Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Therapy Response Evaluation
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
Predictors of Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Eustatia-Rutten, C.F.A.; Corssmit, E.P.M.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Survival and Death Causes in Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 313–319. [Google Scholar] [CrossRef]
- Schlumberger, M.J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 1998, 338, 297–306. [Google Scholar] [CrossRef]
- Park, S.; Kim, W.G.; Song, E.; Oh, H.S.; Kim, M.; Kwon, H.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Dynamic Risk Stratification for Predicting Recurrence in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine Remnant Ablation Therapy. Thyroid 2017, 27, 524–530. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating Risk of Recurrence in Differentiated Thyroid Cancer After Total Thyroidectomy and Radioactive Iodine Remnant Ablation: Using Response to Therapy Variables to Modify the Initial Risk Estimates Predicted by the New American Thyroid Association Staging System. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L.; Kloos, R.T. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J. Clin. Endocrinol. Metab. 2002, 87, 1490–1498. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, W.B.; Park, W.R.; Han, J.M.; Kim, T.Y.; Song, D.E.; Chung, K.-W.; Ryu, J.-S.; Shong, Y.K. Modified dynamic risk stratification for predicting recurrence using the response to initial therapy in patients with differentiated thyroid carcinoma. Eur. J. Endocrinol. 2014, 170, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.Y.; Lee, S.-W.; Kong, E.J.; Kim, K.; Kim, B.I.; Kim, J.; Kim, H.; Park, S.H.; Park, J.; Park, H.L.; et al. Clinicopathologic risk factors of radioactive iodine therapy based on response assessment in patients with differentiated thyroid cancer: A multicenter retrospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liang, J.; Li, T.; Zhao, T.; Lin, Y. Preablative Stimulated Thyroglobulin Correlates to New Therapy Response System in Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Pace, L.; Klain, M.; Salvatore, B.; Nicolai, E.; Zampella, E.; Assante, R.; Pellegrino, T.; Storto, G.; Fonti, R.; Salvatore, M. Prognostic Role of 18F-FDG PET/CT in the Postoperative Evaluation of Differentiated Thyroid Cancer Patients. Clin. Nucl. Med. 2015, 40, 111–115. [Google Scholar] [CrossRef]
- Klain, M.; Pace, L.; Zampella, E.; Mannarino, T.; Limone, S.; Mazziotti, E.; De Simini, G.; Cuocolo, A. Outcome of Patients with Differentiated Thyroid Cancer Treated With 131-Iodine on the Basis of a Detectable Serum Thyroglobulin Level After Initial Treatment. Front. Endocrinol. 2019, 10, 11–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, T.H.; Choe, J.; Kim, J.-H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Jang, H.W.; Kim, Y.N.; et al. Restratification of survival prognosis of N1b papillary thyroid cancer by lateral lymph node ratio and largest lymph node size. Cancer Med. 2017, 6, 2244–2251. [Google Scholar] [CrossRef] [Green Version]
- Perrier, N.D.; Brierley, J.D.; Tuttle, R.M. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 68, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagna, M.G.; Maino, F.; Cipri, C.; Belardini, V.; Theodoropoulou, A.; Cevenini, G.; Pacini, F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur. J. Endocrinol. 2011, 165, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Pitoia, F.; Jerkovich, F. Dynamic risk assessment in patients with differentiated thyroid cancer. Endocr.-Relat. Cancer 2019, 26, R553–R566. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Faculty Opinions recommendation of Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, J.; Li, X.; Liang, Z.; Gao, W.; Liang, J.; Cheng, S.; Lin, Y. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Ki, C.-S.; Kim, H.S.; Kim, K.; Choe, J.-H.; Kim, J.-H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; et al. Refining Dynamic Risk Stratification and Prognostic Groups for Differentiated Thyroid Cancer with TERT Promoter Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 1757–1764. [Google Scholar] [CrossRef]
- Pacini, F.; Capezzone, M.; Elisei, R.; Ceccarelli, C.; Taddei, D.; Pinchera, A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J. Clin. Endocrinol. Metab. 2002, 87, 1499–1501. [Google Scholar] [CrossRef]
- Cailleux, A.F.; Baudin, E.; Travagli, J.P.; Ricard, M.; Schlumberger, M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J. Clin. Endocrinol. Metab. 2000, 85, 175–178. [Google Scholar] [CrossRef]
- Jeon, E.J.; Jung, E.D. Diagnostic Whole-Body Scan May Not Be Necessary for Intermediate-Risk Patients with Differentiated Thyroid Cancer after Low-Dose (30 mCi) Radioactive Iodide Ablation. Endocrinol. Metab. 2014, 29, 33–39. [Google Scholar] [CrossRef]
- Baudin, E.; Cao, C.D.; Cailleux, A.F.; Leboulleux, S.; Travagli, J.P.; Schlumberger, M. Positive Predictive Value of Serum Thyroglobulin Levels, Measured during the First Year of Follow-Up after Thyroid Hormone Withdrawal, in Thyroid Cancer Patients. J. Clin. Endocrinol. Metab. 2003, 88, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Chung, J.-K.; Lim, I.H.; Park, D.J.; Lee, D.S.; Lee, M.C.; Cho, B.Y. Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 172–179. [Google Scholar] [CrossRef]
- Ma, C.; Kuang, A.; Xie, J.; Ma, T. Possible explanations for patients with discordant findings of serum thyroglobulin and 131I whole-body scanning. J. Nucl. Med. 2005, 46, 1473–1480. [Google Scholar] [PubMed]
| Clinical Features | All (n = 606) | Event (n = 42) | No Event (n = 564) | p-Value |
|---|---|---|---|---|
| Age (years) | 44 ± 14 | 47 ± 17 | 44 ± 14 | 0.12 |
| Male gender, n (%) | 106 (17) | 12 (29) | 94 (17) | 0.05 |
| ATA risk, n (%) | - | - | - | - |
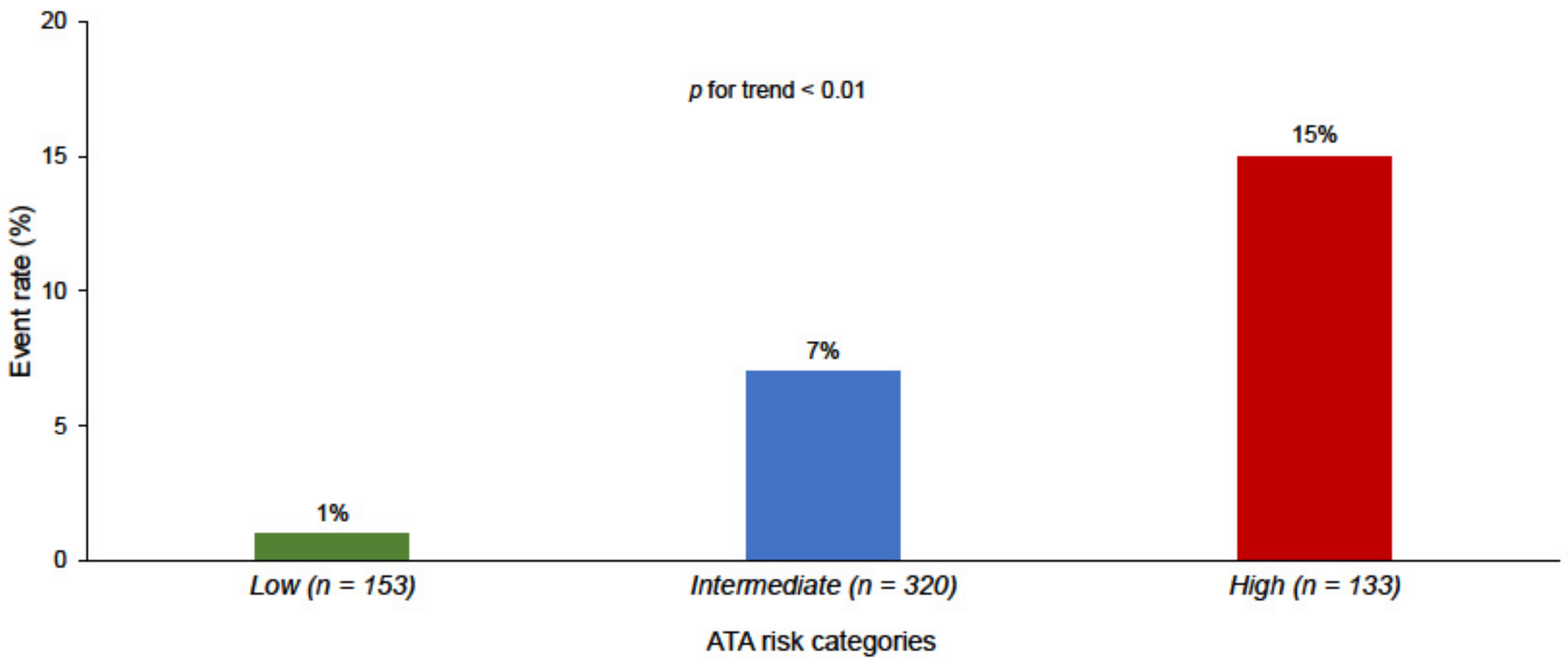
| Low risk | 153 (25) | 2 (4) | 151 (30) | <0.01 |
| Intermediate risk | 320 (53) | 20 (48) | 300 (53) | 0.48 |
| High risk | 133 (22) | 20 (48) | 113 (20) | <0.001 |
| Papillary type, n (%) | 301 (50) | 23 (55) | 278 (49) | 0.49 |
| Follicular type, n (%) | 109 (18) | 5 (12) | 104 (18) | 0.29 |
| Mixed, n (%) | 194 (32) | 13 (31) | 181 (32) | 0.88 |
| Time interval surgery/therapy (days) | 99 ± 46 | 88 ± 44 | 100 ± 46 | 0.10 |
| Administered 131I activity (MBq) | 3589 ± 922 | 3612 ± 814 | 3552 ± 925 | 0.69 |
| Pre-therapy off Tg (ng/mL) | 16 ± 39 | 43 ± 64 | 14 ± 35 | <0.001 |
| Pre-therapy off Tg ≥ 10 ng/mL, n (%) | 198 (33) | 22 (52) | 92 (16) | <0.001 |
| Post-therapy WBS, n (%) | - | - | - | - |
| Thyroid bed | 594 (98) | 42 (100) | 552 (98) | 0.34 |
| Suspicious for lymph nodes | 38 (6) | 4 (10) | 34 (6) | 0.37 |
| 12-Months Evaluation | All (n = 606) | Event (n = 42) | No Event (n = 564) | p-Value |
|---|---|---|---|---|
| Post-therapy Tg (ng/mL) | 4 ± 11 | 17 ± 29 | 4 ± 14 | <0.001 |
| Undetectable Tg/AbTg, n (%) | 97 (31) | 6 (16) | 91 (32) | <0.05 |
| Detectable Tg, n (%) | 342 (56) | 30 (71) | 312 (55) | <0.01 |
| Detectable AbTg, n (%) | 38 (6) | 5 (12) | 33 (6) | 0.12 |
| Normal US, n (%) | 572 (94) | 35 (83) | 537 (95) | <0.001 |
| Abnormal US, n (%) | 34 (6) | 7 (17) | 27 (5) | <0.001 |
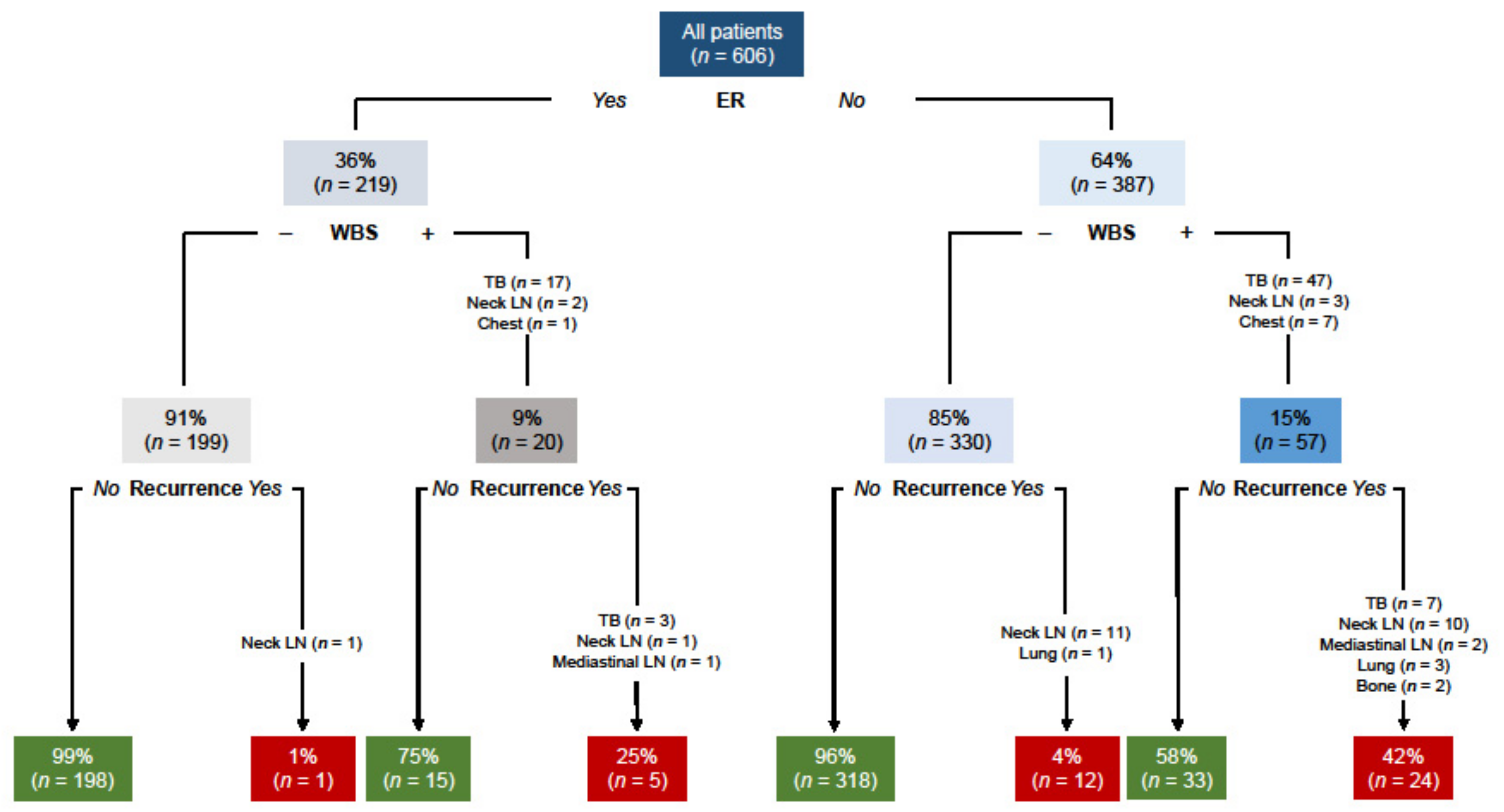
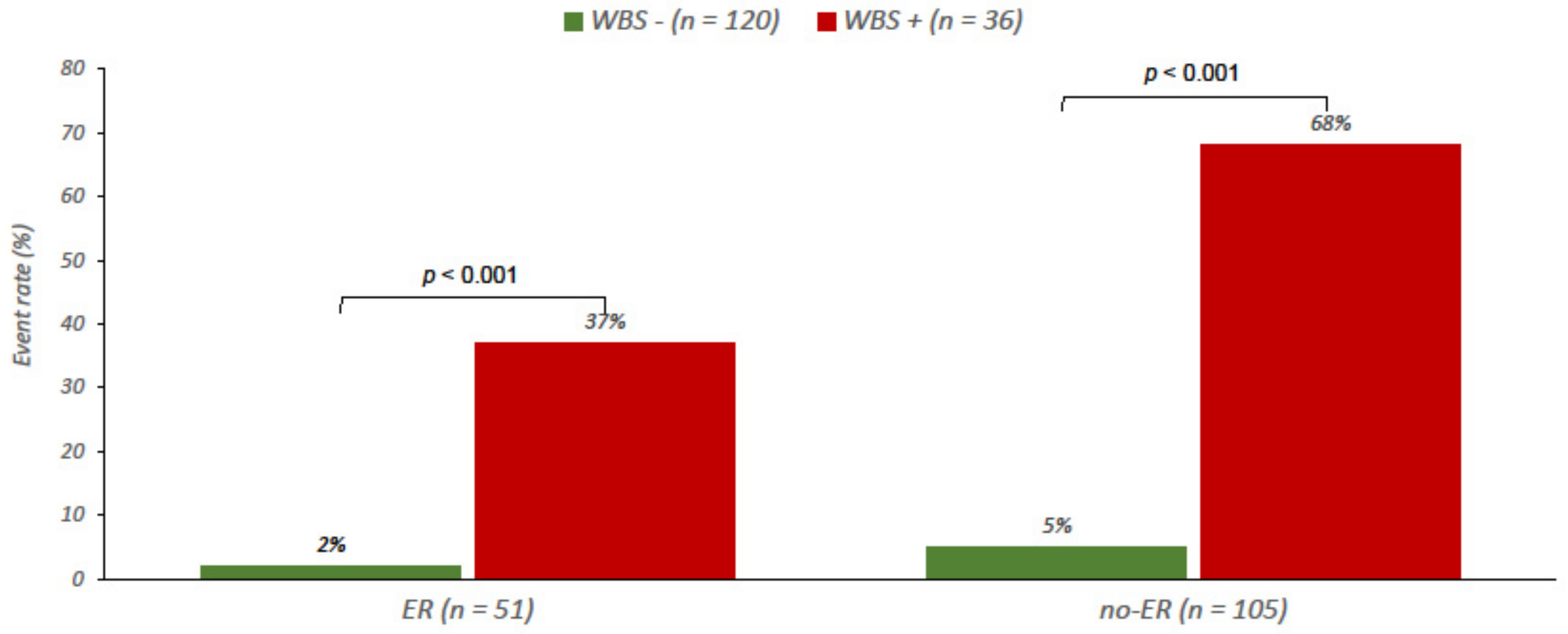
| Uptake at diagnostic WBS, n (%) | 77 (13) | 29 (70) | 48 (9) | <0.001 |
| Thyroid bed, n (%) | 65 (11) | 19 (45) | 46 (8) | <0.001 |
| Suspicious for lymph nodes, n (%) | 2 (0.5) | 2 (5) | 2 (0.5) | <0.001 |
| Extra-neck uptake, n (%) | 8 (1) | 8 (19) | 0 | - |
| ID | Pre Therapy | 12 Months Evaluation | Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATA Risk Category | Tg | Tg | US | Diagnostic WBS Uptake | Response to Therapy | Site | Months | Tg | Treatment | |
| 1 | Intermediate | 184 | 11 | − | TB | BIR | TB | 48 | 70 | RAI |
| 2 | Intermediate | 12 | 6 | − | TB | BIR | TB | 32 | 13 | RAI |
| 3 | Intermediate | 18 | 53 | − | TB | BIR | TB | 67 | 13 | RAI |
| 4 | Intermediate | 72 | 1.5 | − | TB | BIR | TB | 19 | 14 | RAI |
| 5 | Low | 32 | 1 | + | TB | SIR | TB | 18 | 29 | RAI |
| 6 | High | 73 | 1.4 | + | TB | BIR | TB | 90 | 11 | RAI |
| 7 | Intermediate | 36 | 7 | + | TB | IR | TB | 10 | 12 | RAI |
| 8 | Intermediate | 13 | 1.4 | − | TB | IR | TB | 17 | 9.1 | RAI |
| 9 | Low | 41 | 3 | − | TB | IR | TB | 17 | 59 | RAI |
| 10 | Intermediate | 24 | 0.1 | − | TB | ER | TB | 15 | 12 | RAI |
| 11 | Intermediate | 0.5 | 0.2 | − | TB | ER | TB | 17 | 28 | RAI |
| 12 | Intermediate | 51 | 2 | + | TB | SIR | TB and LN | 13 | 7.6 | RAI |
| 13 | High | 36 | 0.5 | − | − | BIR | TB and LN | 29 | 15 | RAI |
| 14 | Intermediate | 1 | 2 | − | − | BIR | TB and LN | 13 | 37 | RAI |
| 15 | Intermediate | 0.5 | 0.3 | − | − | IR | TB and LN | 25 | 8 | RAI |
| 16 | High | 320 | 11 | − | − | IR | TB and LN | 26 | 5.6 | RAI |
| 17 | High | 14 | 4.5 | − | − | IR | Bone | 73 | 330 | RAI |
| 18 | High | 1.1 | 1.4 | − | Thorax | IR | Bone | 19 | 49 | RAI |
| 19 | High | 19 | 2 | − | Thorax | BIR | Lung | 60 | 440 | RAI |
| 20 | Intermediate | 28 | 8 | − | Thorax | BIR | Lung | 11 | 83 | RAI |
| 21 | High | 102 | 5.2 | − | Thorax | BIR | Lung | 21 | 113 | RAI |
| 22 | High | 112 | 1.2 | − | Thorax | IR | Lung | 33 | 62 | RAI |
| 23 | High | 215 | 0.2 | − | Mediastinum | ER | Mediastinal LN | 17 | 30 | RAI |
| 24 | High | 2 | 4 | − | Mediastinum | BIR | Mediastinal LN | 16 | 8 | RAI |
| 25 | High | 67 | 45 | − | Mediastinum | BIR | Paratracheal LN | 25 | 9,7 | RAI |
| 26 | Intermediate | 14 | 0.7 | − | LN | IR | TB and LN | 18 | 4.2 | Surgery + RAI |
| 27 | High | 48 | 0.1 | − | TB | IR | TB and LN | 27 | 1.1 | Surgery + RAI |
| 28 | High | 0.8 | 0.2 | − | LN | ER | LC LN | 112 | 236 | Surgery + RAI |
| 29 | Intermediate | 24 | 0.1 | − | − | ER | LC LN | 12 | 6,1 | Surgery + RAI |
| 30 | Intermediate | 14 | 1.3 | + | − | BIR | LC LN | 56 | 4,7 | Surgery + RAI |
| 31 | High | 13 | 1.5 | − | TB | BIR | LC LN | 12 | 7,1 | Surgery + RAI |
| 32 | High | 64 | 1.2 | + | LN | IR | LC LN | 17 | 6,2 | Surgery + RAI |
| 33 | High | 6 | 5.1 | − | − | BIR | LC LN | 30 | 398 | Surgery + RAI |
| 34 | High | 37 | 20 | − | TB | BIR | LC LN | 12 | 196 | Surgery + RAI |
| 35 | Intermediate | 0.8 | 10 | − | TB | BIR | LC LN | 31 | 5,1 | Surgery + RAI |
| 36 | High | 0.5 | 2.5 | − | − | IR | LC LN | 55 | 2,2 | Surgery + RAI |
| 37 | High | 4 | 1.1 | − | − | IR | LC LN | 16 | 4,1 | Surgery + RAI |
| 38 | High | 3 | 0.6 | − | − | IR | LC LN | 32 | 9 | Surgery + RAI |
| 39 | Intermediate | 31 | 0.1 | + | LN | IR | LC LN | 19 | 10 | Surgery + RAI |
| 40 | Intermediate | 0.5 | 0.9 | − | − | IR | LC LN | 86 | 4,1 | Surgery + RAI |
| 41 | Intermediate | 7 | 8 | − | − | BIR | LC LN | 95 | 4 | Surgery + RAI |
| 42 | Intermediate | 51 | 0.2 | − | TB | ER | LC LN | 10 | 7 | Surgery + RAI |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| ATA risk categories | - | - | - | - |
| Low (reference) (n = 153) | - | <0.001 | - | <0.01 |
| Intermediate (n = 320) | 4.88 (1.14–20.89) | <0.05 | 5.99 (1.33–26.8) | <0.05 |
| High (n = 133) | 11.99 (2.80–51.29) | <0.01 | 14.6 (3.17–67.3) | <0.01 |
| Pre-therapy Tg ≥ 10 ng/mL (n = 198) | 4.87 (2.534–9.37) | <0.001 | 2.07 (2.25–7.78) | <0.001 |
| 12 months response to therapy (Tg + US) | - | - | - | - |
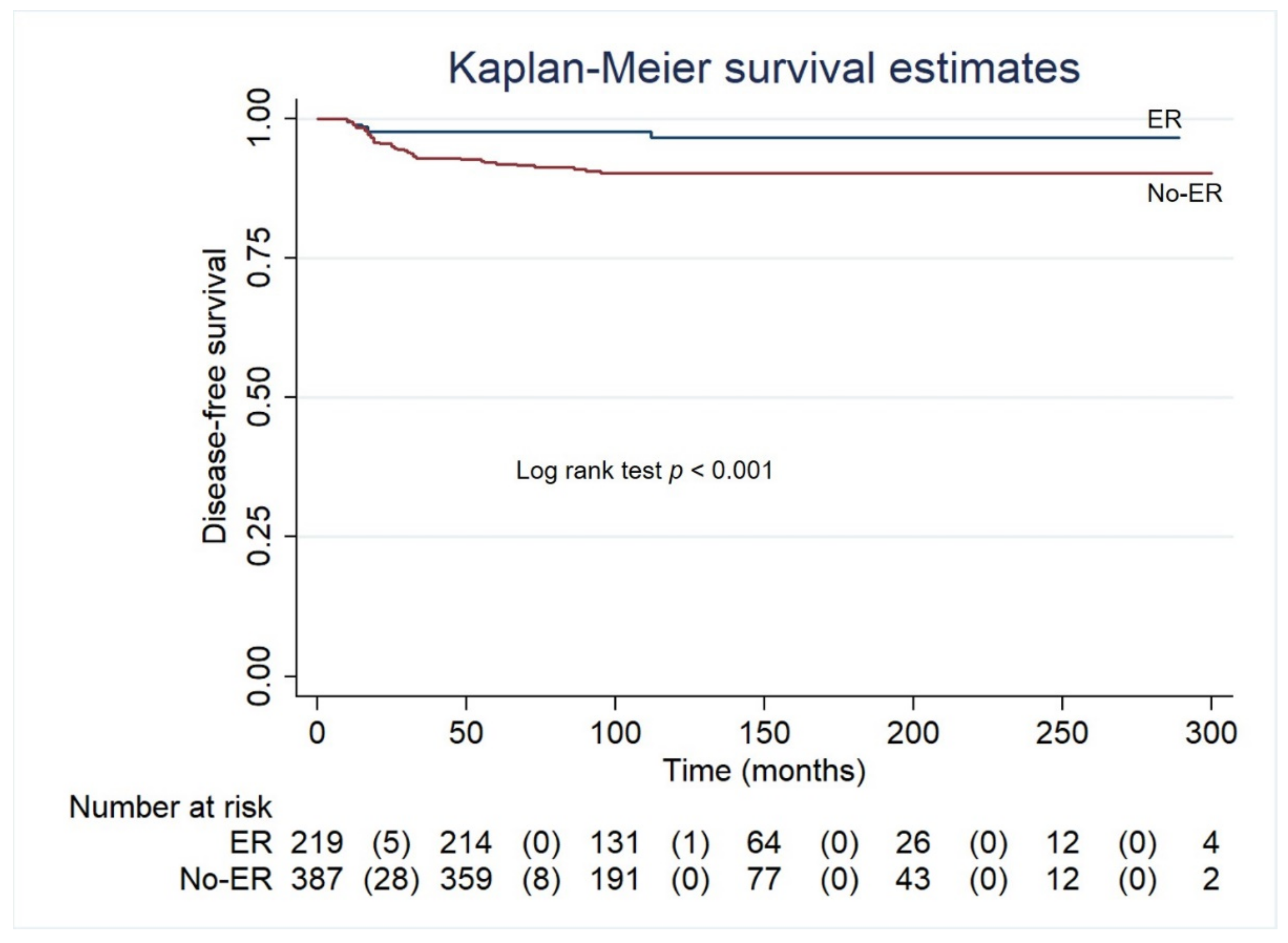
| ER (reference) (n = 219) | - | <0.01 | - | - |
| IR (n = 182) | 3.14 (1.20–8.00) | <0.05 | 3.24 (1.25–8.37) | <0.05 |
| BIR (n = 203) | 3.51 (1.40–8.78) | <0.01 | 2.88 (1.14–7.24) | <0.05 |
| SIR (n = 2) | 8.49 (2.85–25.30) | <0.001 | 17.4 (2.49–29.5) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klain, M.; Zampella, E.; Piscopo, L.; Volpe, F.; Manganelli, M.; Masone, S.; Pace, L.; Salvatore, D.; Schlumberger, M.; Cuocolo, A. Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer. Cancers 2021, 13, 4338. https://doi.org/10.3390/cancers13174338
Klain M, Zampella E, Piscopo L, Volpe F, Manganelli M, Masone S, Pace L, Salvatore D, Schlumberger M, Cuocolo A. Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer. Cancers. 2021; 13(17):4338. https://doi.org/10.3390/cancers13174338
Chicago/Turabian StyleKlain, Michele, Emilia Zampella, Leandra Piscopo, Fabio Volpe, Mariarosaria Manganelli, Stefania Masone, Leonardo Pace, Domenico Salvatore, Martin Schlumberger, and Alberto Cuocolo. 2021. "Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer" Cancers 13, no. 17: 4338. https://doi.org/10.3390/cancers13174338
APA StyleKlain, M., Zampella, E., Piscopo, L., Volpe, F., Manganelli, M., Masone, S., Pace, L., Salvatore, D., Schlumberger, M., & Cuocolo, A. (2021). Long-Term Prognostic Value of the Response to Therapy Assessed by Laboratory and Imaging Findings in Patients with Differentiated Thyroid Cancer. Cancers, 13(17), 4338. https://doi.org/10.3390/cancers13174338









