Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
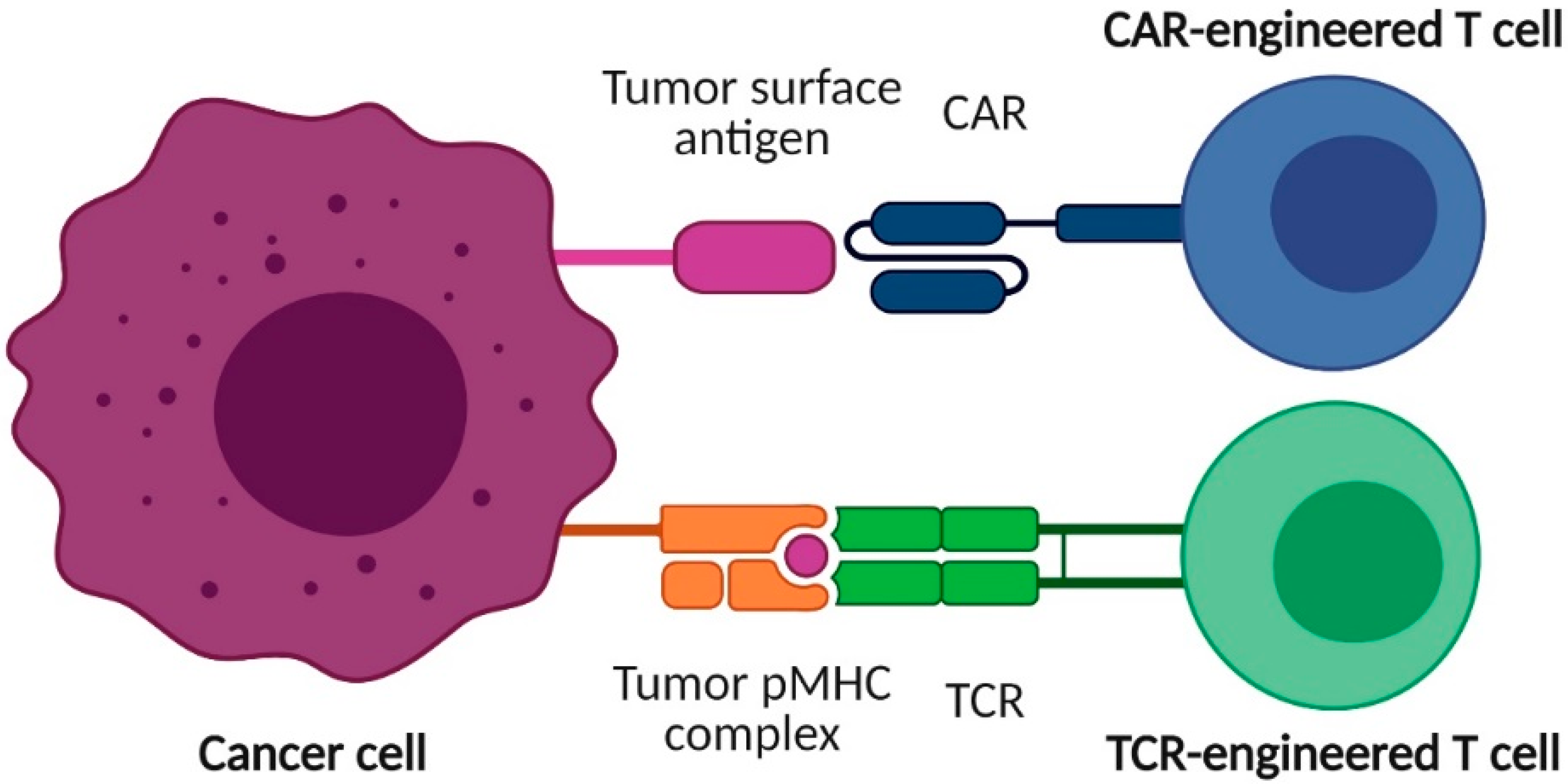
1. Introduction
2. Target Antigens in TCR-T-Cell Therapies for AML
2.1. WT1
2.2. PRAME
2.3. MiHA
3. Characteristics and Results of Clinical Trials Using AML-Directed TCR-T Cells
| Clinical Trial Identifier | Location | Status (First Posted) | Phase | AML Status | Prior Treatment | AML Patients Recruited or Treated (Intended) | Ref. |
|---|---|---|---|---|---|---|---|
| WT1-Specific | |||||||
| NCT01621724 EudraCT-2006-004950-25 | UK | Completed (2012, completed in 2018) | I/II | AML | n.d. | 7 treated (18) | [51] |
| NCT02550535 EudraCT-2014-003111-10 | Belgium Germany UK | Completed (2015, completed in 2018) | I/II | Relapsed/ stable AML | HAT | 10 pt. treated: 6 AML, 3 MDS and 1 TKI- resistant CML (25-30) | [52,53] |
| UMIN000011519 | Japan | Completed (2013, completed in 2018) | I | R/R AML | n.d. | 12 recruited, 8 treated | [54] |
| NCT01640301 | USA | Active, not recruiting (2012) | I/II | High-risk/ relapsed AML | allo-HSCT | 12 treated (45) | [55] |
| NCT02770820 | USA | Active, not recruiting (2016) | I/II | High-risk non-M3 AML | Consolidation chemotherapy | 7 treated (9) | [56] |
| PRAME-Specific | |||||||
| NCT02743611 | USA | Active, not recruiting (2016) | I/II | Relapsed AML | n.d. | (28) | [57] |
| NCT03503968 EudraCT-2017-000440-18 | Germany | Recruiting (2018) | I/II | R/R AML | HAT and/or allo-HSCT | (92) | n.d. |
| EudraCT-2018-000717-20 | Germany | Ongoing (2019) | Long-term follow-up of phase I | R/R AML | HAT and/or allo-HSCT | (52) | n.d. |
| MiHA HA-1H-Specific | |||||||
| EudraCT-2010-024625-20 NTR3454/NL3307 | Netherlands | Completed (2012, prematurely ended in 2018) | I | High-risk AML | allo-HSCT | 9 recruited, 5 treated (20) | [49,58] |
| NCT04464889 EudraCT-2019-002346-20 | Netherlands | Active, not recruiting (2020) | I | R/R AML | allo-HSCT | (29) | n.d. |
| NCT03326921 | USA | Recruiting (2017) | I | Recurrent AML | allo-HSCT | (24) | n.d. |
| Other | |||||||
| NTR6541/NL6357 | Netherlands | Recruiting (2017) | I | R/R AML | n.d. | (18) | [61,62,63] |
| Clinical Trial Identifier | Name of T-Cell Product | TCR | T-Cell Population | Ref. | |
|---|---|---|---|---|---|
| Restriction | High-Affinity/Avidity | ||||
| WT1-Specific | |||||
| NCT01621724 EudraCT-2006-004950-25 | WT1 TCR-001 | HLA-A2 | n.d. | Autologous T cells | [51] |
| NCT02550535 EudraCT-2014-003111-10 | n.d. | HLA-A2 | n.d. (allo-restricted TCR) | Autologous T cells | [52,53] |
| UMIN000011519 | n.d. | HLA-A24 | No | Autologous T cells | [54] |
| NCT01640301 | WT1-TTCR-C4 | HLA-A2 | Yes (from healthy individual) | Donor-derived EBV-specific CD8 T cells | [55] |
| NCT02770820 | WT1-TTCR-C4 | HLA-A2 | Yes (from healthy individual) | Autologous central memory/naïve CD8 T cells EBV-specific T cells | [56] |
| PRAME-specific | |||||
| NCT02743611 | BPX-701 | HLA-A2 | Yes (allo-restricted donor) | Autologous T cells | [57] |
| NCT03503968 EudraCT-2017-000440-18 | MDG1011 | HLA-A2 | n.d. | Autologous T cells | n.d. |
| EudraCT-2018-000717-20 | MDG1011 | HLA-A2 | n.d. | Autologous T cells | n.d. |
| MiHA HA-1H-specific | |||||
| EudraCT-2010-024625-20 NTR3454/NL3307 | n.d. | HLA-A2 | n.d. | Autologous donor-derived CMV- and/or EBV-specific T cells | [49,58] |
| NCT04464889 EudraCT-2019-002346-20 | MDG1021 | HLA-A2 | n.d. | Autologous T cells | n.d. |
| NCT03326921 | n.d. | HLA-A2 | n.d. | CD4 and CD8 memory donor T cells | n.d. |
| Other | |||||
| NTR6541/NL6357 | TEG001 | n.a. (Vγ9Vδ2 TCR) | Yes | Autologous T cells | [61,62,63] |
| Clinical Trial Identifier | Age of Patients | No. Patients per Arm or Cohort | Dosage per Cohort | Additional Treatments | Ref. |
|---|---|---|---|---|---|
| WT1-Specific | |||||
| NCT01621724 EudraCT-2006-004950-25 | 1 pt. 18-64 years 6 pt. ≥ 65 years | Cohort 1: 3 pt. Cohort 2: 4 pt. | Cohort 1: ≤2 × 107 T cells/kg Cohort 2: ≤1 × 108 T cells/kg | Standard conditioning; 106 units/m2 IL-2 | [51] |
| NCT02550535 EudraCT-2014-003111-10 | n.d. | Cohort 1: 7 pt. Cohort 2: 3 pt. (6 AML, 3 MDS and 1 TKI- resistant CML in total) | Cohort 1: ≤2 × 107 T cells/kg Cohort 2: ≤1 × 108 T cells/kg | Subcutaneous low-dose injections of IL-2 (1 × 106 units/m2) | [52,53] |
| UMIN000011519 | 1 pt. 18-64 years 7 pt. ≥ 65 years | Cohort 1: 3 pt. (1 AML and 2 MDS) Cohort 2: 3 pt. (MDS) (+2 pt. extracohort; 1 AML and 1 MDS) Cohort 3: 0 pt. | Cohort 1: two doses of 2 × 108 cells Cohort 2: two doses of 1 × 109 cells Cohort 3: two doses of 5 × 109 cells Cells administered at day 0 and 28 | Subcutaneous injection of 300 μg mutated WT1235-243 peptide at day 30 and 44 | [54] |
| NCT01640301 | 8 pt 18-64 years 4 pt. ≥ 65 years | Treatment arm: 12 pt. Prophylactic arm: 12 pt. | 12/12 pt.; one dose of 1010 T cells/m2 7/12 pt.; second dose of 1010 T cells/m2 (administered if frequency of TCR-T cells was <3% of total peripheral CD8+ T cells) | Subcutaneous low-dose injection of IL-2 | [55] |
| NCT02770820 | 4 pt. 18-64 years 3 pt. ≥ 65 years | Cohort 1: 7 pt. (4/7 pt. completed treatment) | Cohort 1: Two doses (day 0 and day > 21) | Subcutaneous injection of IL-2 | [56] |
| PRAME-Specific | |||||
| NCT02743611 | n.d. | n.d. | Escalating doses from 1.25 × 106 T cells/kg up to 5 × 106 T cells/kg to be explored | Rimiducid (in response to treatment-related toxicity) | [57] |
| NCT03503968 EudraCT-2017-000440-18 | n.d. | n.d. | Cohort 1: target dose of 1 × 105 T cells/kg Cohort 2: target dose of 1 × 106 T cells/kg Cohort 3: target dose of 5 × 106 T cells/kg Optional cohort 4: up to 1 × 107 T cells/kg | n.d. | n.d. |
| EudraCT-2018-000717-20 | n.d. | n.d. | Patients that were treated with MDG1011 TCR-T-cell product in EudraCT-2017-000440-18 trial | n.d. | n.d. |
| MiHA HA-1H-Specific | |||||
| EudraCT-2010-024625-20 NTR3454/NL3307 | 4 pt. 18-64 years 1 pt. ≥ 65 years | Cohort 1: 5 pt. (4 AML and 1 B-LBL) | Cohort 1: two doses of ≥3 × 106 T cells (day 8 and 14 after allo-HSCT) | n.d. | [49,58] |
| NCT04464889 EudraCT-2019-002346-20 | n.d. | n.d. | Cohort 1: target dose of 0.3 × 106 T cells/kg Cohort 2: target dose of 1 × 106 T cells/kg Cohort 3: target dose of 3 × 106 T cells/kg | n.d. | n.d. |
| NCT03326921 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Other | |||||
| NTR6541/NL6357 | n.d. | n.d. | n.d. | n.d. | [61,62,63] |
| Clinical Trial Identifier | Treatment-Related Toxicities (Grade 1–2) | Treatment-Related Serious Adverse Events (Grade 3–4) | Persistence of T Cells | Disease Response | Ref. |
|---|---|---|---|---|---|
| WT1-Specific | |||||
| NCT01621724 EudraCT-2006-004950-25 | No dose-limiting toxicity | Cohort 1: febrile neutropenia (1/3 pt.) | Cohort 1: 2/3 pt. at day 365 Cohort 2: 2/4 pt. at day 365 | Cohort 1: CR (1/3 pt.); no response (2/3 pt.) Cohort 2: CR (3/4 pt.); no response (1/4 pt.) | [51] |
| NCT02550535 EudraCT-2014-003111-10 | No dose-limiting toxicity | Possibly treatment-related cytokine release syndrome (1/10 pt.) | 10/10 pt. at day 28 7/10 pt. at day 29-365 | 6 AML pt.: median survival of 12 months | [52,53] |
| UMIN000011519 | No dose-limiting toxicity Facial edema, dermatitis, fever, phlebitis, arrhythmia, stomatitis (1/8 pt.) Skin reaction at peptide injection site (7/8 pt.) | None | Cohort 1: 2/3 pt. at day 58 Cohort 2: 3/5 pt. at day 58 | Decrease of abnormal erythroblasts in PB (1/8 pt.); Decrease of blasts in BM (2/8 pt.); Stable disease (1/8 pt.); Progressive disease (4/8 pt.) | [54] |
| NCT01640301 | None disclosed | Cytokine release syndrome (2/12 pt.) Lymphopenia (12/12 pt.) Trombocythopenia (2/12 pt.) Neutropenia (2/12 pt.) Anemia (7/12 pt.) | 9/12 pt. at day 28 4/12 pt. at day >365 | No evidence of disease (AML recurrence) at median follow-up of 44 months (12/12 pt.) | [55] |
| NCT02770820 | Not disclosed if treatment related: Fatigue, alanine aminotransferase increased, hyperglycemia (1/6 pt.); Anemia, thrombocytopenia (2/6 pt.); Neutropenia, leukopenia (3/6 pt.); Hypertension (4/6 pt.); Lymphopenia (5/7 pt.) | Not disclosed if treatment related: Death (1/6 pt.) | n.d. | n.d. | [56] |
| PRAME-Specific | |||||
| NCT02743611 | No results available yet | [57] | |||
| NCT03503968 EudraCT-2017-000440-18 | No results available yet | n.d. | |||
| EudraCT-2018-000717-20 | No results available yet | n.d. | |||
| MiHA HA-1H-Specific | |||||
| EudraCT-2010-024625-20 NTR3454/NL3307 | None | None | 3/5 pt. at day 14 after second infusion | Relapsed AML prior to infusion leading to death (1/5 pt.; 1/4 AML pt.); Infections during follow-up leading to death (2/5 pt.; ¼ AML pt.); No AML relapse and alive (2/4 pt.) | [49,58] |
| NCT04464889 EudraCT-2019-002346-20 | No results available yet | n.d. | |||
| NCT03326921 | No results available yet | n.d. | |||
| Other | |||||
| NTR6541/NL6357 | No results available yet | [61,62,63] | |||
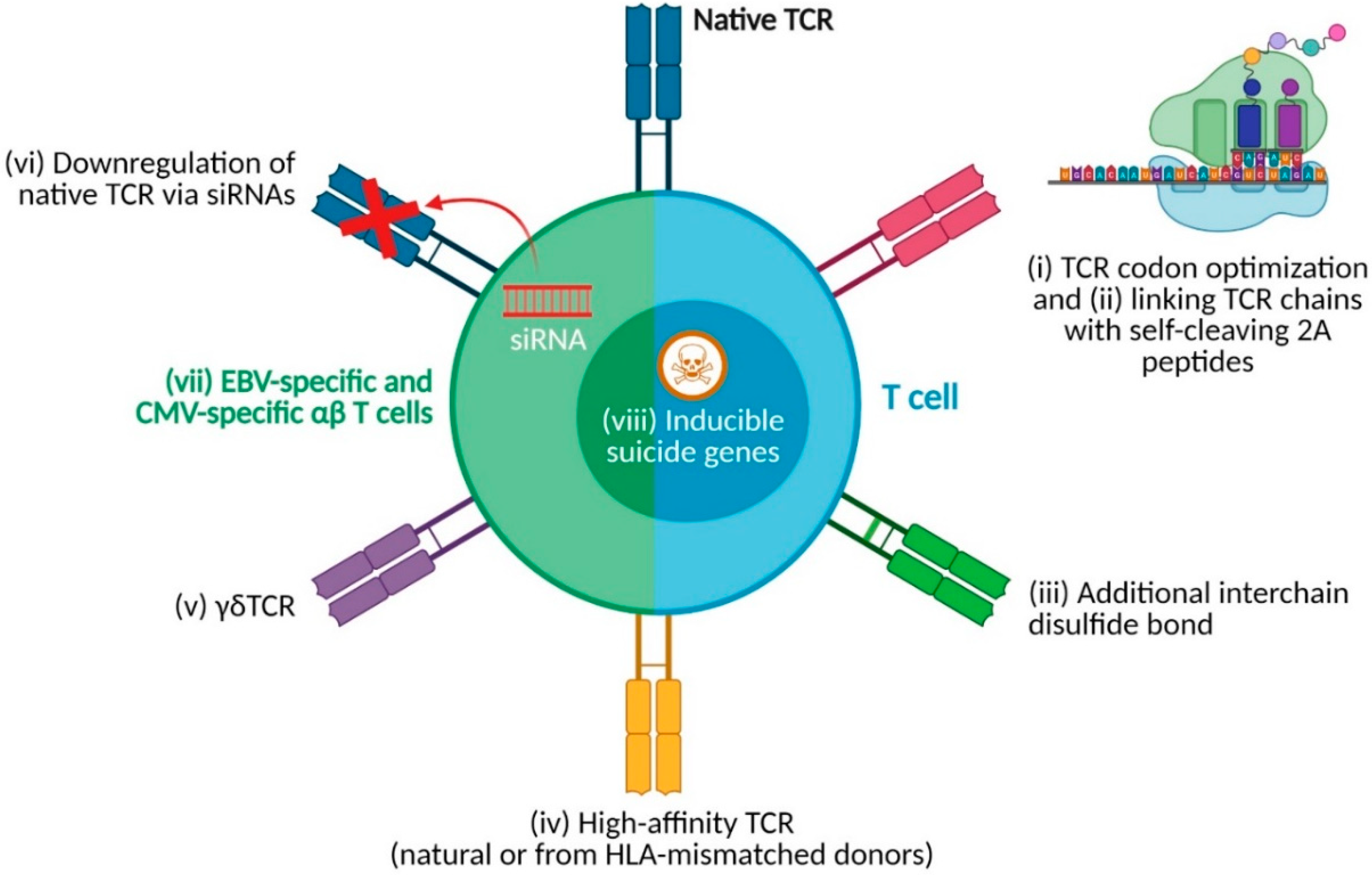
4. Strategies for Enhancing TCR-T-Cell Products
5. Future Directions in TCR-T-Cell Therapy for AML
| Clinical Trial Identifier | Description of Limitations |
|---|---|
| WT1-Specific | |
| NCT01621724 EudraCT-2006-004950-25 | Enrolment into the study was terminated early due to difficulties in the recruitment of patients |
| NCT02550535 EudraCT-2014-003111-10 | Enrolment into the study was terminated early in Germany due to difficulties in the recruitment of patients |
| UMIN000011519 | T-cell numbers for Arm 2 were not feasible for all patients; T-cell products were not feasible for Arm 3 |
| NCT01640301 | None disclosed |
| NCT02770820 | None disclosed |
| PRAME-Specific | |
| NCT02743611 | n.d. |
| NCT03503968/EudraCT-2017-000440-18 | n.d. |
| EudraCT-2018-000717-20 | n.d. |
| MiHA HA-1H-Specific | |
| EudraCT-2010-024625-20 NTR3454/NL3307 | HA-1H TCR-transduced CMV or EBV-specific T-cell products could not be generated for 4 out of 9 patients; TCR-T cells could not be detected (lack of TCR-T-cell expansion) in peripheral blood in 2 out of 5 treated patients at any time during follow-up; 3 out of 5 treated patients died during follow-up for causes not related to treatment; overall feasibility and efficacy of the procedure was too low to warrant further developments of this therapy |
| NCT04464889/EudraCT-2019-002346-20 | n.d. |
| NCT03326921 | n.d. |
| Other | |
| NTR6541/NL6357 | n.d. |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipof, J.J.; Loh, K.P.; O’Dwyer, K.; Liesveld, J.L. Allogeneic Hematopoietic Cell Transplantation for Older Adults with Acute Myeloid Leukemia. Cancers 2018, 10, 179. [Google Scholar] [CrossRef]
- Herold, T.; Rothenberg-Thurley, M.; Grunwald, V.V.; Janke, H.; Goerlich, D.; Sauerland, M.C.; Konstandin, N.P.; Dufour, A.; Schneider, S.; Neusser, M.; et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2020, 34, 3161–3172. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Bene, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Ngai, L.L.; Kelder, A.; Janssen, J.; Ossenkoppele, G.J.; Cloos, J. MRD Tailored Therapy in AML: What We Have Learned So Far. Front. Oncol. 2020, 10, 603636. [Google Scholar] [CrossRef]
- Vago, L.; Gojo, I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 1552–1564. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Versteven, M.; Lichtenegger, F.S.; Roex, G.; Campillo-Davo, D.; Lion, E.; Subklewe, M.; Van Tendeloo, V.F.; Berneman, Z.N.; Anguille, S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van Tendeloo, V.F.; Berneman, Z.N. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia 2012, 26, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bucklein, V.; Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: Current concepts and future developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Pont, M.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 2018, 131, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Timmers, M.; Roex, G.; Wang, Y.; Campillo-Davo, D.; Van Tendeloo, V.F.I.; Chu, Y.; Berneman, Z.N.; Luo, F.; Van Acker, H.H.; Anguille, S. Chimeric Antigen Receptor-Modified T-cell therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front. Immunol. 2019, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Lunning, M.A. CAR T-Cell Therapy in Hematologic Malignancies: A Voyage in Progress. Clin. Pharmacol. Ther. 2020, 107, 112–122. [Google Scholar] [CrossRef]
- Mardiana, S.; Gill, S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 697. [Google Scholar] [CrossRef]
- Hofmann, S.; Schubert, M.L.; Wang, L.; He, B.; Neuber, B.; Dreger, P.; Muller-Tidow, C.; Schmitt, M. Chimeric Antigen Receptor (CAR) T-cell therapy in Acute Myeloid Leukemia (AML). J. Clin. Med. 2019, 8, 200. [Google Scholar] [CrossRef]
- Acharya, U.H.; Walter, R.B. Chimeric Antigen Receptor (CAR)-Modified Immune Effector Cell Therapy for Acute Myeloid Leukemia (AML). Cancers 2020, 12, 3617. [Google Scholar] [CrossRef]
- Ritchie, D.S.; Neeson, P.J.; Khot, A.; Peinert, S.; Tai, T.; Tainton, K.; Chen, K.; Shin, M.; Wall, D.M.; Honemann, D.; et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 2013, 21, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Cummins, K.D.; Gill, S. Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: How close to reality? Haematologica 2019, 104, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Yan, S.; Veomett, N.; Pankov, D.; Zhou, L.; Korontsvit, T.; Scott, A.; Whitten, J.; Maslak, P.; Casey, E.; et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci. Transl. Med. 2013, 5, 176ra133. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Van Morris, K.; Vo, H.H.; Eck, S.; Lin, Y.F.; Rivas, J.M.; Andersson, B.S. T-cell receptor-based therapy: An innovative therapeutic approach for solid tumors. J. Hematol. Oncol. 2021, 14, 102. [Google Scholar] [CrossRef]
- Biernacki, M.A.; Brault, M.; Bleakley, M. T-Cell Receptor-Based Immunotherapy for Hematologic Malignancies. Cancer J. 2019, 25, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y. T cell receptor-engineered T cells for leukemia immunotherapy. Cancer Cell Int. 2019, 19, 2. [Google Scholar] [CrossRef]
- Fisher, J.; Anderson, J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; Van Tendeloo, V.F.; Lion, E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology 2015, 4, e1021538. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Van den Bergh, J.M.; Berneman, Z.N.; Lion, E.; Smits, E.L.; Van Tendeloo, V.F. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 2016, 9, 101. [Google Scholar] [CrossRef]
- Chandran, S.S.; Klebanoff, C.A. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunol. Rev. 2019, 290, 127–147. [Google Scholar] [CrossRef]
- Campillo-Davo, D.; Flumens, D.; Lion, E. The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells 2020, 9, 1720. [Google Scholar] [CrossRef]
- Sugiyama, H. WT1 (Wilms’ tumor gene 1): Biology and cancer immunotherapy. Jpn. J. Clin. Oncol. 2010, 40, 377–387. [Google Scholar] [CrossRef]
- Drakos, E.; Rassidakis, G.Z.; Tsioli, P.; Lai, R.; Jones, D.; Medeiros, L.J. Differential expression of WT1 gene product in non-Hodgkin lymphomas. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 132–137. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Oji, Y.; Horiuchi, T.; Kanda, T.; Kitagawa, M.; Takeuchi, T.; Kawano, K.; Kuwae, Y.; Yamauchi, A.; Okumura, M.; et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006, 19, 804–814. [Google Scholar] [CrossRef]
- Menssen, H.D.; Renkl, H.J.; Rodeck, U.; Maurer, J.; Notter, M.; Schwartz, S.; Reinhardt, R.; Thiel, E. Presence of Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia 1995, 9, 1060–1067. [Google Scholar]
- Niksic, M.; Slight, J.; Sanford, J.R.; Caceres, J.F.; Hastie, N.D. The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum. Mol. Genet. 2004, 13, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.; Fitzgibbon, J.; Paschka, P. The clinical relevance of Wilms Tumour 1 (WT1) gene mutations in acute leukaemia. Hematol. Oncol. 2010, 28, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rampal, R.; Figueroa, M.E. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica 2016, 101, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.C. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer 2011, 2, 95–107. [Google Scholar] [CrossRef]
- Walker, A.; Marcucci, G. Molecular prognostic factors in cytogenetically normal acute myeloid leukemia. Expert Rev. Hematol. 2012, 5, 547–558. [Google Scholar] [CrossRef]
- Epping, M.T.; Wang, L.; Edel, M.J.; Carlee, L.; Hernandez, M.; Bernards, R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 2005, 122, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Steger, B.; Floro, L.; Amberger, D.C.; Kroell, T.; Tischer, J.; Kolb, H.J.; Schmetzer, H.M. WT1, PRAME, and PR3 mRNA Expression in Acute Myeloid Leukemia (AML). J. Immunother. 2020, 43, 204–215. [Google Scholar] [CrossRef]
- Paydas, S.; Tanriverdi, K.; Yavuz, S.; Disel, U.; Baslamisli, F.; Burgut, R. PRAME mRNA levels in cases with acute leukemia: Clinical importance and future prospects. Am. J. Hematol. 2005, 79, 257–261. [Google Scholar] [CrossRef]
- Ding, K.; Wang, X.M.; Fu, R.; Ruan, E.B.; Liu, H.; Shao, Z.H. PRAME Gene Expression in Acute Leukemia and Its Clinical Significance. Cancer Biol. Med. 2012, 9, 73–76. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, H.; Jiang, B.; Li, J.; Lu, X.; Li, L.; Ruan, G.; Liu, Y.; Chen, S.; Huang, X. Expression patterns of WT1 and PRAME in acute myeloid leukemia patients and their usefulness for monitoring minimal residual disease. Leuk. Res. 2009, 33, 384–390. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Letsch, A.; Thiel, E.; Schmittel, A.; Mailaender, V.; Baerwolf, S.; Nagorsen, D.; Keilholz, U. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood 2002, 100, 2132–2137. [Google Scholar] [CrossRef]
- Dao, T.; Korontsvit, T.; Zakhaleva, V.; Jarvis, C.; Mondello, P.; Oh, C.; Scheinberg, D.A. An immunogenic WT1-derived peptide that induces T cell response in the context of HLA-A*02:01 and HLA-A*24:02 molecules. Oncoimmunology 2017, 6, e1252895. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Tan, A.C.; Xiang, S.D.; Goubier, A.; Harland, K.L.; Clemens, E.B.; Plebanski, M.; Kedzierska, K. Understanding CD8(+) T-cell responses toward the native and alternate HLA-A*02:01-restricted WT1 epitope. Clin. Transl. Immunol. 2017, 6, e134. [Google Scholar] [CrossRef] [PubMed]
- Matko, S.; Manderla, J.; Bonsack, M.; Schmitz, M.; Bornhauser, M.; Tonn, T.; Odendahl, M. PRAME peptide-specific CD8(+) T cells represent the predominant response against leukemia-associated antigens in healthy individuals. Eur. J. Immunol. 2018, 48, 1400–1411. [Google Scholar] [CrossRef]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Yong, A.S.; Tawab, A.; Jafarpour, B.; Eniafe, R.; Mielke, S.; Savani, B.N.; Keyvanfar, K.; Li, Y.; Kurlander, R.; et al. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood 2009, 113, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Oostvogels, R.; Lokhorst, H.M.; Mutis, T. Minor histocompatibility Ags: Identification strategies, clinical results and translational perspectives. Bone Marrow Transplant. 2016, 51, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Marijt, W.A.; Heemskerk, M.H.; Kloosterboer, F.M.; Goulmy, E.; Kester, M.G.; van der Hoorn, M.A.; van Luxemburg-Heys, S.A.; Hoogeboom, M.; Mutis, T.; Drijfhout, J.W.; et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc. Natl. Acad. Sci. USA 2003, 100, 2742–2747. [Google Scholar] [CrossRef]
- Cai, J.; Lee, J.; Jankowska-Gan, E.; Derks, R.; Pool, J.; Mutis, T.; Goulmy, E.; Burlingham, W.J. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J. Exp. Med. 2004, 199, 1017–1023. [Google Scholar] [CrossRef]
- Van Balen, P.; Jedema, I.; van Loenen, M.M.; de Boer, R.; van Egmond, H.M.; Hagedoorn, R.S.; Hoogstaten, C.; Veld, S.A.J.; Hageman, L.; van Liempt, P.A.G.; et al. HA-1H T-Cell Receptor Gene Transfer to Redirect Virus-Specific T Cells for Treatment of Hematological Malignancies After Allogeneic Stem Cell Transplantation: A Phase 1 Clinical Study. Front. Immunol. 2020, 11, 1804. [Google Scholar] [CrossRef]
- Torikai, H.; Akatsuka, Y.; Miyauchi, H.; Terakura, S.; Onizuka, M.; Tsujimura, K.; Miyamura, K.; Morishima, Y.; Kodera, Y.; Kuzushima, K.; et al. The HLA-A*0201-restricted minor histocompatibility antigen HA-1H peptide can also be presented by another HLA-A2 subtype, A*0206. Bone Marrow Transplant. 2007, 40, 165–174. [Google Scholar] [CrossRef][Green Version]
- European Union Clinical Trials Register. WT1 TCR Gene Therapy for Leukaemia: A Phase I/II Safety and Toxicity Study—EudraCT 2006-004950-25. Available online: www.clinicaltrialsregister.eu/ctr-search/trial/2006-004950-25/results (accessed on 25 July 2021).
- Morris, E.C.; Tendeiro-Rego, R.; Richardson, R.; Fox, T.A.; Sillito, F.; Holler, A.; Thomas, S.; Xue, S.-A.; Martínez-Dávila, I.A.; Nicholson, E.; et al. A Phase I Study Evaluating the Safety and Persistence of Allorestricted WT1-TCR Gene Modified Autologous T Cells in Patients with High-Risk Myeloid Malignancies Unsuitable for Allogeneic Stem Cell Transplantation. Blood 2019, 134, 1367. [Google Scholar] [CrossRef]
- European Union Clinical Trials Register. A Single Arm Phase I/II Study of the Safety and Efficacy of Gene-modified WT1 TCR Therapy in Patients with Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukaemia (AML) Who Have Failed to Achieve or Maintain an IWG Defined Response Following Hypomethylating Agent Therapy. Available online: www.clinicaltrialsregister.eu/ctr-search/trial/2014-003111-10/results (accessed on 25 July 2021).
- Tawara, I.; Kageyama, S.; Miyahara, Y.; Fujiwara, H.; Nishida, T.; Akatsuka, Y.; Ikeda, H.; Tanimoto, K.; Terakura, S.; Murata, M.; et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 2017, 130, 1985–1994. [Google Scholar] [CrossRef]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Laboratory-Treated (Central Memory/Naive) CD8+ T Cells in Treating Patients with Newly Diagnosed or Relapsed Acute Myeloid Leukemia. Available online: Clinicaltrials.gov/ct2/show/results/NCT02770820 (accessed on 25 July 2021).
- Amir, A.L.; van der Steen, D.M.; van Loenen, M.M.; Hagedoorn, R.S.; de Boer, R.; Kester, M.D.; de Ru, A.H.; Lugthart, G.J.; van Kooten, C.; Hiemstra, P.S.; et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin. Cancer Res. 2011, 17, 5615–5625. [Google Scholar] [CrossRef]
- Van Loenen, M.M.; de Boer, R.; van Liempt, E.; Meij, P.; Jedema, I.; Falkenburg, J.H.; Heemskerk, M.H. A Good Manufacturing Practice procedure to engineer donor virus-specific T cells into potent anti-leukemic effector cells. Haematologica 2014, 99, 759–768. [Google Scholar] [CrossRef]
- Van Loenen, M.M.; de Boer, R.; Hagedoorn, R.S.; van Egmond, E.H.; Falkenburg, J.H.; Heemskerk, M.H. Optimization of the HA-1-specific T-cell receptor for gene therapy of hematologic malignancies. Haematologica 2011, 96, 477–481. [Google Scholar] [CrossRef]
- Styczynski, J.; Tridello, G.; Gil, L.; Ljungman, P.; Hoek, J.; Iacobelli, S.; Ward, K.N.; Cordonnier, C.; Einsele, H.; Socie, G.; et al. Impact of Donor Epstein-Barr Virus Serostatus on the Incidence of Graft-Versus-Host Disease in Patients With Acute Leukemia After Hematopoietic Stem-Cell Transplantation: A Study From the Acute Leukemia and Infectious Diseases Working Parties of the European Society for Blood and Marrow Transplantation. J. Clin. Oncol. 2016, 34, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Straetemans, T.; Kierkels, G.J.J.; Doorn, R.; Jansen, K.; Heijhuurs, S.; Dos Santos, J.M.; van Muyden, A.D.D.; Vie, H.; Clemenceau, B.; Raymakers, R.; et al. GMP-Grade Manufacturing of T Cells Engineered to Express a Defined gammadeltaTCR. Front. Immunol. 2018, 9, 1062. [Google Scholar] [CrossRef]
- Grunder, C.; van Dorp, S.; Hol, S.; Drent, E.; Straetemans, T.; Heijhuurs, S.; Scholten, K.; Scheper, W.; Sebestyen, Z.; Martens, A.; et al. gamma9 and delta2CDR3 domains regulate functional avidity of T cells harboring gamma9delta2TCRs. Blood 2012, 120, 5153–5162. [Google Scholar] [CrossRef] [PubMed]
- Johanna, I.; Straetemans, T.; Heijhuurs, S.; Aarts-Riemens, T.; Norell, H.; Bongiovanni, L.; de Bruin, A.; Sebestyen, Z.; Kuball, J. Evaluating in vivo efficacy—Toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J. Immunother. Cancer 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Mauro, V.P. Codon Optimization in the Production of Recombinant Biotherapeutics: Potential Risks and Considerations. BioDrugs 2018, 32, 69–81. [Google Scholar] [CrossRef]
- Szymczak, A.L.; Workman, C.J.; Wang, Y.; Vignali, K.M.; Dilioglou, S.; Vanin, E.F.; Vignali, D.A. Correction of multi-gene deficiency in vivo using a single ’self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 2004, 22, 589–594. [Google Scholar] [CrossRef]
- Hadpech, S.; Jinathep, W.; Saoin, S.; Thongkum, W.; Chupradit, K.; Yasamut, U.; Moonmuang, S.; Tayapiwatana, C. Impairment of a membrane-targeting protein translated from a downstream gene of a “self-cleaving” T2A peptide conjunction. Protein Expr. Purif. 2018, 150, 17–25. [Google Scholar] [CrossRef]
- Yang, S.; Cohen, C.J.; Peng, P.D.; Zhao, Y.; Cassard, L.; Yu, Z.; Zheng, Z.; Jones, S.; Restifo, N.P.; Rosenberg, S.A.; et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008, 15, 1411–1423. [Google Scholar] [CrossRef]
- Leisegang, M.; Engels, B.; Meyerhuber, P.; Kieback, E.; Sommermeyer, D.; Xue, S.A.; Reuss, S.; Stauss, H.; Uckert, W. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J. Mol. Med. 2008, 86, 573–583. [Google Scholar] [CrossRef]
- Cohen, C.J.; Li, Y.F.; El-Gamil, M.; Robbins, P.F.; Rosenberg, S.A.; Morgan, R.A. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007, 67, 3898–3903. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Fujiwara, H.; Okamoto, S.; An, J.; Nagai, K.; Shirakata, T.; Mineno, J.; Kuzushima, K.; Shiku, H.; Yasukawa, M. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 2011, 118, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef]
- Wilde, S.; Geiger, C.; Milosevic, S.; Mosetter, B.; Eichenlaub, S.; Schendel, D.J. Generation of allo-restricted peptide-specific T cells using RNA-pulsed dendritic cells: A three phase experimental procedure. Oncoimmunology 2012, 1, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, L.T.; Hagedoorn, R.S.; van Loenen, M.M.; Willemze, R.; Falkenburg, J.H.; Heemskerk, M.H. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006, 66, 3331–3337. [Google Scholar] [CrossRef]
- Van der Veken, L.T.; Coccoris, M.; Swart, E.; Falkenburg, J.H.; Schumacher, T.N.; Heemskerk, M.H. Alpha beta T cell receptor transfer to gamma delta T cells generates functional effector cells without mixed TCR dimers in vivo. J. Immunol. 2009, 182, 164–170. [Google Scholar] [CrossRef]
- Osborn, M.J.; Webber, B.R.; Knipping, F.; Lonetree, C.L.; Tennis, N.; DeFeo, A.P.; McElroy, A.N.; Starker, C.G.; Lee, C.; Merkel, S.; et al. Evaluation of TCR Gene Editing Achieved by TALENs, CRISPR/Cas9, and megaTAL Nucleases. Mol. Ther. 2016, 24, 570–581. [Google Scholar] [CrossRef]
- Legut, M.; Dolton, G.; Mian, A.A.; Ottmann, O.G.; Sewell, A.K. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood 2018, 131, 311–322. [Google Scholar] [CrossRef]
- Schober, K.; Muller, T.R.; Gokmen, F.; Grassmann, S.; Effenberger, M.; Poltorak, M.; Stemberger, C.; Schumann, K.; Roth, T.L.; Marson, A.; et al. Orthotopic replacement of T-cell receptor alpha- and beta-chains with preservation of near-physiological T-cell function. Nat. Biomed. Eng. 2019, 3, 974–984. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef] [PubMed]
- Straetemans, T.; Janssen, A.; Jansen, K.; Doorn, R.; Aarts, T.; van Muyden, A.D.D.; Simonis, M.; Bergboer, J.; de Witte, M.; Sebestyen, Z.; et al. TEG001 Insert Integrity from Vector Producer Cells until Medicinal Product. Mol. Ther. 2020, 28, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dotti, G.; Krance, R.A.; Martinez, C.A.; Naik, S.; Kamble, R.T.; Durett, A.G.; Dakhova, O.; Savoldo, B.; Di Stasi, A.; et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015, 125, 4103–4113. [Google Scholar] [CrossRef] [PubMed]
- Schaft, N.; Dorrie, J.; Muller, I.; Beck, V.; Baumann, S.; Schunder, T.; Kampgen, E.; Schuler, G. A new way to generate cytolytic tumor-specific T cells: Electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol. Immunother. 2006, 55, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Harrer, D.C.; Simon, B.; Fujii, S.I.; Shimizu, K.; Uslu, U.; Schuler, G.; Gerer, K.F.; Hoyer, S.; Dorrie, J.; Schaft, N. RNA-transfection of gamma/delta T cells with a chimeric antigen receptor or an alpha/beta T-cell receptor: A safer alternative to genetically engineered alpha/beta T cells for the immunotherapy of melanoma. BMC Cancer 2017, 17, 551. [Google Scholar] [CrossRef]
- Campillo-Davo, D.; Fujiki, F.; Van den Bergh, J.M.J.; De Reu, H.; Smits, E.; Goossens, H.; Sugiyama, H.; Lion, E.; Berneman, Z.N.; Van Tendeloo, V. Efficient and Non-genotoxic RNA-Based Engineering of Human T Cells Using Tumor-Specific T Cell Receptors With Minimal TCR Mispairing. Front. Immunol. 2018, 9, 2503. [Google Scholar] [CrossRef] [PubMed]
- Dengler, R.; Munstermann, U.; al-Batran, S.; Hausner, I.; Faderl, S.; Nerl, C.; Emmerich, B. Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br. J. Haematol. 1995, 89, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.; Zhang, L.; Rojewski, M.; Fekete, N.; Schrezenmeier, H.; Erle, A.; Bullinger, L.; Hofmann, S.; Gotz, M.; Dohner, K.; et al. Leukemic progenitor cells are susceptible to targeting by stimulated cytotoxic T cells against immunogenic leukemia-associated antigens. Int. J. Cancer 2015, 137, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Yong, A.S.; Mielke, S.; Savani, B.N.; Musse, L.; Superata, J.; Jafarpour, B.; Boss, C.; Barrett, A.J. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 2008, 111, 236–242. [Google Scholar] [CrossRef]
- Qazilbash, M.H.; Wieder, E.; Thall, P.F.; Wang, X.; Rios, R.; Lu, S.; Kanodia, S.; Ruisaard, K.E.; Giralt, S.A.; Estey, E.H.; et al. PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia 2017, 31, 697–704. [Google Scholar] [CrossRef]
- Molldrem, J.; Dermime, S.; Parker, K.; Jiang, Y.Z.; Mavroudis, D.; Hensel, N.; Fukushima, P.; Barrett, A.J. Targeted T-cell therapy for human leukemia: Cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood 1996, 88, 2450–2457. [Google Scholar] [CrossRef]
- Medina, D.J.; Gharibo, M.; Savage, P.; Cohler, A.; Kuriyan, M.; Balsara, B.; Anand, M.; Schaar, D.; Krimmel, T.; Saggiomo, K.; et al. A pilot study of allogeneic cellular therapy for patients with advanced hematologic malignancies. Leuk. Res. 2008, 32, 1842–1848. [Google Scholar] [CrossRef]
- Kapp, M.; Stevanovic, S.; Fick, K.; Tan, S.M.; Loeffler, J.; Opitz, A.; Tonn, T.; Stuhler, G.; Einsele, H.; Grigoleit, G.U. CD8+ T-cell responses to tumor-associated antigens correlate with superior relapse-free survival after allo-SCT. Bone Marrow Transplant. 2009, 43, 399–410. [Google Scholar] [CrossRef]
- Steger, B.; Milosevic, S.; Doessinger, G.; Reuther, S.; Liepert, A.; Braeu, M.; Schick, J.; Vogt, V.; Schuster, F.; Kroell, T.; et al. CD4(+)and CD8(+)T-cell reactions against leukemia-associated- or minor-histocompatibility-antigens in AML-patients after allogeneic SCT. Immunobiology 2014, 219, 247–260. [Google Scholar] [CrossRef]
- Rucker-Braun, E.; Link, C.S.; Schmiedgen, M.; Tunger, A.; Vizjak, P.; Teipel, R.; Wehner, R.; Kuhn, D.; Fuchs, Y.F.; Oelschlagel, U.; et al. Longitudinal analyses of leukemia-associated antigen-specific CD8(+) T cells in patients after allogeneic stem cell transplantation. Exp. Hematol. 2016, 44, 1024–1033.e1021. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, C.; Jones, D.; Quintanilla, K.E.; Li, D.; Wang, Y.; Wieder, E.D.; Clise-Dwyer, K.; Alatrash, G.; Mj, Y.; et al. Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy 2010, 12, 1056–1062. [Google Scholar] [CrossRef]
- Sergeeva, A.; Alatrash, G.; He, H.; Ruisaard, K.; Lu, S.; Wygant, J.; McIntyre, B.W.; Ma, Q.; Li, D.; St John, L.; et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood 2011, 117, 4262–4272. [Google Scholar] [CrossRef]
- Sergeeva, A.; He, H.; Ruisaard, K.; St John, L.; Alatrash, G.; Clise-Dwyer, K.; Li, D.; Patenia, R.; Hong, R.; Sukhumalchandra, P.; et al. Activity of 8F4, a T-cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia 2016, 30, 1475–1484. [Google Scholar] [CrossRef]
- Herrmann, A.C.; Im, J.S.; Pareek, S.; Ruiz-Vasquez, W.; Lu, S.; Sergeeva, A.; Mehrens, J.; He, H.; Alatrash, G.; Sukhumalchandra, P.; et al. A Novel T-Cell Engaging Bi-specific Antibody Targeting the Leukemia Antigen PR1/HLA-A2. Front. Immunol. 2018, 9, 3153. [Google Scholar] [CrossRef]
- Ma, Q.; Garber, H.R.; Lu, S.; He, H.; Tallis, E.; Ding, X.; Sergeeva, A.; Wood, M.S.; Dotti, G.; Salvado, B.; et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy 2016, 18, 985–994. [Google Scholar] [CrossRef]
- Greiner, J.; Ringhoffer, M.; Taniguchi, M.; Schmitt, A.; Kirchner, D.; Krahn, G.; Heilmann, V.; Gschwend, J.; Bergmann, L.; Dohner, H.; et al. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp. Hematol. 2002, 30, 1029–1035. [Google Scholar] [CrossRef]
- Tzankov, A.; Strasser, U.; Dirnhofer, S.; Menter, T.; Arber, C.; Jotterand, M.; Rovo, A.; Tichelli, A.; Stauder, R.; Gunthert, U. In situ RHAMM protein expression in acute myeloid leukemia blasts suggests poor overall survival. Ann. Hematol. 2011, 90, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Willemen, Y.; Van den Bergh, J.M.; Bonte, S.M.; Anguille, S.; Heirman, C.; Stein, B.M.; Goossens, H.; Kerre, T.; Thielemans, K.; Peeters, M.; et al. The tumor-associated antigen RHAMM (HMMR/CD168) is expressed by monocyte-derived dendritic cells and presented to T cells. Oncotarget 2016, 7, 73960–73970. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Schmitt, M.; Li, L.; Giannopoulos, K.; Bosch, K.; Schmitt, A.; Dohner, K.; Schlenk, R.F.; Pollack, J.R.; Dohner, H.; et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood 2006, 108, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Li, L.; Ringhoffer, M.; Barth, T.F.; Giannopoulos, K.; Guillaume, P.; Ritter, G.; Wiesneth, M.; Dohner, H.; Schmitt, M. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood 2005, 106, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Casalegno-Garduno, R.; Meier, C.; Schmitt, A.; Spitschak, A.; Hilgendorf, I.; Rohde, S.; Hirt, C.; Freund, M.; Putzer, B.M.; Schmitt, M. Immune responses to RHAMM in patients with acute myeloid leukemia after chemotherapy and allogeneic stem cell transplantation. Clin. Dev. Immunol. 2012, 2012, 146463. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Schmitt, A.; Rojewski, M.T.; Chen, J.; Giannopoulos, K.; Fei, F.; Yu, Y.; Gotz, M.; Heyduk, M.; Ritter, G.; et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 2008, 111, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Schmitt, A.; Giannopoulos, K.; Rojewski, M.T.; Gotz, M.; Funk, I.; Ringhoffer, M.; Bunjes, D.; Hofmann, S.; Ritter, G.; et al. High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica 2010, 95, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Jeremias, I.; Wilde, S.; Leisegang, M.; Starck, L.; Mosetter, B.; Uckert, W.; Heemskerk, M.H.; Schendel, D.J.; Frankenberger, B. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood 2012, 119, 3440–3449. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, S.; Vanhee, S.; Goetgeluk, G.; Verstichel, G.; Van Caeneghem, Y.; Velghe, I.; Philippe, J.; Berneman, Z.N.; Plum, J.; Taghon, T.; et al. RHAMM/HMMR (CD168) is not an ideal target antigen for immunotherapy of acute myeloid leukemia. Haematologica 2012, 97, 1539–1547. [Google Scholar] [CrossRef]
- Depreter, B.; Weening, K.E.; Vandepoele, K.; Essand, M.; De Moerloose, B.; Themeli, M.; Cloos, J.; Hanekamp, D.; Moors, I.; D’Hont, I.; et al. TARP is an immunotherapeutic target in acute myeloid leukemia expressed in the leukemic stem cell compartment. Haematologica 2020, 105, 1306–1316. [Google Scholar] [CrossRef]
- Klar, R.; Schober, S.; Rami, M.; Mall, S.; Merl, J.; Hauck, S.M.; Ueffing, M.; Admon, A.; Slotta-Huspenina, J.; Schwaiger, M.; et al. Therapeutic targeting of naturally presented myeloperoxidase-derived HLA peptide ligands on myeloid leukemia cells by TCR-transgenic T cells. Leukemia 2014, 28, 2355–2366. [Google Scholar] [CrossRef]
- Sandri, S.; De Sanctis, F.; Lamolinara, A.; Boschi, F.; Poffe, O.; Trovato, R.; Fiore, A.; Sartori, S.; Sbarbati, A.; Bondanza, A.; et al. Effective control of acute myeloid leukaemia and acute lymphoblastic leukaemia progression by telomerase specific adoptive T-cell therapy. Oncotarget 2017, 8, 86987–87001. [Google Scholar] [CrossRef]
- Ibisch, C.; Gallot, G.; Vivien, R.; Diez, E.; Jotereau, F.; Garand, R.; Vie, H. Recognition of leukemic blasts by HLA-DPB1-specific cytotoxic T cell clones: A perspective for adjuvant immunotherapy post-bone marrow transplantation. Bone Marrow Transplant. 1999, 23, 1153–1159. [Google Scholar] [CrossRef][Green Version]
- Herr, W.; Eichinger, Y.; Beshay, J.; Bloetz, A.; Vatter, S.; Mirbeth, C.; Distler, E.; Hartwig, U.F.; Thomas, S. HLA-DPB1 mismatch alleles represent powerful leukemia rejection antigens in CD4 T-cell immunotherapy after allogeneic stem-cell transplantation. Leukemia 2017, 31, 434–445. [Google Scholar] [CrossRef]
- Klobuch, S.; Hammon, K.; Vatter-Leising, S.; Neidlinger, E.; Zwerger, M.; Wandel, A.; Neuber, L.M.; Heilmeier, B.; Fichtner, R.; Mirbeth, C.; et al. HLA-DPB1 Reactive T Cell Receptors for Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation. Cells 2020, 9, 1264. [Google Scholar] [CrossRef]
- Biernacki, M.A.; Foster, K.A.; Woodward, K.B.; Coon, M.E.; Cummings, C.; Cunningham, T.M.; Dossa, R.G.; Brault, M.; Stokke, J.; Olsen, T.M.; et al. CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 5127–5141. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Ono, Y.; Hofmann, S.; Schmitt, A.; Mehring, E.; Gotz, M.; Guillaume, P.; Dohner, K.; Mytilineos, J.; Dohner, H.; et al. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood 2012, 120, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Riva, G.; Lagreca, I.; Barozzi, P.; Vallerini, D.; Morselli, M.; Paolini, A.; Bresciani, P.; Colaci, E.; Maccaferri, M.; et al. Characterization and dynamics of specific T cells against nucleophosmin-1 (NPM1)-mutated peptides in patients with NPM1-mutated acute myeloid leukemia. Oncotarget 2019, 10, 869–882. [Google Scholar] [CrossRef]
- Van der Lee, D.I.; Reijmers, R.M.; Honders, M.W.; Hagedoorn, R.S.; de Jong, R.C.; Kester, M.G.; van der Steen, D.M.; de Ru, A.H.; Kweekel, C.; Bijen, H.M.; et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Investig. 2019, 129, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Hobo, W.; Hutten, T.J.A.; Schaap, N.P.M.; Dolstra, H. Immune checkpoint molecules in acute myeloid leukaemia: Managing the double-edged sword. Br. J. Haematol. 2018, 181, 38–53. [Google Scholar] [CrossRef]
- Stahl, M.; Goldberg, A.D. Immune Checkpoint Inhibitors in Acute Myeloid Leukemia: Novel Combinations and Therapeutic Targets. Curr. Oncol. Rep. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campillo-Davo, D.; Anguille, S.; Lion, E. Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia. Cancers 2021, 13, 4519. https://doi.org/10.3390/cancers13184519
Campillo-Davo D, Anguille S, Lion E. Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia. Cancers. 2021; 13(18):4519. https://doi.org/10.3390/cancers13184519
Chicago/Turabian StyleCampillo-Davo, Diana, Sébastien Anguille, and Eva Lion. 2021. "Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia" Cancers 13, no. 18: 4519. https://doi.org/10.3390/cancers13184519
APA StyleCampillo-Davo, D., Anguille, S., & Lion, E. (2021). Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia. Cancers, 13(18), 4519. https://doi.org/10.3390/cancers13184519






