Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy
Abstract
:Simple Summary
Abstract
1. Introduction
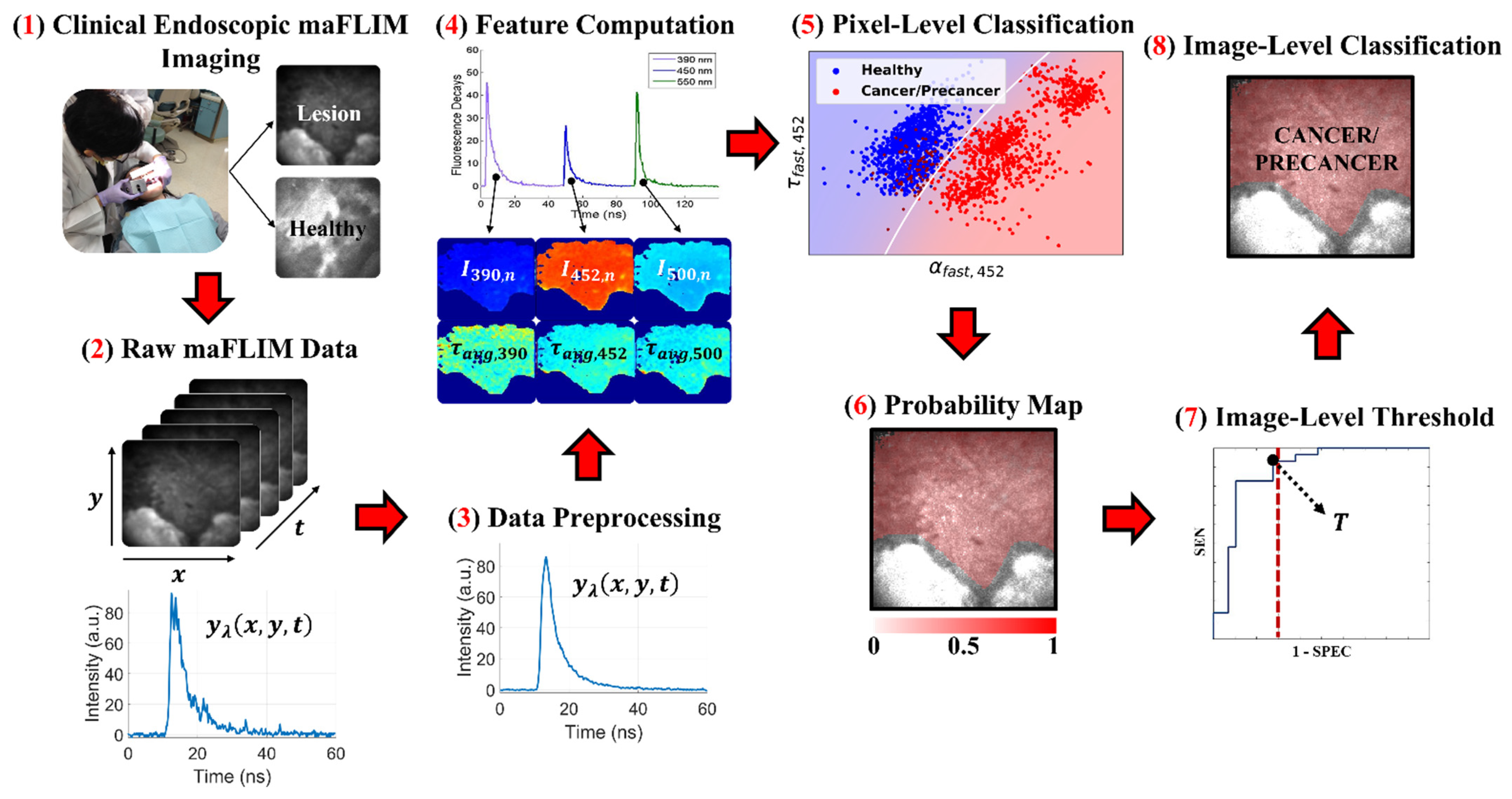
2. Materials and Methods
2.1. Clinical Endoscopic maFLIM Imaging of Oral Lesions
2.1.1. Training Set
2.1.2. Testing Set
2.2. maFLIM Feature Extraction
2.3. Classification Model Optimization Using the Training Set
3. Results
3.1. Classification Model Optimization Using the Training Set
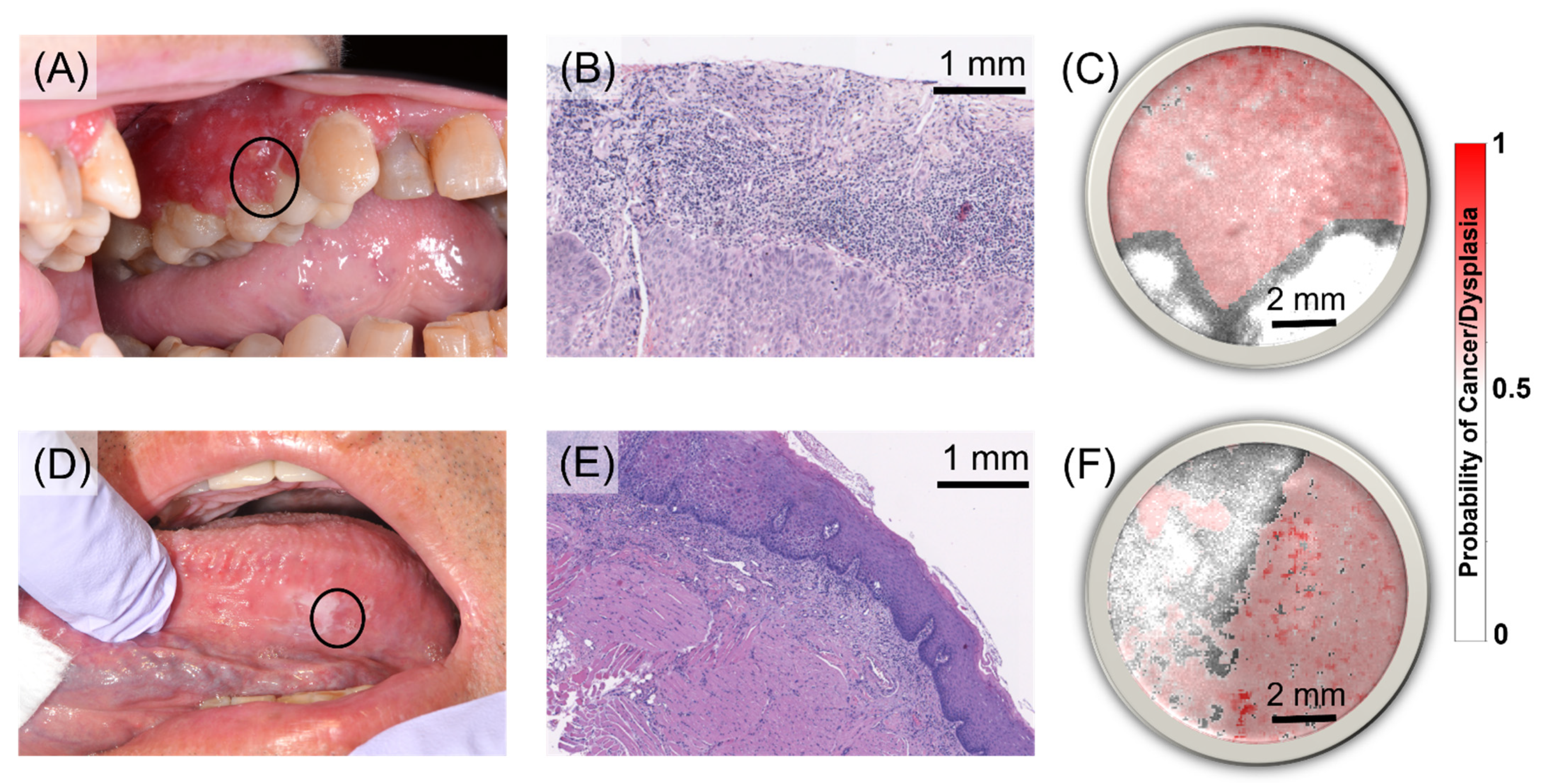
3.2. Independent Classification Performance Quantification in the Testing Set
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas Robbins, K.; Triantafyllou, A.; Suarez, C.; Lopez, F.; Hunt, J.L.; Strojan, P.; Williams, M.D.; Braakhuis, B.J.M.; de Bree, R.; Hinni, M.L.; et al. Surgical margins in head and neck cancer: Intra- and postoperative considerations. Auris Nasus Larynx 2019, 46, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Buchakjian, M.R.; Tasche, K.K.; Robinson, R.A.; Pagedar, N.A.; Sperry, S.M. Association of Main Specimen and Tumor Bed Margin Status With Local Recurrence and Survival in Oral Cancer Surgery. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Shah, A.K. Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review. J. Oral Maxillofac. Pathol. 2018, 22, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, M.G.; Tarabichi, O.; Sethi, R.K.; Parikh, A.S.; Varvares, M.A. Does Clearance of Positive Margins Improve Local Control in Oral Cavity Cancer? A Meta-analysis. Otolaryngol. Head Neck Surg. 2019, 161, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.V.; Sturgis, C.D.; Lai, C.; Maxwell, J.H.; Merzianu, M.; Hernandez-Prera, J.C.; Purgina, B.; Thompson, L.D.R.; Tuluc, M.; Yang, X.; et al. Improving margin revision: Characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017, 75, 184–188. [Google Scholar] [CrossRef]
- Patel, R.S.; Goldstein, D.P.; Guillemaud, J.; Bruch, G.A.; Brown, D.; Gilbert, R.W.; Gullane, P.J.; Higgins, K.M.; Irish, J.; Enepekides, D.J. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck 2010, 32, 1444–1451. [Google Scholar] [CrossRef]
- Szewczyk, M.; Golusinski, W.; Pazdrowski, J.; Masternak, M.; Sharma, N.; Golusinski, P. Positive fresh frozen section margins as an adverse independent prognostic factor for local recurrence in oral cancer patients. Laryngoscope 2018, 128, 1093–1098. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahuja, A.; Sable, N.; Stambuk, H.E. Imaging in oral cancers: A comprehensive review. Oral Oncol. 2020, 104, 104658. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Cho, J.K.; Koo, B.S.; Kwon, M.; Kwon, S.K.; Kwon, S.Y.; Kim, M.S.; Kim, J.K.; Kim, H.; Nam, I.; et al. Guidelines for the Surgical Management of Oral Cancer: Korean Society of Thyroid-Head and Neck Surgery. Clin. Exp. Otorhinolaryngol. 2019, 12, 107–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillone, G.A.; Wang, Z.; Krisciunas, G.P.; Tsai, A.C.; Kannabiran, V.R.; Pistey, R.W.; Zhao, Q.; Rodriguez-Diaz, E.; A’Amar, O.M.; Bigio, I.J. The color of cancer: Margin guidance for oral cancer resection using elastic scattering spectroscopy. Laryngoscope 2017, 127, S1–S9. [Google Scholar] [CrossRef]
- Hamdoon, Z.; Jerjes, W.; McKenzie, G.; Jay, A.; Hopper, C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn. Ther. 2016, 13, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.-J.; Sharma, M.; Sharma, L.; Chao, T.-Y.; Huang, S.-F.; Chang, L.-B.; Wu, S.-L.; Chow, L. Raman Spectroscopy Analysis for Optical Diagnosis of Oral Cancer Detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef]
- Halicek, M.; Fabelo, H.; Ortega, S.; Little, J.V.; Wang, X.; Chen, A.Y.; Callico, G.M.; Myers, L.; Sumer, B.D.; Fei, B. Hyperspectral imaging for head and neck cancer detection: Specular glare and variance of the tumor margin in surgical specimens. J. Med. Imaging 2019, 6, 035004. [Google Scholar] [CrossRef]
- Nayak, G.; Kamath, S.; Pai, K.M.; Sarkar, A.; Ray, S.; Kurien, J.; D’Almeida, L.; Krishnanand, B.; Santhosh, C.; Kartha, V. Principal component analysis and artificial neural network analysis of oral tissue fluorescence spectra: Classification of normal premalignant and malignant pathological conditions. Biopolym. Orig. Res. Biomol. 2006, 82, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, I.; Williams, M.; El-Naggar, A.; Richards-Kortum, R.; Gillenwater, A. Understanding the Biological Basis of Autofluorescence Imaging for Oral Cancer Detection: High-Resolution Fluorescence Microscopy in Viable Tissue. Clin. Cancer Res. 2008, 14, 2396–2404. [Google Scholar] [CrossRef] [Green Version]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [Green Version]
- Skala, M.C.; Riching, K.M.; Bird, D.K.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; Keely, P.J.; Ramanujam, N. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J. Biomed. Opt. 2007, 12, 024014. [Google Scholar] [CrossRef] [Green Version]
- Duran-Sierra, E.; Cheng, S.; Cuenca-Martinez, R.; Malik, B.; Maitland, K.C.; Lisa Cheng, Y.S.; Wright, J.; Ahmed, B.; Ji, J.; Martinez, M.; et al. Clinical label-free biochemical and metabolic fluorescence lifetime endoscopic imaging of precancerous and cancerous oral lesions. Oral Oncol. 2020, 105, 104635. [Google Scholar] [CrossRef]
- Cheng, S.; Cuenca, R.M.; Liu, B.; Malik, B.H.; Jabbour, J.M.; Maitland, K.C.; Wright, J.; Cheng, Y.-S.L.; Jo, J.A. Handheld multispectral fluorescence lifetime imaging system for in vivo applications. Biomed. Opt. Express 2014, 5, 921–931. [Google Scholar] [CrossRef] [Green Version]
- America, L.I.O. (Ed.) Safe Use of Lasers, ANSI Z136.1–2007; American National Standards Institute: New York, NY, USA, 2007. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Tharwat, A.; Gaber, T.; Ibrahim, A.; Hassanien, A.E. Linear discriminant analysis: A detailed tutorial. AI Commun. 2017, 30, 169–190. [Google Scholar] [CrossRef] [Green Version]
- Tharwat, A. Linear vs. quadratic discriminant analysis classifier: A tutorial. Int. J. Appl. Pattern Recognit. 2016, 3, 145–180. [Google Scholar] [CrossRef]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar]
- Ayer, T.; Chhatwal, J.; Alagoz, O.; Kahn, C.E., Jr.; Woods, R.W.; Burnside, E.S. Comparison of logistic regression and artificial neural network models in breast cancer risk estimation. Radiographics 2010, 30, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Brier, G.W. Verification of forecasts expressed in terms of probability. Mon. Weather Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Somol, P.; Novovicová, J.; Pudil, P. Efficient feature subset selection and subset size optimization. In Pattern Recognition Recent Advances; Adam, H., Ed.; InTech Europe: Rijeka, Croatia, 2010; ISBN 978-953-7619-90-9. Available online: https://www.intechopen.com/chapters/10666 (accessed on 14 June 2021).
- Whitney, A.W. A direct method of nonparametric measurement selection. IEEE Trans. Comput. 1971, 100, 1100–1103. [Google Scholar] [CrossRef]
- Pavlova, I.; Sokolov, K.; Drezek, R.; Malpica, A.; Follen, M.; Richards-Kortum, R. Microanatomical and Biochemical Origins of Normal and Precancerous Cervical Autofluorescence Using Laser-scanning Fluorescence Confocal Microscopy. Photochem. Photobiol. 2003, 77, 550–555. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Drezek, R.A.; Sokolov, K.V.; Utzinger, U.; Boiko, I.; Malpica, A.; Follen, M.; Richards-Kortum, R.R. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: Modeling, measurements, and implications. J. Biomed. Opt. 2001, 6, 385–397. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenc, O.I.; Quinn, K.P. Evaluating cell metabolism through autofluorescence imaging of NAD (P) H and FAD. Antioxid. Redox Signal. 2019, 30, 875–889. [Google Scholar] [CrossRef] [PubMed]
- de Veld, D.C.; Skurichina, M.; Witjes, M.J.; Duin, R.P.; Sterenborg, H.J.; Roodenburg, J.L. Clinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopy. J. Biomed. Opt. 2004, 9, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Kanaujia, S.K.; Singh, A.; Pradhan, A. In vivo detection of oral precancer using a fluorescence-based, in-house-fabricated device: A Mahalanobis distance-based classification. Lasers Med. Sci. 2019, 34, 1243–1251. [Google Scholar] [CrossRef]
- Huang, T.-T.; Chen, K.-C.; Wong, T.-Y.; Chen, C.-Y.; Chen, W.-C.; Chen, Y.-C.; Chang, M.-H.; Wu, D.-Y.; Huang, T.-Y.; Nioka, S. Two-channel autofluorescence analysis for oral cancer. J. Biomed. Opt. 2018, 24, 051402. [Google Scholar] [CrossRef]
- Jeng, M.-J.; Sharma, M.; Chao, T.-Y.; Li, Y.-C.; Huang, S.-F.; Chang, L.-B.; Chow, L. Multiclass classification of autofluorescence images of oral cavity lesions based on quantitative analysis. PLoS ONE 2020, 15, e0228132. [Google Scholar] [CrossRef]
- Marsden, M.; Weyers, B.W.; Bec, J.; Sun, T.; Gandour-Edwards, R.F.; Birkeland, A.C.; Abouyared, M.; Bewley, A.F.; Farwell, D.G.; Marcu, L. Intraoperative Margin Assessment in Oral and Oropharyngeal Cancer using Label-free Fluorescence Lifetime Imaging and Machine Learning. IEEE Trans. Biomed. Eng. 2020, 68, 857–868. [Google Scholar] [CrossRef]
- Serafino, M.J.; Applegate, B.E.; Jo, J.A. Direct frequency domain fluorescence lifetime imaging using field programmable gate arrays for real time processing. Rev. Sci. Instrum. 2020, 91, 033708. [Google Scholar] [CrossRef]
- Jeyaraj, P.R.; Nadar, E.R.S. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J. Cancer Res. Clin. Oncol. 2019, 145, 829–837. [Google Scholar] [CrossRef]
- Welikala, R.A.; Remagnino, P.; Lim, J.H.; Chan, C.S.; Rajendran, S.; Kallarakkal, T.G.; Zain, R.B.; Jayasinghe, R.D.; Rimal, J.; Kerr, A.R. Automated detection and classification of oral lesions using deep learning for early detection of oral cancer. IEEE Access 2020, 8, 132677–132693. [Google Scholar] [CrossRef]
- Jubair, F.; Al-karadsheh, O.; Malamos, D.; Al Mahdi, S.; Saad, Y.; Hassona, Y. A novel lightweight deep convolutional neural network for early detection of oral cancer. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]


| Lesion Location | Histopathology Diagnosis | Total Number | ||||
|---|---|---|---|---|---|---|
| Distribution of Imaged Oral Lesions | MiD | MoD | HiD | SCC | ||
| Training Set | Buccal Mucosa | 1 | 1 | 1 | 9 | 12 |
| Tongue | 0 | 0 | 0 | 12 | 12 | |
| Gingiva | 0 | 0 | 2 | 3 | 5 | |
| Lip | 0 | 0 | 0 | 2 | 2 | |
| Mandible | 0 | 0 | 0 | 1 | 1 | |
| Maxilla | 0 | 0 | 0 | 1 | 1 | |
| Floor of Mouth | 0 | 0 | 0 | 1 | 1 | |
| Total Number | 1 | 1 | 3 | 29 | 34 | |
| Testing Set | Tongue | 6 | 1 | 0 | 6 | 13 |
| Gingiva | 1 | 0 | 0 | 5 | 6 | |
| Buccal Mucosa | 0 | 1 | 0 | 2 | 3 | |
| Mandible | 0 | 0 | 0 | 1 | 1 | |
| Total Number | 7 | 2 | 0 | 14 | 23 | |
| Training Set (Doha, Qatar) | Testing Set (Dallas, Texas) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient # | Race | Age | Gender | Histopathology | Patient # | Race | Age | Gender | Histopathology |
| 1 | Indian | 34 | M | SCC | 1 | White | 59 | M | SCC |
| 2 | Egyptian | 67 | M | SCC | 2 | White | 76 | F | SCC |
| 3 | Sri Lankan | 52 | M | SCC | 3 | White | N/A | F | SCC |
| 4 | Nepalese | 47 | M | SCC | 4 | Asian | N/A | F | SCC |
| 5 | Egyptian | 42 | M | SCC | 5 | White | 60 | M | SCC |
| 6 | Nepalese | 35 | M | HiD | 6 | White | N/A | M | MiD |
| 7 | Indian | 50 | M | HiD | 7 | White | 54 | F | MiD |
| 8 | Indian | 51 | M | SCC | 8 | White | 75 | F | MiD |
| 9 | Indian | 43 | M | MoD | 9 | Asian | 58 | M | MiD |
| 10 | Bangladeshi | 59 | M | SCC | 10 | Asian | N/A | M | MiD |
| 11 | Sri Lankan | 55 | M | MiD | 11 | White | 55 | F | MiD |
| 12 | Nepalese | 31 | M | SCC | 12 | White | N/A | M | MiD |
| 13 | Nepalese | 39 | M | SCC | 13 | White | N/A | M | MoD |
| 14 | Indian | 36 | M | SCC | 14 | White | 62 | F | SCC |
| 15 | Pakistani | 36 | M | SCC | 15 | White | 59 | M | SCC |
| 16 | Qatari | 55 | M | SCC | 16 | White | N/A | M | SCC |
| 17 | Indian | 48 | M | SCC | 17 | Asian | 52 | F | SCC |
| 18 | Nepalese | 36 | M | SCC | 18 | White | 83 | F | SCC |
| 19 | Indian | 36 | M | SCC | 19 | White | 55 | M | SCC |
| 20 | Pakistani | 60 | M | SCC | 20 | Black | N/A | F | MoD |
| 21 | Sudanese | 61 | F | SCC | 21 | White | N/A | M | SCC |
| 22 | Sudanese | 60 | F | SCC | 22 | White | 68 | M | SCC |
| 23 | Iranian | 68 | M | SCC | 23 | N/A | 47 | F | SCC |
| 24 | Indian | 41 | M | SCC | |||||
| 25 | Indian | 49 | M | SCC | |||||
| 26 | Nepalese | 45 | N/A | SCC | |||||
| 27 | Somali | 60 | M | SCC | |||||
| 28 | Indian | 50 | M | SCC | |||||
| 29 | Indian | 61 | M | SCC | |||||
| 30 | Indian | 34 | F | SCC | |||||
| 31 | Nepalese | 30 | M | HiD | |||||
| 32 | Filipino | 49 | F | SCC | |||||
| 33 | Iranian | 59 | M | SCC | |||||
| 34 | Pakistani | 69 | M | SCC | |||||
| maFLIM Feature Category | Spectral Band | Total Number | ||
|---|---|---|---|---|
| 390 ± 20 nm | 452 ± 22.5 nm | >500 nm | ||
| Normalized Intensity | 3 | |||
| Absolute Intensity Ratio | / | 6 | ||
| / | ||||
| / | ||||
| Time-Resolved | 12 | |||
| Total Number | 21 | |||
| maFLIM Feature Pool | Classification Model | F1-Score | Sensitivity | Specificity |
|---|---|---|---|---|
| Spectral | LDA | 0.78 | 82% | 71% |
| QDA | 0.74 | 76% | 71% | |
| SVM | 0.79 | 82% | 74% | |
| LOGREG | 0.79 | 85% | 71% | |
| Time-Resolved | LDA | 0.75 | 79% | 68% |
| QDA | 0.83 | 91% | 71% | |
| SVM | 0.73 | 76% | 68% | |
| LOGREG | 0.76 | 79% | 71% | |
| Top three Spectral | SVM | 0.76 | 79% | 71% |
| Top three Time-Resolved | QDA | 0.82 | 91% | 68% |
| Ensemble (Top three Spectral and Time-Resolved) | SVM-QDA | 0.85 | 94% | 74% |
| Confusion Matrices for Best Performing Models | Predicted | ||||||
|---|---|---|---|---|---|---|---|
| SVM (Spectral) | QDA (Time-Resolved) | SVM-QDA (Ensemble) | |||||
| (−) | (+) | (−) | (+) | (−) | (+) | ||
| True | Healthy (n = 34) | 25 | 9 | 24 | 10 | 25 | 9 |
| MiD (n = 1) | 1 | 0 | 0 | 1 | 0 | 1 | |
| MoD (n = 1) | 0 | 1 | 0 | 1 | 0 | 1 | |
| HiD (n = 3) | 1 | 2 | 0 | 3 | 0 | 3 | |
| SCC (n = 29) | 4 | 25 | 3 | 26 | 2 | 27 | |
| Total | 31 | 37 | 27 | 41 | 27 | 41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran-Sierra, E.; Cheng, S.; Cuenca, R.; Ahmed, B.; Ji, J.; Yakovlev, V.V.; Martinez, M.; Al-Khalil, M.; Al-Enazi, H.; Cheng, Y.-S.L.; et al. Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy. Cancers 2021, 13, 4751. https://doi.org/10.3390/cancers13194751
Duran-Sierra E, Cheng S, Cuenca R, Ahmed B, Ji J, Yakovlev VV, Martinez M, Al-Khalil M, Al-Enazi H, Cheng Y-SL, et al. Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy. Cancers. 2021; 13(19):4751. https://doi.org/10.3390/cancers13194751
Chicago/Turabian StyleDuran-Sierra, Elvis, Shuna Cheng, Rodrigo Cuenca, Beena Ahmed, Jim Ji, Vladislav V. Yakovlev, Mathias Martinez, Moustafa Al-Khalil, Hussain Al-Enazi, Yi-Shing Lisa Cheng, and et al. 2021. "Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy" Cancers 13, no. 19: 4751. https://doi.org/10.3390/cancers13194751
APA StyleDuran-Sierra, E., Cheng, S., Cuenca, R., Ahmed, B., Ji, J., Yakovlev, V. V., Martinez, M., Al-Khalil, M., Al-Enazi, H., Cheng, Y. -S. L., Wright, J., Busso, C., & Jo, J. A. (2021). Machine-Learning Assisted Discrimination of Precancerous and Cancerous from Healthy Oral Tissue Based on Multispectral Autofluorescence Lifetime Imaging Endoscopy. Cancers, 13(19), 4751. https://doi.org/10.3390/cancers13194751







