I.; Lahaye, M.J.; Veen, B.J.D.W.D.; Stokkel, M.P.M. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Imaoka, H.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Ohno, I.; Mitsunaga, S.; Watanabe, K.; Umemoto, K.; Kimura, G.; Suzuki, Y.; et al. Alternate Endpoints for Phase II Trials in Advanced Neuroendocrine Tumors. Oncology 2019, 24, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Schmeel, F.; Simon, B.; Luetkens, J.A.; Träber, F.; Meyer, C.; Schmeel, L.C.; Sabet, A.; Ezziddin, S.; Schild, H.H.; Hadizadeh, D.R. Prognostic value of pretreatment diffusion-weighted magnetic resonance imaging for outcome prediction of colorectal cancer liver metastases undergoing 90Y-microsphere radioembolization. J. Cancer Res. Clin. Oncol. 2017, 143, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Drewes, R.; Pech, M.; Powerski, M.; Omari, J.; Heinze, C.; Damm, R.; Wienke, A.; Surov, A. Apparent Diffusion Coefficient Can Predict Response to Chemotherapy of Liver Metastases in Colorectal Cancer. Acad. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jain, M.; Harris, A.B.; Lee, V.S.; Babb, J.; Sigmund, E.; Rueff, L.E.; Taouli, B. T1 Hyperintense Renal Lesions: Characterization with Diffusion-weighted MR Imaging versus Contrast-enhanced MR Imaging. Radiology 2009, 251, 796–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Clinical Features | Classification | Number of Patients |
|---|---|---|
| Liver intervention | None | 27 |
| RFA | 1 | |
| TARE | 4 | |
| TACE | 1 | |
| Systemic therapy | None | 4 |
| PRRT | 12 | |
| Biotherapy | 16 | |
| Chemotherapy | 6 | |
| Extrahepatic metastases | None | 7 |
| Lymph nodes | 15 | |
| Peritoneal | 5 | |
| Bone | 12 | |
| Pulmonal | 1 | |
| Mesenterial | 5 | |
| Other | 2 | |
| Intrahepatic tumor load | <10% | 7 |
| 10–25% | 13 | |
| 25–50% | 9 | |
| 51–75% | 3 | |
| >75% | 0 |
| Tumor Grade | Number | OS | HPFS |
|---|---|---|---|
| G1 | 7 | 57.7 months (95% CI: 42.6–72.7 months) | 29.5 months (95% CI: 18–41 months) |
| G2 | 20 | 50.7 months (95% CI: 40.2–61.3 months) | 26 months (95% CI: 18.3–33.8 months) |
| G3 | 2 | 23.5 months (95% CI: 23.5–23.5 months) | 9.3 months (95% CI: 0.0–22.4 months) |
| Imaging Parameters | Pretreatment | Posttreatment | Change (%) | p-Value |
|---|---|---|---|---|
| Size (mm) 1 | 32.1(±12.4) | 28.8 (±11) | −6 (−22–−2) | 0.009 |
| Tumor ADCmin 2 | 0.74 (±0.24) | 0.89 (±0.29) | 18.3 (−7.6–40.4) | 0.003 |
| Tumor ADCmean 2 | 0.88 (±0.29) | 1.05 (±0.31) | 14.2 (−2–44.6) | 0.003 |
| Liver ADCmean 2 | 0.97 (±0.22) | 0.99 (±0.20) | 0.602 | |
| Tumor SUVmax | 29.4 (±16.8) | 23.2 (±13.3) | −22.5 (−35.8–1.4) | 0.005 |
| Tumor SUVmean | 16.2 (±8.4) | 13.1 (±6.5) | −14.7 (−34.2–2.2) | 0.009 |
| Liver SUVmax | 7.4 (±2.4) | 7.2 (±3.1) | 0.700 | |
| Liver SUVmean | 5.7 (±1.6) | 5.5 (±2.1) | 0.767 | |
| Spleen SUVmax | 21.9 (±10.5) | 19.9 (±9.0) | 0.176 | |
| Spleen SUVmean | 17.3 (±8.4) | 17.5 (18.0) | 0.952 | |
| T/L (max/max) | 4.2 (±2.7) | 3.8 (±3.0) | −18.2 (−41.7–22.5) | 0.566 |
| T/S (max/max) | 1.7 (±1.3) | 1.6(±1.3) | −3.0 (−43.9–37.8) | 0.488 |
| T/L (max/mean) | 5.5 (±3.3) | 4.6 (±3.1) | −18.4 (−44.5–21.9) | 0.080 |
| T/S (max/mean) | 2.2 (± 1.8) | 2.0 (± 1.6) | −13.1 (−37.7–32.1) | 0.355 |
| T/L (mean/mean) | 3.0 (± 1.6) | 2.6 (±1.6) | −17.6 (−38.8–18.7) | 0.048 |
| T/S (mean/mean) | 1.2 (±0.9) | 1.2 (±0.9) | −4.4 (−36.1–29.6) | 0.519 |
| Treatment Response | RECIST 1.1 | mRECIST |
|---|---|---|
| PR | 5 | 21 |
| SD | 24 | 5 |
| PD | 3 | 5 |
| Not analyzed | 0 | 1 1 |
| ΔSUVmean | ΔT/L Ratio (Mean/Mean) | ΔT/L Ratio (Max/Mean) | ΔT/S Ratio (Max/Max) | ΔADCmin | ΔADCmean | |
|---|---|---|---|---|---|---|
| Best cut-off (%) | >−8 | >24 | >19 | >23 | <16 | <18 |
| Sensitivity (%) | 100 | 80 | 80 | 80 | 100 | 100 |
| Specificity (%) | 70 | 93 | 82 | 82 | 63 | 56 |
| AUC | 0.79 | 0.82 | 0.76 | 0.72 | 0.79 | 0.70 |
| ΔSUVmean | ΔT/L Ratio (Mean/Mean) | ΔT/L Ratio (Max/Mean) | ΔT/S Ratio (Max/Max) | ΔADCmin | ΔADCmean | |
|---|---|---|---|---|---|---|
| Best cut-off (%) | >−8 | >24 | >19 | >23 | <22 | <20 |
| Sensitivity (%) | 63 | 50 | 50 | 50 | 75 | 88 |
| Specificity (%) | 67 | 92 | 79 | 79 | 54 | 54 |
| AUC | 0.56 | 0.64 | 0.65 | 0.61 | 0.60 | 0.59 |
| ΔSUVmean | ΔT/L Ratio (Mean/Mean) | ΔT/L Ratio (Max/Mean) | ΔT/S Ratio (Max/Max) | ΔADCmin | ΔADCmean | |
|---|---|---|---|---|---|---|
| Best cut-off (%) | >−8 | >16 | >19 | >23 | <22 | <25 |
| Sensitivity (%) | 56 | 50 | 50 | 50 | 75 | 81 |
| Specificity (%) | 75 | 100 | 94 | 94 | 69 | 63 |
| AUC | 0.57 | 0.63 | 0.64 | 0.7 | 0.66 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingenerf, M.; Kiesl, S.; Karim, S.; Beyer, L.; Ilhan, H.; Rübenthaler, J.; Seidensticker, M.; Ricke, J.; Schmid-Tannwald, C. 68Ga-DOTATATE PET/CT and MRI with Diffusion-Weighted Imaging (DWI) in Short- and Long-Term Assessment of Tumor Response of Neuroendocrine Liver Metastases (NELM) Following Transarterial Radioembolization (TARE). Cancers 2021, 13, 4321. https://doi.org/10.3390/cancers13174321
Ingenerf M, Kiesl S, Karim S, Beyer L, Ilhan H, Rübenthaler J, Seidensticker M, Ricke J, Schmid-Tannwald C. 68Ga-DOTATATE PET/CT and MRI with Diffusion-Weighted Imaging (DWI) in Short- and Long-Term Assessment of Tumor Response of Neuroendocrine Liver Metastases (NELM) Following Transarterial Radioembolization (TARE). Cancers. 2021; 13(17):4321. https://doi.org/10.3390/cancers13174321
Chicago/Turabian StyleIngenerf, Maria, Sophia Kiesl, Salma Karim, Leonie Beyer, Harun Ilhan, Johannes Rübenthaler, Max Seidensticker, Jens Ricke, and Christine Schmid-Tannwald. 2021. "68Ga-DOTATATE PET/CT and MRI with Diffusion-Weighted Imaging (DWI) in Short- and Long-Term Assessment of Tumor Response of Neuroendocrine Liver Metastases (NELM) Following Transarterial Radioembolization (TARE)" Cancers 13, no. 17: 4321. https://doi.org/10.3390/cancers13174321






