Stereotactic Re-Irradiation for Local Recurrence after Radical Prostatectomy and Radiation Therapy: A Retrospective Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and Data Collection
2.2. Treatment Modality
2.3. Outcomes
2.4. Statistical Analysis
2.5. Review of the Literature
3. Results
3.1. Patients Baseline Characteristics
3.2. SBRT Characteristics
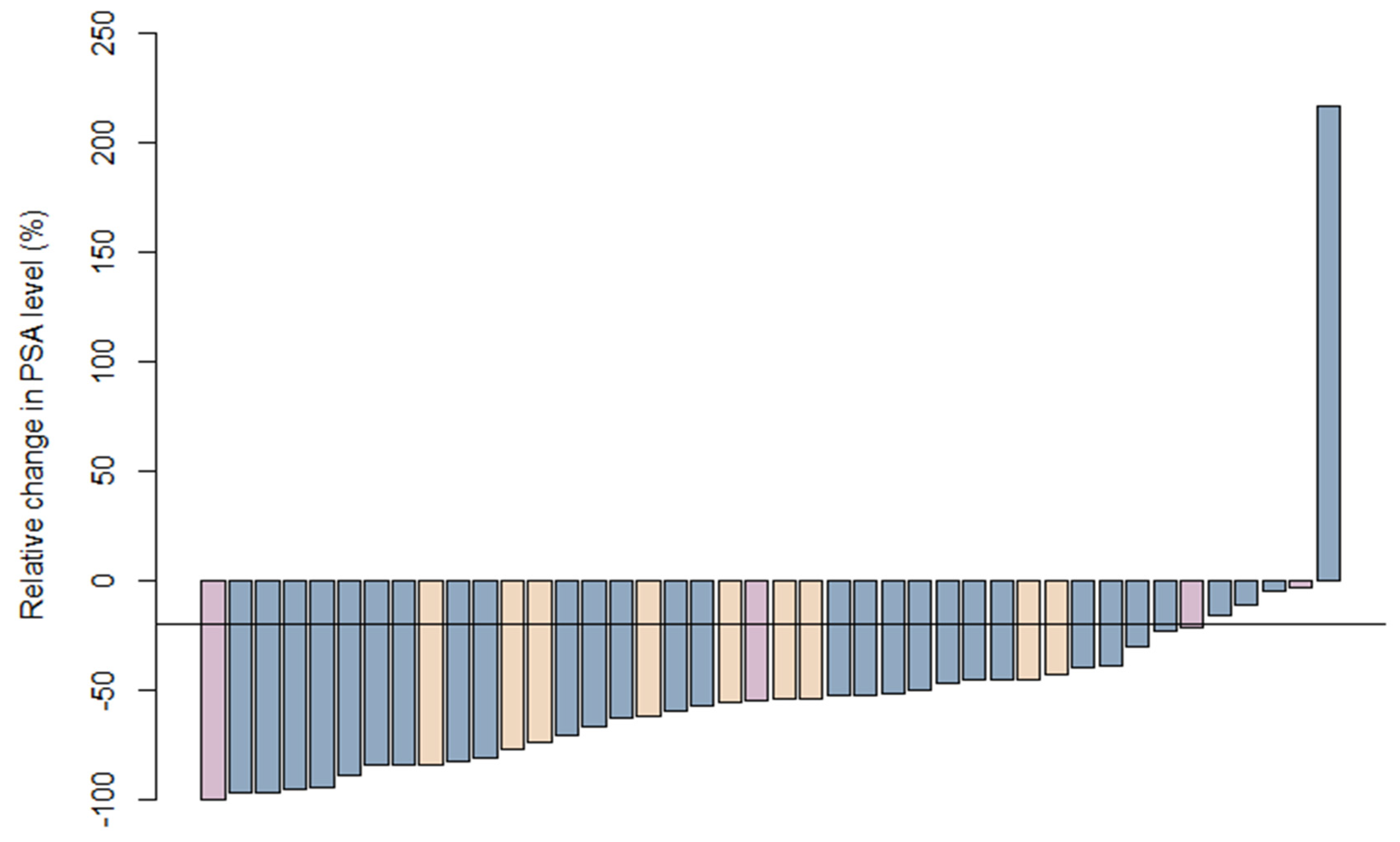
3.3. Initial Response
3.4. Safety
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet, N.; Van den Bergh, R.C.N.; Briers, E.; Cornford, P.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Eur. Assoc. Urol. 2020, 1–182. [Google Scholar] [CrossRef]
- Amling, C.L.; Blute, M.L.; Bergstralh, E.J.; Seay, T.M.; Slezak, J.; Zincke, H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: Continued risk of biochemical failure after 5 years. J. Urol. 2000, 164, 101–105. [Google Scholar] [CrossRef]
- Han, M.; Partin, A.W.; Pound, C.R.; Epstein, J.I.; Walsh, P.C. Long-term Biochemical Disease-free and Cancer-specific Survival Following Anatomic Radical Retropubic Prostatectomy: The 15-Year Johns Hopkins Experience. Urol. Clin. N. Am. 2001, 28, 555–565. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Caileaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.L.; Supiot, S.; Belkacemi, Y.; Peiffert, D.; Allouache, N.; et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Pisansky, T.M.; Thompson, I.M.; Valicenti, R.K.; D’Amico, A.V.; Selvarajah, S. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J. Urol. 2019, 202, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Carrie, C.; Hasbini, A.; de Laroche, G.; Richaud, P.; Guerif, S.; Latorzeff, I.; Supiot, S.; Bosset, M.; Lagrange, J.-L.; Beckendorf, V.; et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016, 17, 747–756. [Google Scholar] [CrossRef]
- Loriot, Y.; Supiot, S.; Beauval, J.B.; Schlürmann, F.; Pasticier, G.; Sargos, P.; Barthélémy, P.; Pignot, G.; Maillet, D.; Vincendeau, S.; et al. Management of non-metastatic castrate-resistant prostate cancer: A systematic review. Cancer Treat. Rev. 2018, 70, 223–231. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R.; et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen Deprivation Therapy for Prostate Cancer. JAMA 2005, 294, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, N.J.; Cooper, B.T. Hypofractionated radiation therapy for prostate cancer: Biologic and technical considerations. Am. J. Clin. Exp. Urol. 2014, 2, 286–293. [Google Scholar] [PubMed]
- Dasu, A.; Toma-Dasu, I. Prostate alpha/beta revisited—An analysis of clinical results from 14,168 patients. Acta Oncol. 2012, 51, 963–974. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 18 July 2021).
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Olivier, J.; Basson, L.; Puech, P.; Lacornerie, T.; Villers, A.; Wallet, J.; Lartigau, E.; Pasquier, D. Stereotactic Re-irradiation for Local Recurrence in the Prostatic Bed After Prostatectomy: Preliminary Results. Front. Oncol. 2019, 9, 71. [Google Scholar] [CrossRef]
- Detti, B.; Bonomo, P.; Masi, L.; Doro, R.; Cipressi, S.; Iermano, C.; Bonucci, I.; Franceschini, D.; di Brina, L.; Baki, M. CyberKnife stereotactic radiotherapy for isolated recurrence in the prostatic bed. World J. Urol. 2016, 34, 311–317. [Google Scholar] [CrossRef]
- Zerini, D.; Jereczek-Fossa, B.A.; Fodor, C.; Bazzani, F.; Maucieri, A.; Ronchi, S.; Ferrario, S.; Colangione, S.P.; Gerardi, M.A.; Caputo, M.; et al. Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br. J. Radiol. 2015, 88. [Google Scholar] [CrossRef] [Green Version]
- Volpe, S.; Jereczek-Fossas, B.A.; Zerini, D.; Rojas, D.P.; Fodor, C.; Vavassori, A.; Romanelli, P.; Vigorito, S.; Rondi, E.; Comi, S.; et al. Case series on multiple prostate re-irradiation for locally recurrent prostate cancer: Something ventured, something gained. Neoplasma 2019, 66, 308–314. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.R.; Di Brina, L.; Mancosu, P.; Franzese, C.; Iftode, C.; Franceschini, D.; Clerici, E.; Tozzi, A.; Navarria, P.; Scorsetti, M. Reirradiation of Locally Recurrent Prostate Cancer With Volumetric Modulated Arc Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Di Cataldo, V.; Simontacchi, G.; Detti, B.; Bonomo, P.; Masi, L.; Desideri, I.; Greto, D.; Francolini, G.; Carfora, V.; et al. Robotic Stereotactic Retreatment for Biochemical Control in Previously Irradiated Patients Affected by Recurrent Prostate Cancer. Clin. Oncol. 2018, 30, 93–100. [Google Scholar] [CrossRef]
- Arcangeli, S.; Gambardella, P.; Agolli, L.; Monaco, A.; Dognini, J.; Regine, G.; Donato, V. Stereotactic Body Radiation Therapy Salvage Reirradiation of Radiorecurrent Prostatic Carcinoma Relapsed in the Prostatic Bed. Tumori J. 2015, 101, e57–e59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gou, Z.; Wu, R.; Yuan, Y.; Yu, G.; Zhao, Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skelet. Radiol. 2019, 48, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Patena, E.; Giżewska, A.; Dziuk, M.; Miśko, J.; Budzyńska, A.; Walęcka-Mazur, A. Head-to-Head Comparison of 18F-Prostate-Specific Membrane Antigen-1007 and 18F-Fluorocholine PET/CT in Biochemically Relapsed Prostate Cancer. Clin. Nucl. Med. 2019, 44, e629–e633. [Google Scholar] [CrossRef] [PubMed]
- Paymani, Z.; Rohringer, T.; Vali, R.; Loidl, W.; Alemohammad, N.; Geinitz, H.; Langsteger, W.; Beheshti, M. Diagnostic Performance of [18F]Fluorocholine and [68Ga]Ga-PSMA PET/CT in Prostate Cancer: A Comparative Study. J. Clin. Med. 2020, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Ascalone, L.; Ziegler, B.; Hohenhorst, J.L.; Simon, R.; Berliner, C.; van Leeuwen, F.W.B.; ven der Poel, H.; Giesel, F.; Graefen, M.; et al. Salvage Surgery in Patients with Local Recurrence After Radical Prostatectomy. Eur. Urol. 2021, 79, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.L.; Gassa, F.; Rouvière, O.; Desmettre, O.; Bringeon, G.; Charret, J.; Serre, A.A.; Pommier, P. Salvage low-dose-rate brachytherapy for local recurrences after prostatectomy and adjuvant or salvage external beam irradiation: Feasibility study on five patients and literature review. Brachytherapy 2021, 20, 19–28. [Google Scholar] [CrossRef]


| Characteristics | Available Data | Overall |
|---|---|---|
| Age at diagnosis | 48 | 61 (48–75) |
| D’Amico initial risk | 45 | |
| Low | 3 (6.2%) | |
| Intermediate | 14 (29.2%) | |
| High | 28 (58.3%) | |
| Gleason score | 46 | |
| 6 | 12 (25%) | |
| 7 | 33 (68.8%) | |
| 8 | 1 (2.1%) | |
| ISUP grade | 43 | |
| ISUP 1 | 12 (25%) | |
| ISUP 2 | 19 (39.6%) | |
| ISUP 3 | 11 (22.9%) | |
| ISUP 4 | 1 (2.1%) | |
| PSA (ng/mL) | 41 | 9 (4.8–38) |
| pT3a or above | 42 | 25 (52.1%) |
| pN1 | 45 | 6 (12.5%) |
| Positive margins | 47 | 9 (18.8%) |
| Time to irradiation (month) | 47 | 26 (2–108) |
| RT indication | 48 | |
| Adjuvant | 8 (16.7%) | |
| Salvage | 40 (82.3%) | |
| Dose delivered to the prostatic bed (Gy) | 41 | 66 (60–75) |
| Pelvic node irradiation | 48 | 5 (10.4%) |
| ADT associated with irradiation | 48 | 4 (8.3%) |
| Characteristics | Available Data | Overall |
|---|---|---|
| Delay since first irradiation (month) | 47 | 102 (33–210) |
| PSA prior to SBRT (ng/mL) | 48 | 2.6 (0.2–10.4) |
| ADT during SBRT | 48 | 15 (31.2%) |
| Among which… | ||
| Long term ADT (>3 months before SBRT) | 9 (18.8%) | |
| ADT beginning along the SBRT | 6 (12.5%) | |
| Exams before SBRT | 47 | |
| Choline PET-CT alone | 11 (23%) | |
| Choline PET-CT + MRI | 28 (59%) | |
| Choline PET-CT + PSMA PET/CT | 4 (8.5%) | |
| Choline PET-CT + PSMA PET/CT + MRI | 2 (4.2%) | |
| PSMA PET-CT + MRI | 1 (2.1%) | |
| MRI + CT scan + bone scintigraphy | 1 (2.1%) | |
| Total dose (Gy) | 48 | 31.5 (20–37.2) |
| Fractionation (days) | 48 | 5 (3–6) |
| SBRT course | 48 | |
| 30 Gy in 5 fractions | 18 (37.5%) | |
| 36 Gy in 6 fractions | 16 (33.3%) | |
| Other | 13 (27.1%) |
| Available Data | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Acute rectal toxicity | 47 | 2 (4.3%) | 1 (2.1%) | - |
| Acute bladder toxicity | 47 | 5 (10.6%) | 2 (4.3%) | - |
| Late proctitis | 44 | 4 (9.1%) | 3 (6.8%) | - |
| Late cystitis | 44 | 8 (18.2%) | 4 (9.1%) | 5 (11.4%) |
| Late urinary incontinence | 45 | 7 (15.6%) | 3 (6.7%) | 3 (6.7%) |
| Chronic abdominal pain | 44 | 3 (6.8%) | - | 1 (2.3%) |
| Late Toxicity after SBRT | SBRT Prescription | ADT during SBRT | Time Since First Radiotherapy (Months) | Late Toxicity Post First Radiotherapy | ||
|---|---|---|---|---|---|---|
| Incontinence | Cystitis | Incontinence | Cystitis | |||
| 0 | 3 | 37.25 Gy in 5 | no | 75 | 1 | 1 |
| 3 | 0 | 35 Gy in 5 | no | 95 | 3 | 0 |
| 0 | 3 | 36 Gy in 6 | no | 158 | 0 | 0 |
| 3 | - | 36 Gy in 6 | no | 126 | 1 | 1 |
| 3 | 3 | 30 Gy in 5 | yes | - | 0 | 0 |
| 0 | 3 | 36 Gy in 6 | no | 135 | 1 | 1 |
| 0 | 3 | 36 Gy in 6 | yes | 76 | 0 | 0 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| HR | IC95% | p-value | HR | IC95% | p-Value | |
| Time since first RT | 1.01 | [1.00; 1.02] | 0.16 | 1.00 | [0.99;1.02] | 0.58 |
| PSA level before SBRT | 1.19 | [1.01; 1.41] | 0.04 | 1.18 | [0.96;1.45] | 0.12 |
| No ADT (reference) | 1 | - | - | - | - | - |
| ADT over 3 months before SBRT | 2.03 | [0.63; 6.58] | 0.24 | - | - | - |
| ADT starting at SBRT or less than three months before | 0.91 | [0.25; 3.38] | 0.89 | - | - | - |
| SBRT course: | ||||||
| - 30 Gy in 5 fractions | 0.57 | [0.20; 1.68] | 0.31 | - | - | - |
| - 36 Gy in 6 fractions | 0.33 | [0.09; 1.25] | 0.10 | - | - | - |
| - others (reference) | 1 | - | - | - | - | - |
| Initial Gleason | ||||||
| - 6 (reference) | 1 | - | - | 1 | - | - |
| - 7 | 0.70 | [0.26; 1.9] | 0.48 | 1.05 | [0.36; 3.08] | 0.93 |
| - 8 | 1.08 | [0.13; 9.36] | 0.94 | 2.40 | [0.24; 24.87] | 0.46 |
| Ref. | Patients | Dose | ADT | BR | 1-Year BRFS | 2-Years BRFS | Grade 3 Acute Toxicity | Grade 3 Late Toxicity |
|---|---|---|---|---|---|---|---|---|
| Olivier et al. [19] | 12 | 36 Gy in 6 | 2 (17%) | 10 (83%) | 0.79 | 0.56 | 0 | 0 |
| Detti et al. [20] | 8 | 30 Gy in 5 | 1 (12%) | 7 (88%) | 0.62 | - | 0 | 0 |
| Zerini et al. [21] | 10 | 25–30 Gy in 5–10 | 3 (30%) | - | 40% * | - | 0 | 0 |
| Volpe et al. [22] | 2 | 25–30 Gy in 5–6 | 0 | 2 (100%) | 1 | 1 | 0 | 0 |
| D’Agostino et al. [23] | 8 | 25–30 Gy in 5 | 0 | 8 (100%) | 81.6% * | 41.7% * | 1 hematuria | 1 urethral obstruction |
| Loi et al. [24] | 22 | 30 Gy in 5 | 17 (78%) | 19 (86%) | 80% * | - | 1 urine retention | 1 hematuria |
| Arcangeli et al. [25] | 1 | 30 Gy in 5 | 1 (100%) | 1 (100%) | - | - | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perennec, T.; Vaugier, L.; Toledano, A.; Scher, N.; Thomin, A.; Pointreau, Y.; Janoray, G.; De Crevoisier, R.; Supiot, S. Stereotactic Re-Irradiation for Local Recurrence after Radical Prostatectomy and Radiation Therapy: A Retrospective Multicenter Study. Cancers 2021, 13, 4339. https://doi.org/10.3390/cancers13174339
Perennec T, Vaugier L, Toledano A, Scher N, Thomin A, Pointreau Y, Janoray G, De Crevoisier R, Supiot S. Stereotactic Re-Irradiation for Local Recurrence after Radical Prostatectomy and Radiation Therapy: A Retrospective Multicenter Study. Cancers. 2021; 13(17):4339. https://doi.org/10.3390/cancers13174339
Chicago/Turabian StylePerennec, Tanguy, Loig Vaugier, Alain Toledano, Nathaniel Scher, Astrid Thomin, Yoann Pointreau, Guillaume Janoray, Renaud De Crevoisier, and Stéphane Supiot. 2021. "Stereotactic Re-Irradiation for Local Recurrence after Radical Prostatectomy and Radiation Therapy: A Retrospective Multicenter Study" Cancers 13, no. 17: 4339. https://doi.org/10.3390/cancers13174339
APA StylePerennec, T., Vaugier, L., Toledano, A., Scher, N., Thomin, A., Pointreau, Y., Janoray, G., De Crevoisier, R., & Supiot, S. (2021). Stereotactic Re-Irradiation for Local Recurrence after Radical Prostatectomy and Radiation Therapy: A Retrospective Multicenter Study. Cancers, 13(17), 4339. https://doi.org/10.3390/cancers13174339







