Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human
Abstract
:Simple Summary
Abstract
1. Current Ovarian Cancer Standard-of-Care and Limitations
1.1. Maintenance Therapies
1.2. Disease Recurrence and Side Effects from Standard-of-Care
2. Cancer Vaccine Strategies
2.1. Tumor-Associated Antigen and Tumor Neoantigen Peptide Vaccines
2.2. DNA and RNA Vaccines
2.3. Viral Vector Vaccines
2.4. DC-Based and Whole Tumor Cell-Based Vaccines
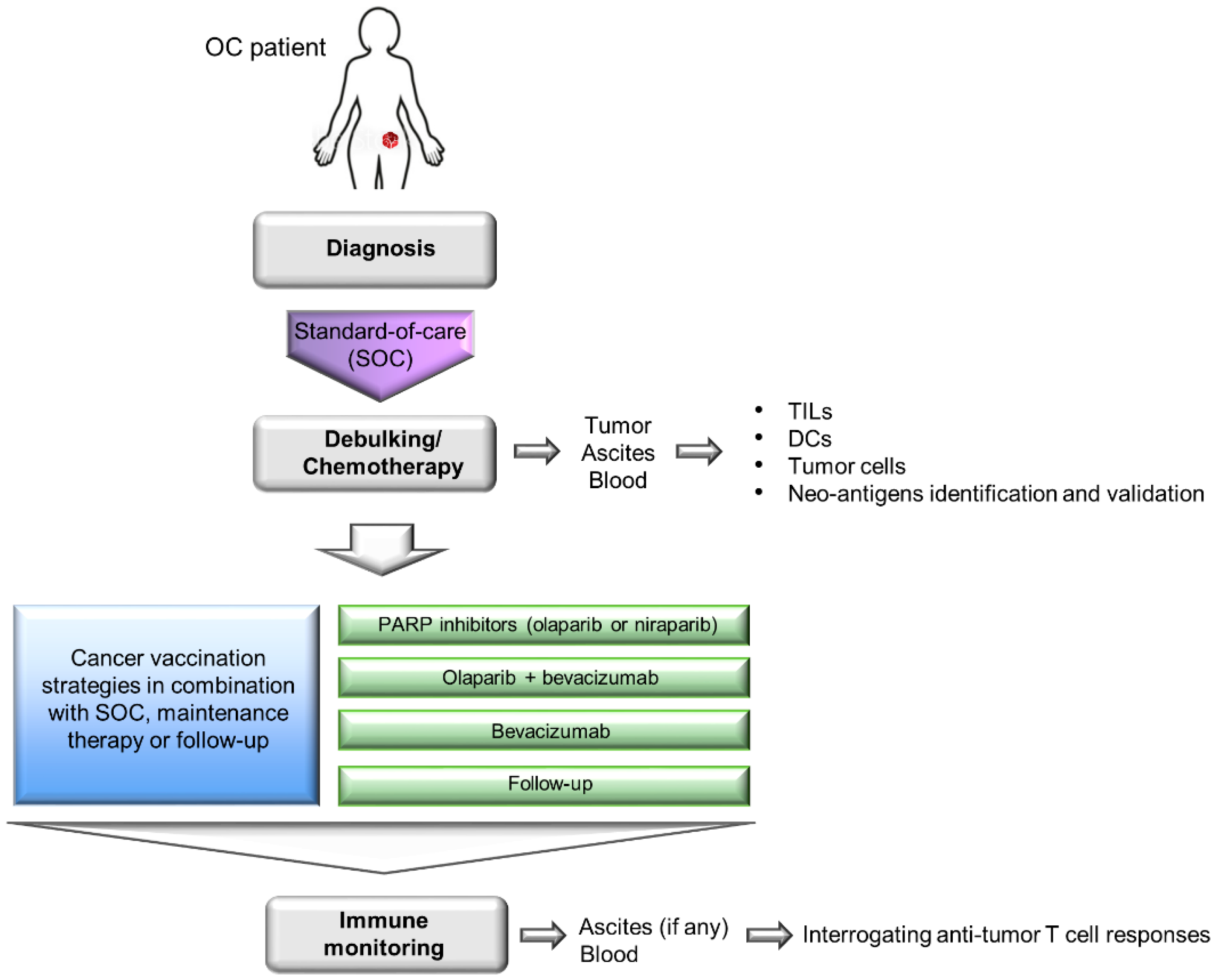
3. Integrating Cancer Vaccines into OC Standard-of-Care Regimen
Combining Cancer Vaccines with Immunomodulatory Agents
4. Preclinical Ovarian Tumor Animal Models as Tools for Clinical Translation
4.1. Syngeneic ID8 Tumor Model
4.2. Orthotopic Patient-Derived Xenograft (PDX)
4.3. Genetically Engineered Mouse Models (GEMMs)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Fader, A.N.; Rose, P.G. Role of surgery in ovarian carcinoma. J. Clin. Oncol. 2007, 25, 2873–2883. [Google Scholar] [CrossRef] [Green Version]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.C.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC CHORUS trial. J. Clin. Oncol. 2013, 31, 5500. [Google Scholar] [CrossRef]
- Piccart, M.J.; Bertelsen, K.; Stuart, G.; Cassidy, J.; Mangioni, C.; Simonsen, E.; James, K.; Kaye, S.; Vergote, I.; Blom, R.; et al. Long-term follow-up confirms a survival advantage of the paclitaxel–cisplatin regimen over the cyclophosphamide–cisplatin combination in advanced ovarian cancer. Int. J. Gynecol. Cancer 2003, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.J.; Sonke, G.S.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Elit, L.; Oliver, T.K.; Covens, A.; Kwon, J.; Fung, M.F.-K.; Hirte, H.W.; Oza, A.M. Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer. Cancer 2007, 109, 692–702. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [Green Version]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Yamamoto, S.; Konishi, I.; Mandai, M.; Kuroda, H.; Komatsu, T.; Nanbu, K.; Sakahara, H.; Mori, T. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: Correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br. J. Cancer 1997, 76, 1221–1227. [Google Scholar] [CrossRef]
- Shen, G.H.; Ghazizadeh, M.; Kawanami, O.; Shimizu, H.; Jin, E.; Araki, T.; Sugisaki, Y. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br. J. Cancer 2000, 83, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, A.; Weindel, K.; Ochs, A.; Marth, C.; Zmija, J.; Schumacher, P.; Unger, C.; Marmé, D.; Gastl, G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999, 85, 178–187. [Google Scholar] [CrossRef]
- Randall, L.M.; Monk, B.J. Bevacizumab toxicities and their management in ovarian cancer. Gynecol. Oncol. 2010, 117, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, A.T.; Ross, L.; Holash, J.; Nakanishi, M.; Hu, L.; Hofmann, J.I.; Yancopoulos, G.D.; Jaffe, R.B. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 2003, 9, 5721–5728. [Google Scholar] [PubMed]
- Vaughan, S.; Coward, J.I.; Bast, R.C.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Rubin, S.C.; Blackwood, M.A.; Bandera, C.; Behbakht, K.; Benjamin, I.; Rebbeck, T.R.; Boyd, J. BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: Relationship to family history and implications for genetic testing. Am. J. Obstet. Gynecol. 1998, 178, 670–677. [Google Scholar] [CrossRef]
- Lancaster, J.M.; Wooster, R.; Mangion, J.; Phelan, C.M.; Cochran, C.; Gumbs, C.; Seal, S.; Barfoot, R.; Collins, N.; Bignell, G.; et al. BRCA2 mutations in primary breast and ovarian cancers. Nat. Genet. 1996, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Chiu, J.W.; Koller, B.H.; Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell 1999, 4, 511–518. [Google Scholar] [CrossRef]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254.e236. [Google Scholar] [CrossRef] [Green Version]
- Moschetta, M.; George, A.; Kaye, S.B.; Banerjee, S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. 2016, 27, 1449–1455. [Google Scholar] [CrossRef]
- Paride, L.; Emidio, C.; Andrea, C.; Roberto, P.; Antonio, M. From Polypharmacology to Target Specificity: The Case of PARP Inhibitors. Curr. Top. Med. Chem. 2013, 13, 2939–2954. [Google Scholar] [CrossRef]
- De Vos, M.; Schreiber, V.; Dantzer, F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem. Pharmacol. 2012, 84, 137–146. [Google Scholar] [CrossRef]
- Kirby, I.T.; Cohen, M.S. Small-Molecule Inhibitors of PARPs: From Tools for Investigating ADP-Ribosylation to Therapeutics. In Activity-Based Protein Profiling; Cravatt, B.F., Hsu, K.-L., Weerapana, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 211–231. [Google Scholar]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Deeks, E.D. Olaparib: First Global Approval. Drugs 2015, 75, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Niraparib: First Global Approval. Drugs 2017, 77, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Rucaparib: First Global Approval. Drugs 2017, 77, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Cannistra, S.A. Cancer of the Ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Roberto Sorio, R.; Vergote, I.; Witteveen, P.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Cowin, P.A.; George, J.; Fereday, S.; Loehrer, E.; Van Loo, P.; Cullinane, C.; Etemadmoghadam, D.; Ftouni, S.; Galletta, L.; Anglesio, M.S.; et al. LRP1B Deletion in High-Grade Serous Ovarian Cancers Is Associated with Acquired Chemotherapy Resistance to Liposomal Doxorubicin. Cancer Res. 2012, 72, 4060–4073. [Google Scholar] [CrossRef] [Green Version]
- Etemadmoghadam, D.; George, J.; Cowin, P.A.; Cullinane, C.; Kansara, M.; Australian Ovarian Cancer Study, G.; Gorringe, K.L.; Smyth, G.K.; Bowtell, D.D.L. Amplicon-Dependent CCNE1 Expression Is Critical for Clonogenic Survival after Cisplatin Treatment and Is Correlated with 20q11 Gain in Ovarian Cancer. PLoS ONE 2010, 5, e15498. [Google Scholar] [CrossRef]
- Stronach, E.A.; Chen, M.; Maginn, E.N.; Agarwal, R.; Mills, G.B.; Wasan, H.; Gabra, H. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia 2011, 13, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norquist, B.; Wurz, K.A.; Pennil, C.C.; Garcia, R.; Gross, J.; Sakai, W.; Karlan, B.Y.; Taniguchi, T.; Swisher, E.M. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 2011, 29, 3008–3015. [Google Scholar] [CrossRef] [Green Version]
- Lheureux, S.; Bruce, J.P.; Burnier, J.V.; Karakasis, K.; Shaw, P.A.; Clarke, B.A.; Yang, S.Y.; Quevedo, R.; Li, T.; Dowar, M.; et al. Somatic BRCA1/2 Recovery as a Resistance Mechanism After Exceptional Response to Poly (ADP-ribose) Polymerase Inhibition. J. Clin. Oncol. 2017, 35, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoiemma, P.P.; Reyes, C.; Wang, L.-P.; McLane, M.W.; Feldman, M.D.; Tanyi, J.L.; Powell, D.J., Jr. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 2016, 143, 120–127. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.M.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2008, 58, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Tomšová, M.; Melichar, B.; Sedláková, I.; Šteiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; IARC: Lyon, France, 2014. [Google Scholar]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Spira, A.; Hansen, A.R.; Harb, W.A.; Curtis, K.K.; Koga-Yamakawa, E.; Origuchi, M.; Li, Z.; Ertik, B.; Shaib, W.L. Multicenter, Open-Label, Phase I Study of DSP-7888 Dosing Emulsion in Patients with Advanced Malignancies. Target. Oncol. 2021, 16, 461–469. [Google Scholar] [CrossRef]
- Rahma, O.E.; Ashtar, E.; Czystowska, M.; Szajnik, M.E.; Wieckowski, E.; Bernstein, S.; Herrin, V.E.; Shams, M.A.; Steinberg, S.M.; Merino, M.; et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: Subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol. Immunother. 2012, 61, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Herrin, V.E.; Achtar, M.S.; Steinberg, S.M.; Whiteside, T.L.; Wieckowski, E.; Czystowska, M.; Visus, C.; Berzofsky, J.A.; Khleif, S.N. A randomized phase II p53 vaccine trial comparing subcutaneous direct administration with intravenous peptide-pulsed dendritic cells in high risk ovarian cancer patients. J. Clin. Oncol. 2007, 25, 3011. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Karkada, M.; Morse, M.A.; Nemunaitis, J.J.; Chatta, G.; Kaufman, H.; Odunsi, K.; Nigam, R.; Sammatur, L.; MacDonald, L.D.; et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J. Transl. Med. 2012, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Berinstein, N.L.; Karkada, M.; Oza, A.M.; Odunsi, K.; Villella, J.A.; Nemunaitis, J.J.; Morse, M.A.; Pejovic, T.; Bentley, J.; Buyse, M.; et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology 2015, 4, e1026529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.A.; Byrd, K.; Vreeland, T.J.; Clifton, G.T.; Jackson, D.O.; Hale, D.F.; Herbert, G.S.; Myers, J.W.; Greene, J.M.; Berry, J.S.; et al. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019, 8, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, T.J.; Litton, J.K.; Qiao, N.; Philips, A.V.; Alatrash, G.; Hale, D.F.; Jackson, D.O.; Peace, K.M.; Greene, J.M.; Berry, J.S.; et al. Phase Ib trial of folate binding protein (FBP)-derived peptide vaccines, E39 and an attenuated version, E39′: An analysis of safety and immune response. Clin. Immunol. 2018, 192, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.P.; Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Hueman, M.T.; Mittendorf, E.A.; Murray, J.L.; Amin, A.; Craig, D.; von Hofe, E.; et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J. Clin. Oncol. 2008, 26, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Chianese-Bullock, K.A.; Irvin, W.P., Jr.; Petroni, G.R.; Murphy, C.; Smolkin, M.; Olson, W.C.; Coleman, E.; Boerner, S.A.; Nail, C.J.; Neese, P.Y.; et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J. Immunother. 2008, 31, 420–430. [Google Scholar] [CrossRef]
- Sabbatini, P.; Tsuji, T.; Ferran, L.; Ritter, E.; Sedrak, C.; Tuballes, K.; Jungbluth, A.A.; Ritter, G.; Aghajanian, C.; Bell-McGuinn, K.; et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res. 2012, 18, 6497–6508. [Google Scholar] [CrossRef] [Green Version]
- Diefenbach, C.S.; Gnjatic, S.; Sabbatini, P.; Aghajanian, C.; Hensley, M.L.; Spriggs, D.R.; Iasonos, A.; Lee, H.; Dupont, B.; Pezzulli, S.; et al. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin. Cancer Res. 2008, 14, 2740–2748. [Google Scholar] [CrossRef] [Green Version]
- Zamarin, D.; Walderich, S.; Holland, A.; Zhou, Q.; Iasonos, A.E.; Torrisi, J.M.; Merghoub, T.; Chesebrough, L.F.; McDonnell, A.S.; Gallagher, J.M.; et al. Safety, immunogenicity, and clinical efficacy of durvalumab in combination with folate receptor alpha vaccine TPIV200 in patients with advanced ovarian cancer: A phase II trial. J. Immunother. Cancer 2020, 8, e000829. [Google Scholar] [CrossRef]
- Morse, M.A.; Secord, A.A.; Blackwell, K.; Hobeika, A.C.; Sinnathamby, G.; Osada, T.; Hafner, J.; Philip, M.; Clay, T.M.; Lyerly, H.K.; et al. MHC class I-presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T-cell responses against breast and ovarian cancer. Clin. Cancer Res. 2011, 17, 3408–3419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.A.; Brady, W.E.; Lankes, H.A.; Rose, P.G.; Kelley, J.L.; De Geest, K.; Crispens, M.A.; Resnick, K.E.; Howell, S.B. A phase II study of a urokinase-derived peptide (A6) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2012, 125, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef] [Green Version]
- Odunsi, K.; Matsuzaki, J.; James, S.R.; Mhawech-Fauceglia, P.; Tsuji, T.; Miller, A.; Zhang, W.; Akers, S.N.; Griffiths, E.A.; Miliotto, A.; et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol. Res. 2014, 2, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Chelariu-Raicu, A.; Nick, A.; Urban, R.; Gordinier, M.; Leuschner, C.; Bavisotto, L.; Molin, G.Z.D.; Whisnant, J.K.; Coleman, R.L. A multicenter open-label randomized phase II trial of paclitaxel plus EP-100, a novel LHRH receptor-targeted, membrane-disrupting peptide, versus paclitaxel alone for refractory or recurrent ovarian cancer. Gynecol. Oncol. 2021, 160, 418–426. [Google Scholar] [CrossRef]
- Dijkgraaf, E.M.; Santegoets, S.J.; Reyners, A.K.; Goedemans, R.; Nijman, H.W.; van Poelgeest, M.I.; van Erkel, A.R.; Smit, V.T.; Daemen, T.A.; van der Hoeven, J.J.; et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget 2015, 6, 32228–32243. [Google Scholar] [CrossRef] [Green Version]
- Vermeij, R.; Leffers, N.; Hoogeboom, B.N.; Hamming, I.L.; Wolf, R.; Reyners, A.K.; Molmans, B.H.; Hollema, H.; Bart, J.; Drijfhout, J.W.; et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: A single-arm phase II study. Intl. J. Cancer 2012, 131, E670–E680. [Google Scholar] [CrossRef]
- Sabbatini, P.J.; Ragupathi, G.; Hood, C.; Aghajanian, C.A.; Juretzka, M.; Iasonos, A.; Hensley, M.L.; Spassova, M.K.; Ouerfelli, O.; Spriggs, D.R.; et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin. Cancer Res. 2007, 13, 4170–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatini, P.J.; Kudryashov, V.; Ragupathi, G.; Danishefsky, S.J.; Livingston, P.O.; Bornmann, W.; Spassova, M.; Zatorski, A.; Spriggs, D.; Aghajanian, C.; et al. Immunization of ovarian cancer patients with a synthetic Lewis(y)-protein conjugate vaccine: A phase 1 trial. Int. J. Cancer 2000, 87, 79–85. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Deng, W.; Chen, L.M.; Lucci, J.A., 3rd; Behbakht, K.; Spirtos, N.M.; Muller, C.Y.; Benigno, B.B.; Powell, M.A.; Berry, E.; et al. A phase II randomized, double-blind trial of a polyvalent Vaccine-KLH conjugate (NSC 748933 IND# 14384) + OPT-821 versus OPT-821 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer who are in second or third complete remission: An NRG Oncology/GOG study. Gynecol. Oncol. 2019, 155, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Wada, H.; Muro, K.; Niwa, Y.; Ueda, S.; Miyata, H.; Takiguchi, S.; Sugino, S.H.; Miyahara, Y.; Ikeda, H.; et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J. Transl. Med. 2013, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.L.; Quinn, M.A.; Grant, P.T.; Allen, D.G.; Jobling, T.W.; White, S.C.; Zhao, A.; Karanikas, V.; Vaughan, H.; Pietersz, G.; et al. A phase 2, single-arm study of an autologous dendritic cell treatment against mucin 1 in patients with advanced epithelial ovarian cancer. J. Immunother. Cancer 2014, 2, 16. [Google Scholar] [CrossRef]
- Morse, M.A.; Hobeika, A.; Osada, T.; Niedzwiecki, D.; Marcom, P.K.; Blackwell, K.L.; Anders, C.; Devi, G.R.; Lyerly, H.K.; Clay, T.M. Long term disease-free survival and T cell and antibody responses in women with high-risk Her2+ breast cancer following vaccination against Her2. J. Transl. Med. 2007, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef]
- Liao, J.B.; Cecil, D.; Dang, Y.; Baker, K.K.; Ovenell, K.J.; Reichow, J.; Parker, S.; Higgins, D.; Childs, J.; Broussard, E.K.; et al. Vaccination targeting insulin-like growth factor binding protein-2 (IGFBP-2) in advanced ovarian cancer: Safety and immunogenicity. J. Clin. Oncol. 2016, 34, 5542. [Google Scholar] [CrossRef]
- Alvarez, R.D.; Sill, M.W.; Davidson, S.A.; Muller, C.Y.; Bender, D.P.; DeBernardo, R.L.; Behbakht, K.; Huh, W.K. A phase II trial of intraperitoneal EGEN-001, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: A gynecologic oncology group study. Gynecol. Oncol. 2014, 133, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Gribben, J.G.; Ryan, D.P.; Boyajian, R.; Urban, R.G.; Hedley, M.L.; Beach, K.; Nealon, P.; Matulonis, U.; Campos, S.; Gilligan, T.D.; et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin. Cancer Res. 2005, 11, 4430–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P., Jr.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, N.R.; Frankel, P.; Ruel, C.; Kilpatrick, J.; Tsai, W.; Kos, F.; Kaltcheva, T.; Leong, L.; Morgan, R.; Chung, V.; et al. p53-Reactive T Cells Are Associated with Clinical Benefit in Patients with Platinum-Resistant Epithelial Ovarian Cancer After Treatment with a p53 Vaccine and Gemcitabine Chemotherapy. Clin. Cancer Res. 2018, 24, 1315–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohn, D.E.; Nuovo, G.; Coffey, M.C.; O’Malley, D.; Villalona-Calero, M.A.; Grever, M.R.; Deam, D.; Zwiebel, J.A.; Phelps, M.A. Phase I/II trial of reovirus serotype 3–Dearing strain in patients with recurrent ovarian cancer. J. Clin. Oncol. 2010, 28, TPS253. [Google Scholar] [CrossRef]
- Kim, K.H.; Dmitriev, I.; O’Malley, J.P.; Wang, M.; Saddekni, S.; You, Z.; Preuss, M.A.; Harris, R.D.; Aurigemma, R.; Siegal, G.P.; et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin. Cancer Res. 2012, 18, 3440–3451. [Google Scholar] [CrossRef] [Green Version]
- McNeish, I.A.; Michael, A.; Twelves, C.; Glasspool, R.; Ajaz, M.A.; Morrison, R.; Xeniou, O.; Brown, R.; Fisher, K.; Blanc, C. A phase I/II study of Enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus, administered intraperitoneally (IP): Dose finding and proof of concept in platinum-resistant epithelial ovarian cancer. J. Clin. Oncol. 2015, 33, TPS5611. [Google Scholar] [CrossRef]
- Cohn, D.E.; Sill, M.W.; Walker, J.L.; O’Malley, D.; Nagel, C.I.; Rutledge, T.L.; Bradley, W.; Richardson, D.L.; Moxley, K.M.; Aghajanian, C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 146, 477–483. [Google Scholar] [CrossRef]
- Kimball, K.J.; Preuss, M.A.; Barnes, M.N.; Wang, M.; Siegal, G.P.; Wan, W.; Kuo, H.; Saddekni, S.; Stockard, C.R.; Grizzle, W.E.; et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin. Cancer Res. 2010, 16, 5277–5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heery, C.R.; Palena, C.; McMahon, S.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Dirmeier, U.; Cordes, L.; Marté, J.; Dahut, W.; et al. Phase I Study of a Poxviral TRICOM-Based Vaccine Directed against the Transcription Factor Brachyury. Clin. Cancer Res. 2017, 23, 6833–6845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.A.; Clay, T.M.; Hobeika, A.C.; Osada, T.; Khan, S.; Chui, S.; Niedzwiecki, D.; Panicali, D.; Schlom, J.; Lyerly, H.K. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin. Cancer Res. 2005, 11, 3017–3024. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.L.; Gulley, J.L.; Arlen, P.M.; Beetham, P.K.; Tsang, K.Y.; Slack, R.; Hodge, J.W.; Doren, S.; Grosenbach, D.W.; Hwang, J.; et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J. Clin. Oncol. 2005, 23, 720–731. [Google Scholar] [CrossRef]
- Mohebtash, M.; Tsang, K.-Y.; Madan, R.A.; Huen, N.-Y.; Poole, D.J.; Jochems, C.; Jones, J.; Ferrara, T.; Heery, C.R.; Arlen, P.M.; et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin. Cancer Res. 2011, 17, 7164–7173. [Google Scholar] [CrossRef] [Green Version]
- Odunsi, K.; Matsuzaki, J.; Karbach, J.; Neumann, A.; Mhawech-Fauceglia, P.; Miller, A.; Beck, A.; Morrison, C.D.; Ritter, G.; Godoy, H.; et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc. Natl. Acad. Sci. USA 2012, 109, 5797–5802. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Barve, M.; Matthews, C.M.; Koon, E.C.; Heffernan, T.P.; Fine, B.; Grosen, E.; Bergman, M.K.; Fleming, E.L.; DeMars, L.R.; et al. Phase II study of Vigil®® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 2016, 143, 504–510. [Google Scholar] [CrossRef]
- Chu, C.S.; Boyer, J.; Schullery, D.S.; Gimotty, P.A.; Gamerman, V.; Bender, J.; Levine, B.L.; Coukos, G.; Rubin, S.C.; Morgan, M.A.; et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012, 61, 629–641. [Google Scholar] [CrossRef]
- Gray, H.J.; Benigno, B.; Berek, J.; Chang, J.; Mason, J.; Mileshkin, L.; Mitchell, P.; Moradi, M.; Recio, F.O.; Michener, C.M.; et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J. Immunother. Cancer 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Chiang, L.-L.C.; Chiffelle, J.; Thierry, A.-H.; Baumgartener, P.; Huber, F.; Göpfert, C.; Taurrsio, D.; Tissot, S.; Torigian, D.A.; et al. Personalized cancer vaccine strategy elicits polyfunctional T cells and demonstrates clinical benefits in ovarian cancer. NPJ Vaccines 2021, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Bartunkova, J.; Cibula, D.; Knapp, P.; Namestkova, Z.; Spisek, R.; Waclav, J.; Mallmann, P. Autologous dendritic cell vaccination (DCVAC/OvCa) added to standard of care therapy in three open-label randomized phase 2 studies in women with advanced stage ovarian cancer (OC). J. Clin. Oncol. 2014, 32, TPS3134. [Google Scholar] [CrossRef]
- Novellino, L.; Castelli, C.; Parmiani, G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol. Immunother. 2005, 54, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Stroobant, V.; Van den Eynde, B.J.; van der Bruggen, P. Database of T cell-defined human tumor antigens: The 2013 update. Cancer Immun. 2013, 13, 15. [Google Scholar]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Heemskerk, B.; Kvistborg, P.; Schumacher, T.N.M. The cancer antigenome. EMBO J. 2013, 32, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wolfel, T.; Hauer, M.; Schneider, J.; Serrano, M.; Wolfel, C.; Klehmann-Hieb, E.; De Plaen, E.; Hankeln, T.; Meyer zum Buschenfelde, K.H.; Beach, D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 1995, 269, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Wick, D.A.; Webb, J.R.; Nielsen, J.S.; Martin, S.D.; Kroeger, D.R.; Milne, K.; Castellarin, M.; Twumasi-Boateng, K.; Watson, P.H.; Holt, R.A.; et al. Surveillance of the Tumor Mutanome by T Cells during Progression from Primary to Recurrent Ovarian Cancer. Clin. Cancer Res. 2014, 20, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Martin, S.D.; Brown, S.D.; Wick, D.A.; Nielsen, J.S.; Kroeger, D.R.; Twumasi-Boateng, K.; Holt, R.A.; Nelson, B.H. Low Mutation Burden in Ovarian Cancer May Limit the Utility of Neoantigen-Targeted Vaccines. PLoS ONE 2016, 11, e0155189. [Google Scholar] [CrossRef]
- van Buuren, M.M.; Calis, J.J.; Schumacher, T.N. High sensitivity of cancer exome-based CD8 T cell neo-antigen identification. Oncoimmunology 2014, 3, e28836. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.-C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Bräunlein, E.; Krackhardt, A.M. Identification and Characterization of Neoantigens As Well As Respective Immune Responses in Cancer Patients. Front. Immunol. 2017, 8, 1702. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Minati, R.; Perreault, C.; Thibault, P. A Roadmap toward the Definition of Actionable Tumor-Specific Antigens. Front. Immunol. 2020, 11, 583287. [Google Scholar] [CrossRef]
- Amirouchene-Angelozzi, N.; Swanton, C.; Bardelli, A. Tumor Evolution as a Therapeutic Target. Cancer Discov. 2017, 7, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Pearson, A.; Smyth, E.; Babina, I.S.; Herrera-Abreu, M.T.; Tarazona, N.; Peckitt, C.; Kilgour, E.; Smith, N.R.; Geh, C.; Rooney, C.; et al. High-Level Clonal FGFR Amplification and Response to FGFR Inhibition in a Translational Clinical Trial. Cancer Discov. 2016, 6, 838–851. [Google Scholar] [CrossRef] [Green Version]
- De Mattos-Arruda, L.; Vazquez, M.; Finotello, F.; Lepore, R.; Porta, E.; Hundal, J.; Amengual-Rigo, P.; Ng, C.K.Y.; Valencia, A.; Carrillo, J.; et al. Neoantigen prediction and computational perspectives towards clinical benefit: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Richters, M.M.; Xia, H.; Campbell, K.M.; Gillanders, W.E.; Griffith, O.L.; Griffith, M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, A.-M.; Nielsen, M.; Jurtz, V.; Barra, C.M.; Hadrup, S.R.; Szallasi, Z.; Eklund, A.C. An Analysis of Natural T Cell Responses to Predicted Tumor Neoepitopes. Front. Immunol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumont, C.M.; Vincent, K.; Hesnard, L.; Audemard, É.; Bonneil, É.; Laverdure, J.-P.; Gendron, P.; Courcelles, M.; Hardy, M.-P.; Côté, C.; et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci. Transl. Med. 2018, 10, eaau5516. [Google Scholar] [CrossRef] [Green Version]
- Bijker, M.S.; van den Eeden, S.J.F.; Franken, K.L.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur. J. Immunol. 2008, 38, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Lefebvre, F.; Filali-Mouhim, A.; Cameron, M.J.; Goulet, J.P.; Haddad, E.K.; Breton, G.; Trumpfheller, C.; Pollak, S.; Shimeliovich, I.; et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 2011, 208, 2357–2366. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: An historical perspective and view to the future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Uchijima, M.; Yoshida, A.; Nagata, T.; Koide, Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J. Immunol. 1998, 161, 5594–5599. [Google Scholar] [PubMed]
- Trollet, C.; Pereira, Y.; Burgain, A.; Litzler, E.; Mezrahi, M.; Seguin, J.; Manich, M.; Popoff, M.R.; Scherman, D.; Bigey, P. Generation of high-titer neutralizing antibodies against botulinum toxins A, B, and E by DNA electrotransfer. Infect. Immun. 2009, 77, 2221–2229. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.; Vanvarenberg, K.; Préat, V.; Vandermeulen, G. Codon-Optimized P1A-Encoding DNA Vaccine: Toward a Therapeutic Vaccination against P815 Mastocytoma. Mol. Ther. Nucleic Acids 2017, 8, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, B.; Wang, J.; Zhao, G.; Wang, X.; Li, J.; Wang, B. Sequential administration of cytokine genes to enhance cellular immune responses and CD4 (+) T memory cells during DNA vaccination. Hum. Vaccin. Immunother. 2012, 8, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Dörrie, J.; Schaft, N.; Schuler, G.; Schuler-Thurner, B. Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells-An Update. Pharmaceutics 2020, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Duperret, E.K.; Perales-Puchalt, A.; Stoltz, R.; Hiranjith, G.H.; Mandloi, N.; Barlow, J.; Chaudhuri, A.; Sardesai, N.Y.; Weiner, D.B. A Synthetic DNA, Multi-Neoantigen Vaccine Drives Predominately MHC Class I CD8(+) T-cell Responses, Impacting Tumor Challenge. Cancer Immunol. Res. 2019, 7, 174–182. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Larocca, C.; Schlom, J. Viral vector-based therapeutic cancer vaccines. Cancer, J. 2011, 17, 359–371. [Google Scholar] [CrossRef]
- Hodge, J.W.; Sabzevari, H.; Yafal, A.G.; Gritz, L.; Lorenz, M.G.; Schlom, J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999, 59, 5800–5807. [Google Scholar]
- Tsang, K.Y.; Palena, C.; Yokokawa, J.; Arlen, P.M.; Gulley, J.L.; Mazzara, G.P.; Gritz, L.; Gómez Yafal, A.; Ogueta, S.; Greenhalgh, P.; et al. Analyses of Recombinant Vaccinia and Fowlpox Vaccine Vectors Expressing Transgenes for Two Human Tumor Antigens and Three Human Costimulatory Molecules. Clin. Cancer Res. 2005, 11, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Jäger, E.; Karbach, J.; Gnjatic, S.; Neumann, A.; Bender, A.; Valmori, D.; Ayyoub, M.; Ritter, E.; Ritter, G.; Jäger, D.; et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc. Natl. Acad. Sci. USA 2006, 103, 14453–14458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, D.W.; Adams, S.; Bhardwaj, N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 2004, 104, 2235–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lech, M.; Gröbmayr, R.; Weidenbusch, M.; Anders, H.-J. Tissues Use Resident Dendritic Cells and Macrophages to Maintain Homeostasis and to Regain Homeostasis upon Tissue Injury: The Immunoregulatory Role of Changing Tissue Environments. Mediators Inflamm. 2012, 2012, 951390. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.-L.; Kandalaft, L.E. In vivo cancer vaccination: Which dendritic cells to target and how? Cancer Treat. Rev. 2018, 71, 88–101. [Google Scholar] [CrossRef]
- Anderson, M.M.; Hazen, S.L.; Hsu, F.F.; Heinecke, J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Investig. 1997, 99, 424–432. [Google Scholar]
- Marcinkiewicz, J.; Chain, B.M.; Olszowska, E.; Olszowski, S.; Zgliczynski, J.M. Enhancement of immunogenic properties of ovalbumin as a result of its chlorination. Int. J. Biochem. 1991, 23, 1393–1395. [Google Scholar] [CrossRef]
- Prokopowicz, Z.M.; Arce, F.; Biedron, R.; Chiang, C.L.-L.; Ciszek, M.; Katz, D.R.; Nowakowska, M.; Zapotoczny, S.; Marcinkiewicz, J.; Chain, B.M. Hypochlorous Acid: A Natural Adjuvant That Facilitates Antigen Processing, Cross-Priming, and the Induction of Adaptive Immunity. J. Immunol. 2010, 184, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.L.; Kandalaft, L.E.; Tanyi, J.; Hagemann, A.R.; Motz, G.T.; Svoronos, N.; Montone, K.; Mantia-Smaldone, G.M.; Smith, L.; Nisenbaum, H.L.; et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013, 19, 4801–4815. [Google Scholar] [CrossRef] [Green Version]
- Sarivalasis, A.; Boudousquié, C.; Balint, K.; Stevenson, B.J.; Gannon, P.O.; Iancu, E.M.; Rossier, L.; Martin Lluesma, S.; Mathevet, P.; Sempoux, C.; et al. A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma. J. Transl. Med. 2019, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Senzer, N.; Barve, M.; Kuhn, J.; Melnyk, A.; Beitsch, P.; Lazar, M.; Lifshitz, S.; Magee, M.; Oh, J.; Mill, S.W.; et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol. Ther. 2012, 20, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocconi, R.P.; Grosen, E.A.; Ghamande, S.A.; Chan, J.K.; Barve, M.A.; Oh, J.; Tewari, D.; Morris, P.C.; Stevens, E.E.; Bottsford-Miller, J.N.; et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020, 21, 1661–1672. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Paroli, M.; Bellati, F.; Videtta, M.; Focaccetti, C.; Mancone, C.; Donato, T.; Antonilli, M.; Perniola, G.; Accapezzato, D.; Napoletano, C.; et al. Discovery of chemotherapy-associated ovarian cancer antigens by interrogating memory T cells. Int. J. Cancer. 2014, 134, 1823–1834. [Google Scholar] [CrossRef]
- Coleman, S.; Clayton, A.; Mason, M.D.; Jasani, B.; Adams, M.; Tabi, Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005, 65, 7000–7006. [Google Scholar] [CrossRef] [Green Version]
- Hato, S.V.; Khong, A.; de Vries, I.J.; Lesterhuis, W.J. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef] [Green Version]
- Chan, O.T.; Yang, L.X. The immunological effects of taxanes. Cancer Immunol. Immunother. 2000, 49, 181–185. [Google Scholar] [CrossRef]
- Meza-Perez, S.; Randall, T.D. Immunological Functions of the Omentum. Trends Immunol. 2017, 38, 526–536. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Li, Y. Milky spots: Omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol. 2016, 37, 5715–5726. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Barve, M.; Senzer, N.; Aaron, P.; Manning, L.; Wallraven, G.; Bognar, E.; Stanbery, L.; Horvath, S.; Manley, M.; et al. Long-term follow-up of Phase 2A trial results involving advanced ovarian cancer patients treated with Vigil® in frontline maintenance. Gynecol. Oncol. Rep. 2020, 34, 100648. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef] [Green Version]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti–PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Piha-Paul, S.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Morosky, A.; Yang, P.; Ruman, J.; Matei, D. Pembrolizumab in patients with programmed death ligand 1–positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019, 152, 243–250. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res. 2013, 73, 3591–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Flies, D.B.; Marjon, N.A.; Mantia-Smaldone, G.; Ronner, L.; Gimotty, P.A.; Adams, S.F. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 1257. [Google Scholar] [CrossRef] [Green Version]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef]
- Morse, C.B.; Voillet, V.; Bates, B.M.; Chiu, E.Y.; Garcia, N.M.; Gottardo, R.; Greenberg, P.D.; Anderson, K.G. Development of a clinically relevant ovarian cancer model incorporating surgical cytoreduction to evaluate treatment of micro-metastatic disease. Gynecol. Oncol. 2021, 160, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Walton, J.; Blagih, J.; Ennis, D.; Leung, E.; Dowson, S.; Farquharson, M.; Tookman, L.A.; Orange, C.; Athineos, D.; Mason, S.; et al. CRISPR/Cas9-Mediated Trp53 and Brca2 Knockout to Generate Improved Murine Models of Ovarian High-Grade Serous Carcinoma. Cancer Res. 2016, 76, 6118–6129. [Google Scholar] [CrossRef] [Green Version]
- Walton, J.B.; Farquharson, M.; Mason, S.; Port, J.; Kruspig, B.; Dowson, S.; Stevenson, D.; Murphy, D.; Matzuk, M.; Kim, J.; et al. CRISPR/Cas9-derived models of ovarian high grade serous carcinoma targeting Brca1, Pten and Nf1, and correlation with platinum sensitivity. Sci. Rep. 2017, 7, 16827. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.B.; Kim, H.; Rafail, S.; Omran, D.K.; Medvedev, S.; Kinose, Y.; Rodriguez-Garcia, A.; Flowers, A.J.; Xu, H.; Schwartz, L.E.; et al. An autologous humanized patient-derived-xenograft platform to evaluate immunotherapy in ovarian cancer. Gynecol. Oncol. 2020, 156, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef]
- Bankert, R.B.; Balu-Iyer, S.V.; Odunsi, K.; Shultz, L.D.; Kelleher, R.J., Jr.; Barnas, J.L.; Simpson-Abelson, M.; Parsons, R.; Yokota, S.J. Humanized Mouse Model of Ovarian Cancer Recapitulates Patient Solid Tumor Progression, Ascites Formation, and Metastasis. PLoS ONE 2011, 6, e24420. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, M.; Chou, F.S.; Link, K.A.; Mizukawa, B.; Perry, R.L.; Carroll, M.; Mulloy, J.C. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 2010, 24, 1785–1788. [Google Scholar] [CrossRef] [Green Version]
- Stuckelberger, S.; Drapkin, R. Precious GEMMs: Emergence of faithful models for ovarian cancer research. J. Pathol. 2018, 245, 129–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, P.J.; Weeraratna, A.T. Genetically-defined ovarian cancer mouse models. J. Pathol. 2016, 238, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniati, E.; Berlato, C.; Gopinathan, G.; Heath, O.; Kotantaki, P.; Lakhani, A.; McDermott, J.; Pegrum, C.; Delaine-Smith, R.M.; Pearce, O.M.T.; et al. Mouse Ovarian Cancer Models Recapitulate the Human Tumor Microenvironment and Patient Response to Treatment. Cell Rep. 2020, 30, 525–540.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| NCT ID | Cancer Type(s) | Treatments | No. of Patients Enrolled | Study Outcome | References |
|---|---|---|---|---|---|
| Tumor-Associated Antigen (TAA) Peptides | |||||
| NCT02498665 | Ovarian cancer (OC), prostate cancer (PC), non-small cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), sarcoma, melanoma, acute myeloid leukemia, myelodysplastic syndromes, glioblastoma multiform | WT1 protein-derived peptide vaccine (DSP-7888) | 24 | 4 stable disease (SD), 16 progressive disease (PD) and 4 not evaluated. Overall survival (OS) was 180 months, and median progression-free survival (PFS) was 52 months | [51] |
| NCT02270372 | OC, breast cancer (BC) | Peptide vaccine incorporating a synthetic glycolipopeptide MUC1 antigen (M40Tn6) and novel synthetic toll-like receptor (TLR)-4 agonist (PET lipid A) in a liposomal formulation (ONT-10), varlilumab | 28 | No published results | na |
| NCT00019084 | OC, BC, CRC, PC, cervical cancer, lung cancer | Mutant p53/RAS peptide-pulsed DC vaccine, sargramostim, therapeutic autologous tumor-infiltrating lymphocytes | 17 BC, 13 OC | OC patients showed median OS of 40.8 and 29.6 months for arm A and B, respectively, and the median PFS were 4.2 and. 8.7 months for arm A and B, respectively. | [52] |
| NCT00019916 | OC, BC | p53 peptide, aldesleukin (recombinant IL-2) | 21 | 13 and 7 patients received subcutaneous (SQ arm) and intravenous (IV arm) vaccination, respectively. The mean OS on the SQ arm was 70.4 months and on the IV arm was 72.9 months. | [53] |
| NCT01095848 | OC, BC, PC | vaccine containing 7 tumor-specific HLA-A2-restricted peptides and a universal T-helper peptide, liposome and Montanide ISA51 VG (DPX-0907) | 22 | 14 PD; patients were within the median progression-free survival period for their previous treatment | [54] |
| NCT01416038 | OC, fallopian cancer, peritoneal cancer | DPX-Survivac (targeting survivin antigen), low dose cyclophosphamide | 19 | 12 of 18 patients (67%) remained without clinical progression at 6-months follow up | [55] |
| NCT01580696 | OC, fallopian cancer, peritoneal cancer | Folate-binding protein (FBP) epitope E39 peptide (100–500 mcg), sargramostim, an attenuated peptide of E39 (J65) as booster | 51 | Disease-free survival (DFS) improved in the 1000 μg group after treatment of primary disease (90.0% vs. CG: 42.9%, p = 0.007), but not in recurrent patients. | [56] |
| NCT02019524 | OC, BC | E39 peptide, J65 vaccine | 39 | Increase in E39-specific cytolytic T cells (CTLs) were detected following vaccination and both epitopes were safe | [57] |
| NCT00003002 | OC, BC, lung cancer | HER-2/neu peptide, sargramostim (recombinant GM-CSF) | 60 | No published results | na |
| NCT00005023 | OC, BC, lung cancer | HER-2/neu peptide, sargramostim | 15 | Significant increases in patients’ pre- to post-vaccine delayed-type hypersensitivity (DTH) responses that were correlated with peptides vaccine doses. A reduction of GM-CSF did not affect DTH responses. | [58] |
| NCT00091273 | OC, peritoneal cancer | Multipeptide (MAGE-A, FBP and HER-2/neu) vaccine, sargramostim, incomplete Freund’s adjuvant | 9 | Most frequent side-effects: injection site pain, fatigue and head ache | [59] |
| NCT00616941 | OC, fallopian tube cancer, primary peritoneal cancer | NY-ESO-1 overlapping peptide (OLP)4 emulsified in Montanide ISA51, Poly-ICLC | 28 | Six patients showed no evidence of disease (NED), and PFS ranging from 17–46 months | [60] |
| NCT00066729 | OC, fallopian tube cancer, primary peritoneal cancer | NY-ESO-1 peptide vaccine, incomplete Freund’s adjuvant | 9 | Median time of disease progression/recurrence from start of vaccination: 19.0 mo, 1 patient complete regression of metastatic disease after 10 immunizations | [61] |
| NCT02764333 | OC | Proteins derived from the folate receptor-alpha admix with GM-CSF (TPIV200), durvalumab | 27 | Median OS of 21 months, and median PFS of 2.8 months | [62] |
| NCT00437502 | OC, fallopian tube cancer, primary peritoneal cancer | Multipeptide vaccine, Montanide ISA-51, sargramostim | 8 | Median OS not reached | [63] |
| NCT00939809 | OC, fallopian tube cancer, primary peritoneal cancer, ovarian clear cell cystadenocarcinoma, ovarian endometrioid adenocarcinoma | Urokinase-Derived Peptide A6 | 31 | Median PFS was 2 months, and 1 hemorrhage death possibly related to study | [64] |
| NCT01606241 | Recurrent OC, BC, fallopian tube cancer, primary peritoneal cancer | Multiepitope folate receptor-alpha peptide vaccine, cyclophosphamide | 22 | Median PFS was 528 days (~17.6 months) in patients who were in first remission. Median OS not reached for patients who were in second remission. | [65] |
| NCT01673217 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | NY-ESO-1 peptide vaccine, pegylated liposomal doxorubicin hydrochloride, sargramostim, incomplete Freund’s adjuvant, decitabine | 10 | 6 SD | [66] |
| NCT01485848 | Recurrent OC | Synthetic targeted cytolytic peptide conjugated to luteinizing hormone-releasing hormone (LHRH)-alpha receptors on surfaces of tumor cells (EP-100) | 44 | No difference in response rate was detected with the addition of EP-100 to paclitaxel in the overall patient population | [67] |
| NCT01639885 | Recurrent OC | p53-synthetic long peptide (SLP), IFN-α2b | 15 | 4 SD, 2 partial response (PR), 10 PD following computerized tomography (CT) scan | [68] |
| NCT00844506 | OC | p53-SLP, cyclophosphamide | 10 | 2 SD | [69] |
| NCT00006041 | OC, fallopian tube cancer, peritoneal cancer | MUC1-keyhole limpet hemocyanin (KLH) conjugate vaccine, adjuvant QS21 | 11 | 8 of 9 patients developed responses against to at least 3 different tumor antigens | [70] |
| NCT01248273 | OC, fallopian tube cancer, primary peritoneal cancer | Globo-H-GM2-sTn-TF-Tn-KLH conjugate, adjuvant QS21 | 25 | Median PFS of 6 months, and 5 patients remained in complete clinical remission (CCR) at 18-months follow up | [71] |
| NCT03332576 | OC, fallopian tube cancer, primary peritoneal cancer | DPX-Survivac, low dose cyclophosphamide | 19 | No published results | na |
| NCT00857545 | Stage I-IV OC, fallopian tube cancer, primary peritoneal cancer | Polyvalent vaccine-KLH conjugate vaccine (i.e., Globo-H-KLH, Tn-mucin 1 [MUC1]-32mer-KLH, and Thompson Friedreich antigen [TF]-KLH plus OPT-821), saponin-based immunoadjuvant OBI-821 | 171 | KLH + OPT-821 was not superior to OPT-821 alone (hazard ratio [HR]: 0.98; 2-sided 95% CI, 0.71–1.36). The median OS for KLH + OPT-821 and OPT-821 were 47 and 46 months, respectively. | [72] |
| NCT01003808 | Solid tumors | Peptide vaccine containing nanoparticles of cholesteryl hydrophobized pullulan [CHP] complexed with the cancer-testis antigen NY-ESO-1 protein (IMF-001) | 25 | No tumor shrinkage was observed. Patients receiving 200 μg of CHP-NY-ESO-1 survived longer than patients receiving 100 μg of CHP-NY-ESO-1, even those who exhibited unresponsiveness to previous therapies or had higher tumor burdens. | [73] |
| NCT01617629 | OC | Peptide cancer vaccine containing nanoparticles of cholesteryl hydrophobized pullulan [CHP] complexed with the cancer-testis antigen NY-ESO-1 protein (CHP-NY-ESO-1 peptide vaccine IMF-001) | 28 | 4 patients showed CA125 response or stabilization | [74] |
| NCT00005956 | OC, BC, gastric cancer (GC) | HER-2/neu intracellular domain protein-pulsed DC vaccine | 9 | 1 SD for 3 months, and showed 1 tumor size reduction | [75] |
| RNA and DNA vaccines (alone or DC-based) | |||||
| NCT00004604 | OC, BC, CRC, GC, hepatocellular carcinoma (HCC), PC, gallbladder cancer, extrahepatic bile duct cancer, head and neck cancer, testicular germ cell tumor | Carcinoembryonic antigen (CEA) RNA-pulsed DC cancer vaccine | 24 | 1 complete response (CR), 2 PR, 3 SD and 18 PD | [76] |
| NCT01322802 | Stage III-IV OC and ovarian germ cell tumor | A multiepitope plasmid DNA vaccine containing mammalian expression vector pUMVC3, encoding epitopes of human insulin-like growth factor-binding protein 2 (hIGFBP-2) [pUMVC3-Higfbp-2 vaccine] | 25 | OS rate at 2-years was 82% | [77] |
| NCT01118052 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | PEG-PEI-cholesterol lipopolymer-encased IL-12 DNA plasmid vector (GEN-1) | 16 | 7 SD, 9 PD, median PFS and OS were 2.89 and 9.17 months, respectively | [78] |
| NCT00381173 | OC, BC, colorectal carcinoma (CRC), PC and RCC | Plasmid DNA encoding for cytochrome P450 Family 1 Subfamily B Member 1 (CYP1B1) and encapsulated in biodegradable poly-DL-lactide-coglycolide microparticles (ZYC300), cyclophosphamide | 22 | 3 SD, PD observed in 10 patients who did not respond to CYP1B1 | [79] |
| Viral vector vaccine | |||||
| NCT00408590 | OC, primary peritoneal cancer | CEA-expressing oncolytic measles virus, oncolytic measles virus encoding thyroidal sodium iodide symporter (MV-NIS) | 37 | MV-NIS showed a median of OS 26.5 months, while MV-CEA showed a median OS of 12.15 months | [80,81] |
| NCT02275039 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | Modified vaccinia virus ankara vaccine expressing p53, gemcitabine hydrochloride | 11 | 3 SD, 1 PR, and PFS 3 months | [82] |
| NCT00602277 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | Non-pathogenic isolate of the unmodified Reovirus (REOLYSIN®®; Pelareorep) | 70 | No published results (conference paper) | [83] |
| NCT00964756 | OC | An infectivity-enhanced adenovirus expressing a therapeutic thymidine kinase suicide gene and a somatostatin receptor (Ad5.SSTR/TK.RGD), ganciclovir | 12 | 5 SD, 7 PD | [84] |
| NCT02028117 | Recurrent platinum-resistant OC | A group B Ad11p/Ad3 chimeric oncolytic adenovirus (Enadenotucirev) | No information | No published results (conference paper) | [85] |
| NCT01199263 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | Pelareorep, paclitaxel | 108 | Median PFS of 4.4 months | [86] |
| NCT00562003 | OC, primary peritoneal cancer | Replication-competent oncolytic adenovirus 5 carring a 24-bp deletion in E1A gene (Ad5-delta24RGD) | 21 | 4 SD, 6 PD | [87] |
| NCT01536054 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | Replication-defective recombinant canarypox virus [ALVAC(2)] encoding NY-ESO and the TRIad of COstimulatory Molecules (B7-1, intracellular adhesion molecule-1 [ICAM-1] and leukocyte function-associated antigen-3 [LFA-3]; also called TRICOM) (ALVAC(2)-NY-ESO-1 (M)/TRICOM vaccine), sirolimus, sargramostim | 7 | No published results | na |
| NCT02179515 | OC, BC, PC, lung Cancer, other tumors | Replication-deficient, attenuated derivative of the vaccinia virus strain Ankara expressing a CD8+ T cell epitope of brachyury and TRICOM (MVA Brachyury-TRICOM) | 38 | 34 of 38 patients completed all three doses of therapy; 21 PD and 17 SD observed. | [88] |
| NCT00004032 | Recurrent OC | Canarypox viral vector carrying the gene for human B7.1 (CD80 antigen) (ALVAC-hB7.1), recombinant interferon gamma | No information | No published results | na |
| NCT00027534 | OC, BC, CRC, GC, HCC, PC, gallbladder cancer, head and neck cancer, testicular germ cell tumor | Recombinant fowlpox virus vector encoding CEA and TRICOM (fowlpox-CEA-B7-1/ICAM-1/LFA-3rF-CEA(6D)TRICOM) | 14 | 1 patient had a decrease in the CEA level, and 5 showed SD. CEA-specific T cells were detected in 10 patients. | [89] |
| NCT00028496 | OC, BC, CRC, GC, HCC, PC, cholangiocarcinoma, ovarian endometroid adenocarcinoma | Fowlpox-CEA-B7-1/ICAM-1/LFA-3rF-CEA(6D)TRICOM, sargramostim | 58 | 9 SD for 4 months, 14 SD for >6 months, 1 complete response (CR) | [90] |
| NCT00088413 | OC, BC, CRC, adenocarcinoma | Recombinant vaccinia (PANVAC-V) and recombinant fowlpox (PANVAC-F) expressing MUC1, CEA and TRICOM, sargramostim | 26 | Median OS of 15.0 months | [91] |
| NCT00112957 | OC, fallopian tube cancer, primary peritoneal cancer | Recombinant vaccinia-expressing NY-ESO-1 (rV-NY-ESO-1) and recombinant fowlpox-expressing NY-ESO-1 (rF-NY-ESO-1) vaccines | 22 | Median progression-free survival (PFS) was 21 months, and median OS was 48 months | [92] |
| NCT00803569 | OC, fallopian tube cancer, primary peritoneal cancer | ALVAC(2)-NY-ESO-1(M)/TRICOM vaccine, sargramostim | No information | No published results | na |
| NCT03127098 | OC, BC, PC, CRC, thyroid cancer | Virus expressing CEA (ETBX-011), IL-15 superagonist complex (ALT-803; ALT-803) | No information | No published results | na |
| Whole tumor lysate (WTL) or cell vaccine (alone or DC-based) | |||||
| NCT01312389 | OC, fallopian tube cancer, primary peritoneal cancer | Autologous oxidized WTL (OC-L) emulsified with Montanide ISA 51 VG, Ampligen | No information | No published results | na |
| NCT01551745 | Stage III-IV OC | Vigil™, bevacizumab | No information | No published results | na |
| NCT01867086 | Stage III-IV OC | Vigil™ vaccine, carboplatinum, taxol | 42 | In the Vigil®® arm, a PFS mean of 826 days (27.5 months) and median of 604 days (20.1 months) were observed. In the control arm, a PFS mean of 481 days (16 months) and median of 377 days (12.6 months) were observed. | [93] |
| NCT00478452 | OC, fallopian tube cancer, primary peritoneal cancer | Autologous DCs pulsed with killed autologous tumor cells (DC-Ova), cyclophosphamide | 11 | 6 NED at 36 months. The 3-years PFS was 80% and 3-years OS was 100%. | [94] |
| NCT00683241 | OC, primary peritoneal cancer | Autologous tumor lysate-pulsed DCs (DCVac-L) | No information | No published results | na |
| NCT01068509 | OC | Autologous DCs pulsed with mannosylated-MUC1 fusion protein (M-FP) (Cvac) | 56 | PFS of 13 months observed | [95] |
| NCT01132014 | OC | Autologous DCs pulsed with oxidized WTL (OCDC) | 67 | 2 PR, 14 SD | [96,97] |
| NCT03657966 | Recurrent OC | Autologous DCs pulsed with allogeneic apoptotic tumor cells (DCVAC/OvC), OC standard-of-care chemotherapy | No information | No published results (conference paper) | [98] |
| NCT ID | Cancer Type(s) | Treatments | Status | No. of Patients to Enrol |
|---|---|---|---|---|
| Neoantigen peptides | ||||
| NCT04024878 | Ovarian cancer (OC) | Neoantigen peptide vaccine, nivolumab | Recruiting | 30 |
| NCT04713514 | Platinum-sensitive and recurrent OC | A multi-neoepitope vaccine covering relevant OC TAAs including p53 (OSE2101), pembrolizumab | Not yet recruiting | 180 |
| Tumor-associated antigen (TAA) peptides | ||||
| NCT02737787 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer | Wilms tumor 1 (WT1) and NY-ESO-1 peptides, nivolumab | Recruiting | 20 |
| NCT01376505 | OC, breast cancer (BC), colorectal cancer (CRC), gastrointestinal stroma cancer (GIST) | HER-2/neu peptide | Recruiting | 100 |
| NCT03761914 | OC, acute myelogenous leukemia, CRC, triple-negative BC, non-small cell lung cancer (NSCLC) | A multivalent multipeptides of >20 epitopes of WT1 protein (Galinpepimut-S), pembrolizumab | Recruiting | 90 |
| NCT04853017 | Minimal residual disease, OC, CRC, NSCLC, pancreatic adenocarcinoma (PC), cholangiocarcinoma, bile duct cancer, gallbladder carcinoma | Lipid-conjugated oligonucleotide [Amph-CpG-7909] admixed with lipid-conjugated KRAS/NRAS-derived peptides [Amph-Peptides]) (ELI-002) | Recruiting | 159 |
| NCT00194714 | HER-2/neu-positive stage IV OC or BC | HER-2/neu peptide | Active, not recruiting | 26 |
| NCT02111941 | OC, fallopian tube cancer, primary peritoneal cancer, ovarian clear cell cystadenocarcinoma, ovarian endometrioid adenocarcinoma | Multiepitope folate receptor-alpha peptides-loaded DC vaccine | Active, not recruiting | 19 |
| RNA and DNA vaccines | ||||
| NCT04163094 | OC | Liposome-formulated mRNA vaccine encoding for three OC TAAs (W_ova1), neoadjuvant chemotherapy | Recruiting | 10 |
| NCT00436254 | HER-2/neu-positive stage III-IV OC, OC germ cell tumor, BC | DNA plasmid vaccine (pNGVL3-hICD), sargramostim (recombinant granulocyte-marcophage colony stimulating factor [GM-CSF]) | Active, not recruiting | 66 |
| Viral vector vaccine | ||||
| NCT04246671 | OC, BC, CRC, NSCLC, PC, hepatocellular cancer (HCC), gastric cancer (GC), chordoma, prostate cancer, Merkel cell carcinoma | Modified Vaccinia Ankara-BN (MVA-BN) viral vector vaccine expressing Brachyury and HER-2/neu proteins (TAEK-VAC-HerBy) | Recruiting | 45 |
| NCT03113487 | Recurrent platinum-resistant OC, fallopian tube cancer, primary peritoneal cancer | Modified Vaccinia Virus Ankara viral vector vaccine expressing p53 protein, pembrolizumab, gemcitabine hydrochloride | Recruiting | 28 |
| NCT03120624 | Recurrent OC, fallopian tube cancer, primary peritoneal cancer, endometrioid adenocarcinoma | Recombinant Vesicular Stomatitis Virus-expressing human interferon-beta and sodium-iodide symporter (VSV-hIFNbeta-NIS), ruxolitinib phosphate | Recruiting | 77 |
| NCT04282044 | OC, triple-negative BC, CRC, HCC, GC, osteosarcoma | Activated cytokine-induced killer (CIK) cells infected with an oncolytic virus (CRX-100) | Recruiting | 24 |
| NCT03225989 | OC, CRC, PC, biliary carcinoma | Oncolytic adenovirus expresses transgenes trimerized membrane-bound isoleucine zipper (TMZ) TMZ-CD40L and 41BBL (delolimogene mupadenorepvec; LOAd703) | Recruiting | 50 |
| NCT02364713 | OC, fallopian tube cancer, primary peritoneal cancer | Mesenchymal Stem Cells infected with oncolytic Measles Virus encoding for thyroidal sodium iodide symporter (MV-NIS) | Recruiting | 66 |
| NCT02068794 | OC, fallopian tube cancer, primary peritoneal cancer, endometrioid adenocarcinoma | Mesenchymal Stem Cells infected with MV-NIS | Recruiting | 57 |
| NCT03663712 | Platinum-resistant ovarian cancer, stage IV peritoneal carcinomatosis | Type I genetically modified oncolytc Herpes Simplex Virus (Talimogene Laherparepvec; TVEC) | Recruiting | 24 |
| NCT02759588 | OC, fallopian tube cancer, primary peritoneal cancer | Triple-modified and attenuated Vaccinia virus (Lister strain) [GL-ONC1], chemotherapy, bevacizumab | Active, not recruiting | 64 |
| Whole tumor lysate (WTL) or cell vaccine (alone or DC-based) | ||||
| NCT00722228 | OC and solid tumors of stage II, III and IV | Autologous or allogeneic tumor cell vaccine | Recruiting | 50 |
| NCT03556566 | OC | Tableted vaccine (V3-OVA) prepared from autologous hydrolyzed, inactivated blood and tumors. | Recruiting | 20 |
| NCT03671720 | Advanced and metastatic cancers | Autologous dendritic cell (DC) pulsed with autologous whole tumor lysate | Recruiting | 10 |
| NCT04212377 | Endometrial cancer | Myeloid and plasmacytoid DC (nDC) pulsed with WTL, MUC1 and survivin peptides | Recruiting | 8 |
| NCT04834544 | OC, fallopian tube cancer, primary peritoneal cancer | Autologous DCs pulsed with allogeneic apoptotic tumor cells (DCVAC/OvC) | Recruiting | 75 |
| NCT03735589 | Stage II fallopian tube cancer | Alpha-type-1 polarized dendritic cells pulsed with autologous tumor + autologous natural killer cell-like cytolytic T cells | Not yet recruiting | 18 |
| NCT03905902 | OC, fallopian tube cancer, primary peritoneal cancer | DCVAC/OvCa, standard-of-care platinum-based chemotherapy (carboplatin, gemcitabine, paclitaxel, pegylated liposomal doxorubicin), bevacizumab | Not yet recruiting | 678 |
| NCT04614051 | OC | Autologous DCs pulsed with ovarian cancer-specific antigen(s) (Cellgram-DC) | Not yet recruiting | 10 |
| NCT04739527 | OC | Irradiated mature allogenic DCs (DCP-001) | Not yet recruiting | 17 |
| NCT01309230 | OC | Modified autologous tumor cells expressing GM-CSF (Vigil™) | Active, not recruiting | 44 |
| NCT02033616 | Stage III-IV OC, fallopian tube cancer, primary peritoneal cancer | Autologous DCs pulsed with autologous tumor cells | Active, not recruiting | 99 |
| NCT00660101 | OC | 2,4-dinitrophenyl (DNP) keyhole limpet hemocyanin-Modified autologous tumor cell vaccine (OVax®®) | Unknown status | 34 |
| NCT00703105 | OC and solid tumors of stage II-IV | DCs pulsed with autologous WTL or HLA-A2-restricted MUC1 and WT1 peptides | Unknown status | 36 |
| DC-tumor cell fusion vaccine | ||||
| NCT00799110 | OC, fallopian tube cancer, primary peritoneal cancer | DC-tumor cell fusion vaccine, sargramostim, imiquimod | Active, not recruiting | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.L.-L.; Rovelli, R.; Sarivalasis, A.; Kandalaft, L.E. Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human. Cancers 2021, 13, 4553. https://doi.org/10.3390/cancers13184553
Chiang CL-L, Rovelli R, Sarivalasis A, Kandalaft LE. Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human. Cancers. 2021; 13(18):4553. https://doi.org/10.3390/cancers13184553
Chicago/Turabian StyleChiang, Cheryl Lai-Lai, Raphaël Rovelli, Apostolos Sarivalasis, and Lana E. Kandalaft. 2021. "Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human" Cancers 13, no. 18: 4553. https://doi.org/10.3390/cancers13184553
APA StyleChiang, C. L.-L., Rovelli, R., Sarivalasis, A., & Kandalaft, L. E. (2021). Integrating Cancer Vaccines in the Standard-of-Care of Ovarian Cancer: Translating Preclinical Models to Human. Cancers, 13(18), 4553. https://doi.org/10.3390/cancers13184553






