Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Treatment
2.3. Assessments
2.4. Outcomes
2.5. Statistical Considerations
3. Results
3.1. Patient Characteristics
3.2. Study Treatment
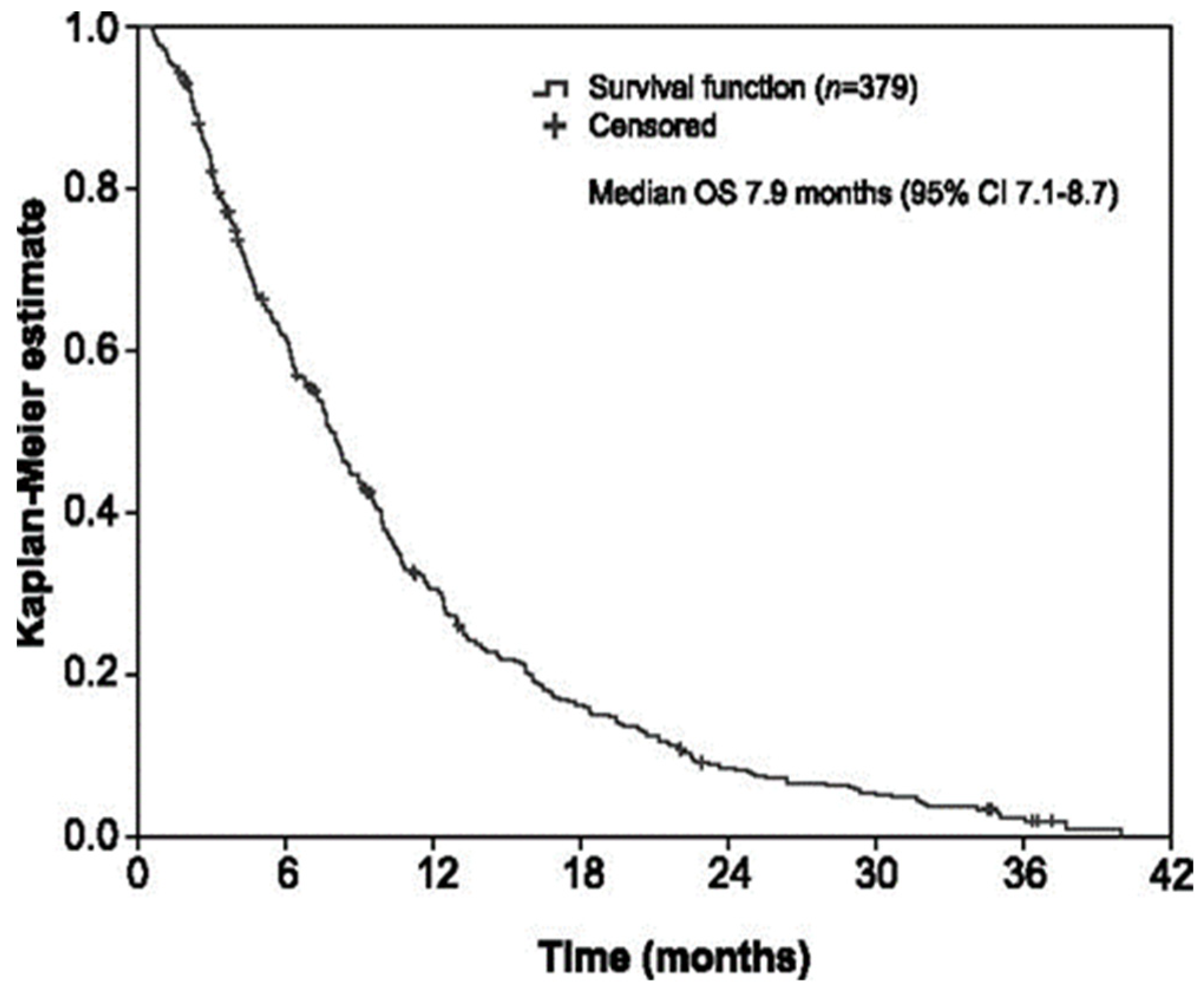
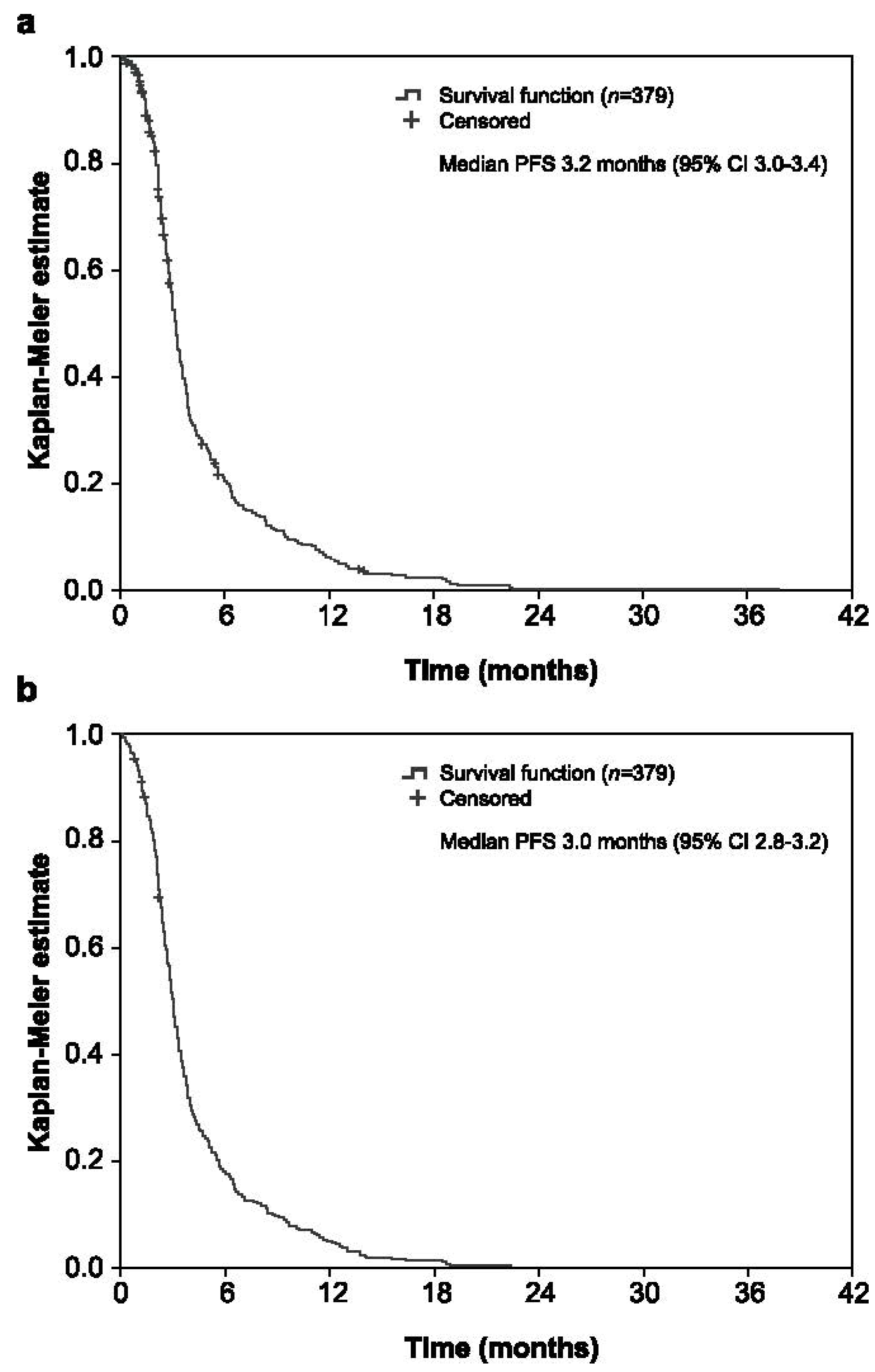
3.3. Efficacy
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 4.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 15 July 2020).
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Emura, T.; Suzuki, N.; Yamaguchi, M.; Ohshimo, H.; Fukushima, M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int. J. Oncol. 2004, 25, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Sakamoto, K.; Okabe, H.; Fujioka, A.; Yamamura, K.; Nakagawa, F.; Nagase, H.; Yokogawa, T.; Oguchi, K.; Ishida, K.; et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol. Rep. 2014, 32, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, M.; Suzuki, N.; Emura, T.; Yano, S.; Kazuno, H.; Tada, Y.; Yamada, Y.; Asao, T. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2′-deoxyribonucleosides. Biochem. Pharmacol. 2000, 59, 1227–1236. [Google Scholar] [CrossRef]
- Emura, T.; Suzuki, N.; Fujioka, A.; Ohshimo, H.; Fukushima, M. Potentiation of the antitumor activity of α, α, α-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int. J. Oncol. 2005, 27, 449–455. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Longo-Muñoz, F.; Argiles, G.; Tabernero, J.; Cervantes, A.; Gravalos, C.; Pericay, C.; Gil-Calle, S.; Mizuguchi, H.; Carrato-Mena, A.; Limón, M.L.; et al. Efficacy of trifluridine and tipiracil (TAS-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from Spain: Results of a subgroup analysis of the phase 3 RECOURSE trial. Clin. Transl. Oncol. 2017, 19, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Mayer, R.J.; Laurent, S.; Winkler, R.; Grávalos, C.; Benavides, M.; Longo-Munoz, F.; Portales, F.; Ciardiello, F.; Siena, S.; et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur. J. Cancer 2018, 90, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Cleary, J.; Van Cutsem, E.; Mayer, R.; Ohtsu, A.; Shinozaki, E.; Falcone, A.; Yamazaki, K.; Nishina, T.; Garcia-Carbonero, R.; et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann. Oncol. 2020, 31, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Argiles, G.; Sobrero, A.F.; Borg, C.; Ohtsu, A.; Mayer, R.J.; Vidot, L.; Moreno Vera, S.R.; Van Cutsem, E. Effect of trifluridine/tipiracil in patients treated in RECOURSE by prognostic factors at baseline: An exploratory analysis. ESMO Open 2020, 5, e000752. [Google Scholar] [CrossRef]
- Andersen, S.E.; Andersen, I.B.; Jensen, B.V.; Pfeiffer, P.; Ota, T.; Larsen, J.S. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta Oncol. 2019, 58, 1149–1157. [Google Scholar] [CrossRef]
- Fernandez Montes, A.; Vazquez Rivera, F.; Martinez Lago, N.; Covela Rua, M.; Cousillas Castineiras, A.; Gonzalez Villarroel, P.; de la Camara Gomez, J.; Mendez Mendez, J.C.; Salgado Fernandez, M.; Candamio Folgar, S.; et al. Efficacy and safety of trifluridine/tipiracil in third-line and beyond for the treatment of patients with metastatic colorectal cancer in routine clinical practice: Patterns of use and prognostic nomogram. Clin. Transl. Oncol. 2020, 22, 351–359. [Google Scholar] [CrossRef]
- Carriles, C.; Jimenez-Fonseca, P.; Sánchez-Cánovas, M.; Pimentel, P.; Carmona-Bayonas, A.; García, T.; Carbajales-Álvarez, M.; Lozano-Blázquez, A. Trifluridine/tipiracil (TAS-102) for refractory metastatic colorectal cancer in clinical practice: A feasible alternative for patients with good performance status. Clin. Transl. Oncol. 2019, 21, 1781–1785. [Google Scholar] [CrossRef]
- Skuja, E.; Gerina-Berzina, A.; Hegmane, A.; Zvirbule, Z.; Vecvagare, E.; Purkalne, G. Duration of previous treatment as a prognostic factor in metastatic colorectal cancer treated with trifluridine/tipiracil. Mol. Clin. Oncol. 2018, 8, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Kwakman, J.J.M.; Vink, G.; Vestjens, J.H.; Beerepoot, L.V.; De Groot, J.W.; Jansen, R.L.; Opdam, F.L.; Boot, H.; Creemers, G.J.; Van Rooijen, J.M.; et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: Real-life data from The Netherlands. Int. J. Clin. Oncol. 2018, 23, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremolini, C.; Rossini, D.; Martinelli, E.; Pietrantonio, F.; Lonardi, S.; Noventa, S.; Tamburini, E.; Frassineti, G.L.; Mosconi, S.; Nichetti, F.; et al. Trifluridine/tipiracil (TAS-102) in refractory metastatic colorectal cancer: A multicenter register in the frame of the Italian compassionate use program. Oncologist 2018, 23, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, A.; Yamada, T.; Matsumoto, S.; Sakurazawa, N.; Kawano, Y.; Shinozuka, E.; Sekiguchi, K.; Suzuki, H.; Yoshida, H. Pretreatment neutrophil–to–lymphocyte ratio predicts survival after TAS-102 treatment of patients with metastatic colorectal cancer. Anticancer Res. 2019, 39, 4343–4350. [Google Scholar] [CrossRef]
- Giuliani, J.; Bonetti, A. The onset of grade ≥3 neutropenia is associated with longer overall survival in metastatic colorectal cancer patients treated with trifluridine/tipiracil. Anticancer Res. 2019, 39, 3967–3969. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 26 May 2020).
- Panageas, K.S.; Ben-Porat, L.; Dickler, M.N.; Chapman, P.B.; Schrag, D. When you look matters: The effect of assessment schedule on progression-free survival. J. Natl. Cancer Inst. 2007, 99, 428–432. [Google Scholar] [CrossRef]
- Cicero, G.; De Luca, R.; Dieli, F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. OncoTargets Ther. 2018, 11, 3059–3063. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, T.; Fukuoka, S.; Masuishi, T.; Takashima, A.; Kumekawa, Y.; Kajiwara, T.; Yamazaki, K.; Esaki, T.; Makiyama, A.; Denda, T.; et al. Prognostic scores for evaluating the survival benefit of regorafenib or trifluridine/tipiracil in patients with metastatic colorectal cancer: An exploratory analysis of the REGOTAS study. Int. J. Clin. Oncol. 2020, 25, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Miceli, R.; Rimassa, L.; Lonardi, S.; Aprile, G.; Mennitto, A.; Marmorino, F.; Bozzarelli, S.; Antonuzzo, L.; Tamburini, E.; et al. Estimating 12-week death probability in patients with refractory metastatic colorectal cancer: The Colon Life nomogram. Ann. Oncol. 2017, 28, 555–561. [Google Scholar] [CrossRef]
- Tanaka, A.; Sadahiro, S.; Suzuki, T.; Okada, K.; Saito, G.; Miyakita, H. Retrospective study of regorafenib and trifluridine/tipiracil efficacy as a third-line or later chemotherapy regimen for refractory metastatic colorectal cancer. Oncol. Lett. 2018, 16, 6589–6597. [Google Scholar] [CrossRef]
- Adenis, A.; De La Fouchardiere, C.; Paule, B.; Burtin, P.; Tougeron, D.; Wallet, J.; Dourthe, L.-M.; Etienne, P.-L.; Mineur, L.; Clisant, S.; et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016, 16, 412. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wu, Y.-S.; Lin, H.; Wang, Y.; Li, L.; Zhang, T. Efficacy and safety of TAS-102 in refractory metastatic colorectal cancer: A meta-analysis. Cancer Manag. Res. 2018, 10, 2915–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Value |
|---|---|
| Age (years) | |
| Median (IQR) | 65 (58–71) |
| ≥65 years, n (%) | 189 (49.9) |
| ≥70 years, n (%) | 108 (28.5) |
| Male, n (%) | 226 (59.6) |
| Caucasian, n (%) | 355 (93.7) |
| ECOG performance status, n (%) | |
| 0 | 117 (30.9) |
| 1 | 255 (67.3) |
| 2 | 7 (1.8) |
| Site of primary tumour, n (%) | |
| Colon | 222 (58.6) |
| Rectum | 129 (34.0) |
| Colon and rectum | 27 (7.1) |
| Unknown | 1 (0.3) |
| Primary tumour surgery, n (%) | 316 (83.4) |
| Timing of metastases from initial diagnosis, n (%) | |
| Synchronous (≤6 months) | 231 (60.9) |
| Metachronous (>6 months) | 148 (39.1) |
| Site of metastasis (frequency ≥10%), n (%) a | |
| Liver | 262 (69.1) |
| Lung | 256 (67.5) |
| Peritoneum | 93 (24.5) |
| Distant node | 70 (18.5) |
| Bone | 29 (7.7) |
| Number of metastatic sites, n (%) | |
| ≤2 | 279 (73.6) |
| ≥3 | 100 (26.4) |
| Lines of treatment, n (%) | |
| <3 | 126 (33.2) |
| ≥3 | 253 (66.8) |
| Previous therapies in ≥1 line, n (%) a | |
| Fluoropyrimidines | 379 (100) |
| Oxaliplatin | 333 (87.9) |
| Irinotecan | 378 (99.7) |
| Anti-VEGF | 339 (89.4) |
| Anti-EGFR | 117 (46.7) |
| Regorafenib | 60 (15.8) |
| Other | 83 (21.9) |
| Time from the initial diagnosis to start trifluridine/tipiracil (years), median (IQR) | 3.3 (2.2–5.3) |
| Time from metastasis diagnosis to start trifluridine/tipiracil (years) | |
| Median (IQR) | 2.5 (1.7–4.2) |
| ≥18 months, n (%) | 320 (84.4) |
| Molecular Status | Value |
|---|---|
| Microsatellite instability, n (%) | |
| No | 128 (33.8) |
| Yes | 50 (13.2) |
| Unknown | 201 (53.0) |
| KRAS status, n (%) | |
| Wild type | 179 (47.2) |
| Mutated | 175 (46.2) |
| Unknown | 25 (6.6) |
| RAS (KRAS + NRAS) status, n (%) | |
| Wild type | 88 (23.2) |
| Mutated | 190 (50.1) |
| Unknown | 101 (26.6) |
| BRAF status, n (%) | |
| Wild type | 71 (18.7) |
| Mutated | 7 (1.8) |
| Unknown | 301 (79.4) |
| PI3K status, n (%) | |
| Wild type | 24 (6.3) |
| Mutated | 6 (1.6) |
| Unknown | 349 (92.1) |
| HER2 status, n (%) | |
| Positive | 2 (0.5) |
| Negative | 22 (5.8) |
| Unknown | 355 (93.7) |
| Characteristics | OS (Months) | Cox 1 | Cox 2 | ||
|---|---|---|---|---|---|
| Median (95% CI) | HR (95% CI) | p | HR (95% CI) | p | |
| Number of metastatic sites | |||||
| ≤2 | 8.6 (7.5–9.7) | 0.6 (0.5–0.8) | <0.001 | 0.6 (0.5–0.8) | <0.001 |
| ≥3 a | 5.6 (4.7–6.6) | ||||
| Liver metastasis | |||||
| No | 10.7 (8.8–12.7) | 0.7 (0.5–0.9) | 0.004 | - | - |
| Yes a | 6.6 (5.6–7.5) | ||||
| Alkaline phosphatase level | |||||
| <300 IU | 9.8 (8.5–11.0) | 0.6 (0.4–0.8) | <0.001 | 0.5 (0.4–0.7) | <0.001 |
| ≥300 IU a | 4.1 (2.5–5.7) | ||||
| Dose reductions | |||||
| Without dose reductions a | 6.4 (5.1–7.6) | - | - | 0.6 (0.4–0.8) | <0.001 |
| With dose reductions | 10.7 (8.8–12.7) | ||||
| Neutrophil/lymphocyte ratio | |||||
| <5 | 9.0 (8.0–10.0) | - | - | 0.5 (0.4–0.7) | <0.001 |
| ≥5 a | 4.1 (3.0–5.1) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Alfonso, P.; Muñoz, A.; Jiménez-Castro, J.; Jiménez-Fonseca, P.; Pericay, C.; Longo-Muñoz, F.; Reyna-Fortes, C.; Argilés-Martínez, G.; González-Astorga, B.; Gómez-Reina, M.J.; et al. Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study. Cancers 2021, 13, 4514. https://doi.org/10.3390/cancers13184514
García-Alfonso P, Muñoz A, Jiménez-Castro J, Jiménez-Fonseca P, Pericay C, Longo-Muñoz F, Reyna-Fortes C, Argilés-Martínez G, González-Astorga B, Gómez-Reina MJ, et al. Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study. Cancers. 2021; 13(18):4514. https://doi.org/10.3390/cancers13184514
Chicago/Turabian StyleGarcía-Alfonso, Pilar, Andrés Muñoz, Jerónimo Jiménez-Castro, Paula Jiménez-Fonseca, Carles Pericay, Federico Longo-Muñoz, Carmen Reyna-Fortes, Guillem Argilés-Martínez, Beatriz González-Astorga, María José Gómez-Reina, and et al. 2021. "Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study" Cancers 13, no. 18: 4514. https://doi.org/10.3390/cancers13184514
APA StyleGarcía-Alfonso, P., Muñoz, A., Jiménez-Castro, J., Jiménez-Fonseca, P., Pericay, C., Longo-Muñoz, F., Reyna-Fortes, C., Argilés-Martínez, G., González-Astorga, B., Gómez-Reina, M. J., Ruiz-Casado, A., Rodríguez-Salas, N., López-López, R., Carmona-Bayonas, A., Conde-Herrero, V., Aranda, E., & on behalf of the ROS Study Group. (2021). Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study. Cancers, 13(18), 4514. https://doi.org/10.3390/cancers13184514






