Histone Deacetylase (HDAC)-1, -2, -4, and -6 in Uveal Melanomas: Associations with Clinicopathological Parameters and Patients’ Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
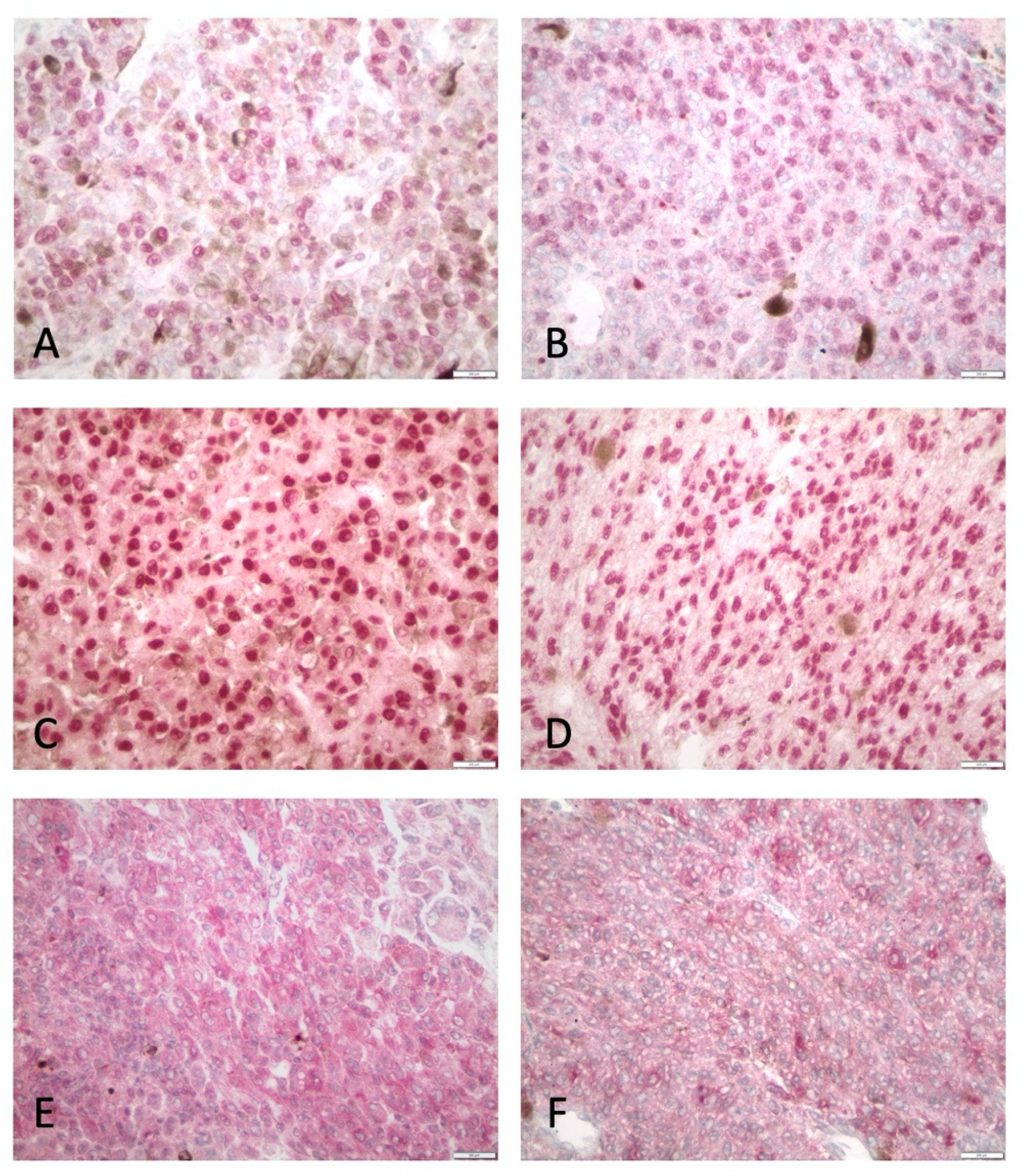
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Study Population
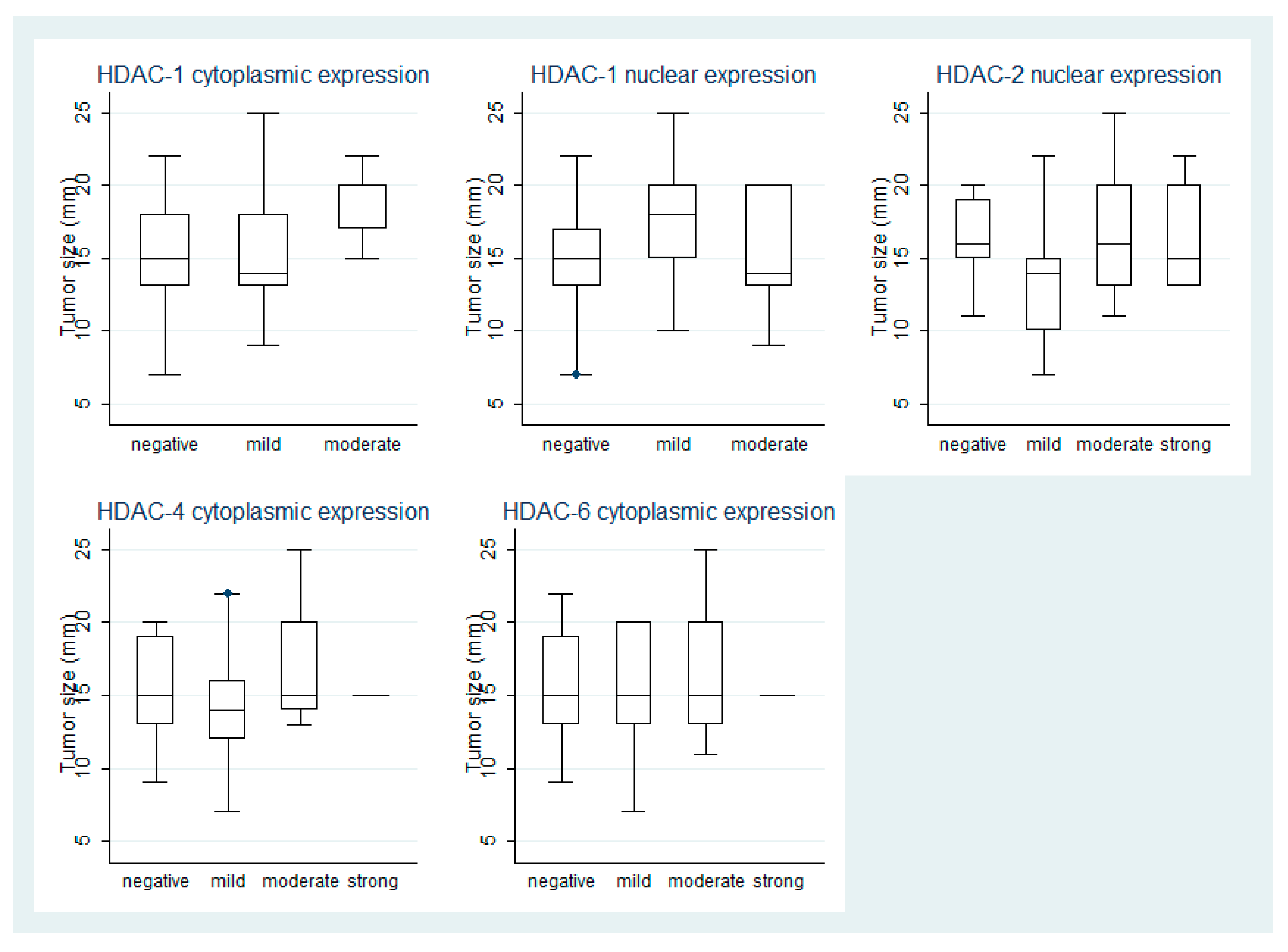
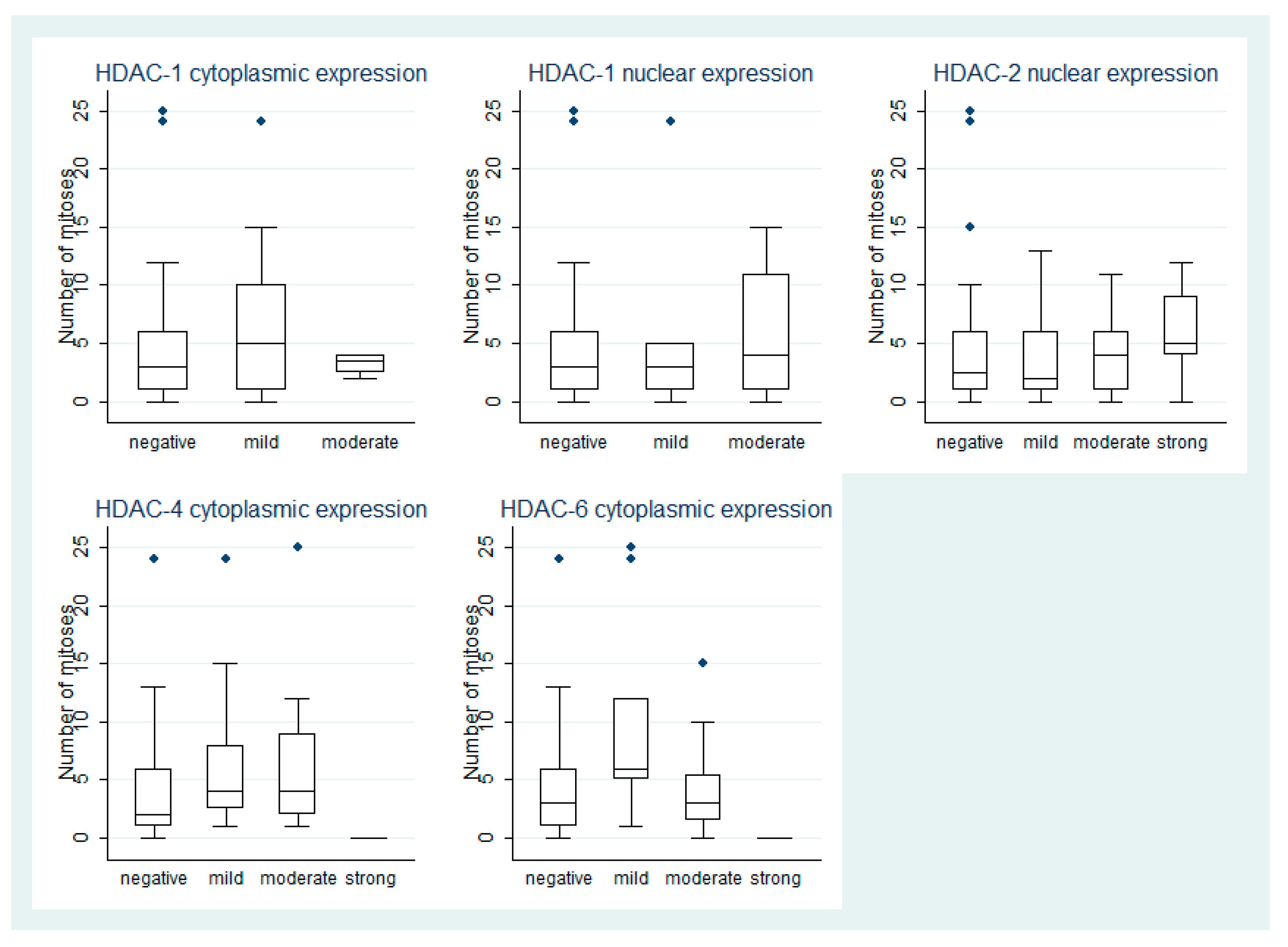
3.2. HDAC-1 IHC Expression and Association with Clinicopathological Parameters
3.3. HDAC-2 IHC Expression and Association with Clinicopathological Parameters
3.4. HDAC-4 IHC Expression and Association with Clinicopathological Parameters
3.5. HDAC-6 IHC Expression and Association with Clinicopathological Parameters
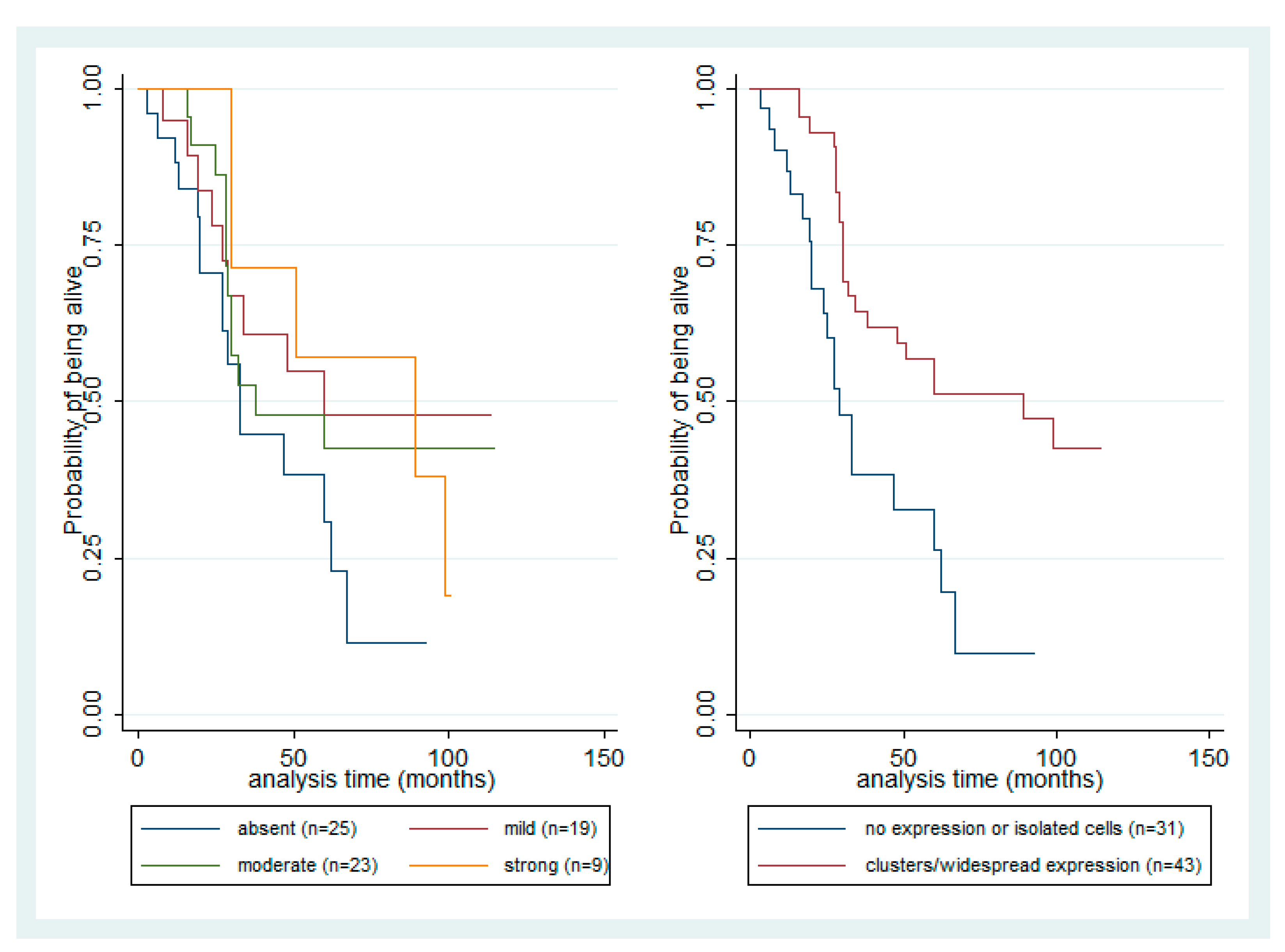
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Roth, S.Y.; Denu, J.M.; Allis, D.C. Histone acetyltransfereases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Marks, P.A.; Miller, T.; Richon, V.M. Histone Deacetylases. Curr. Opin. Pharmacol. 2003, 3, 344–351. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y. The diverse function of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef]
- De Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Guarente, L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004, 73, 417–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutas, D.; Theocharis, S.; Tsourouflis, D. Unraveling the Epigenetic Role and Clinical Impact of Histone Deacetylases in Neoplasia. Diagnostics 2021, 11, 1346. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar]
- Gajdzis, M.; Theocharis, S.; Klijanienko, J.; Cassoux, N.; Gardrat, S.; Donizy, P.; Kaczmarek, R.; Gajdzis, P. The Prognostic Values of PARP-1 Expression in Uveal Melanoma. Cells 2021, 10, 285. [Google Scholar] [CrossRef]
- Gardrat, S.; Houy, A.; Brooks, K.; Cassoux, N.; Barnhill, R.; Dayot, S.; Bièche, I.; Raynal, V.; Baulande, S.; Marais, R.; et al. Definition of Biologically Distinct Groups of Conjunctival Melanomas According to Etiological Factors and Implications for Precision Medicine. Cancers 2021, 13, 3836. [Google Scholar] [CrossRef]
- Kouraklis, G.; Theocharis, S. Histone deacetylase inhibitors and anticancer therapy. Curr. Med. Chem. Anticancer Agents 2002, 2, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Dettoraki, M.; Androudi, S.; Kalogeropoulos, D.; Lavaris, A.; Garmpis, N.; Damaskos, C.; Garmpi, A.; Tsatsos, M. The Role of Histone Deacetylase Inhibitors in Uveal Melanoma: Current Evidence. Anticancer Res. 2018, 38, 3817–3824. [Google Scholar] [CrossRef]
- Gajdzis, M.; Theocharis, S.; Gajdzis, P.; Cassoux, N.; Gardrat, S.; Donizy, P.; Klijanienko, J.; Kaczmarek, R. Ephrin Receptors (Eph): EphA1, EphA5, and EphA7 Expression in Uveal Melanoma-Associations with Clinical Parameters and Patient Survival. Life 2020, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Jochemsen, A.G.; Versluis, M.; Wierenga, A.P.A.; Nemati, F.; van der Velden, P.A.; Kroes, W.G.M.; Verdijk, R.M.; Luyten, G.P.M.; Jager, M.J. HDAC Inhibition Increases HLA Class I Expression in Uveal Melanoma. Cancers 2020, 12, 3690. [Google Scholar] [CrossRef]
- Souri, Z.; Jochemsen, A.G.; Wierenga, A.P.A.; Kroes, W.G.M.; Verdijk, R.M.; van der Velden, P.A.; Luyten, G.P.M.; Jager, M.J. Expression of HDACs 1, 3 and 8 Is Upregulated in the Presence of Infiltrating Lymphocytes in Uveal Melanoma. Cancers 2021, 13, 4146. [Google Scholar] [CrossRef] [PubMed]
- Levinzon, L.; Madigan, M.; Nguyen, V.; Hasic, E.; Conway, M.; Cherepanoff, S. Tumour Expression of Histone Deacetylases in Uveal Melanoma. Ocul. Oncol. Pathol. 2019, 5, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pouloudi, D.; Manou, M.; Sarantis, P.; Tsoukalas, N.; Tsourouflis, G.; Dana, E.; Karamouzis, M.V.; Klijanienko, J.; Theocharis, S. Clinical Significance of Histone Deacetylase (HDAC)-1, -2, -4 and -6 Expression in Salivary Gland Tumors. Diagnostics 2021, 11, 517. [Google Scholar] [CrossRef]
- Giaginis, C.; Damaskos, C.; Koutsounas, I.; Zizi-Serbetzoglou, A.; Tsoukalas, N.; Patsouris, E.; Kouraklis, G.; Theocharis, S. Histone deacetylase (HDAC)-1, -2, -4 and-6 expression in human pancreatic adenocarcinoma: Associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Gastroenterol. 2015, 15, 148. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, S.; Klijanienko, J.; Giaginis, C.; Rodriguez, J.; Jouffroy, T.; Girod, A.; Alexandrou, P.; Sastre-Garau, X. Histone deacetylase-1 and -2 expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients survival. J. Oral. Pathol. Med. 2011, 40, 706–714. [Google Scholar] [CrossRef]
- Pacheco, M.; Nielsen, T.O. Histone deacetylase 1 and 2 in mesenchymal tumors. Mod. Pathol. 2012, 25, 222–230. [Google Scholar] [CrossRef]
- Seo, J.; Min, S.K.; Park, H.R.; Kim, D.H.; Kwon, M.J.; Kim, L.S.; Ju, Y.S. Expression of Histone Deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in Invasive Ductal Carcinomas of the Breast. J. Breast Cancer 2014, 17, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.T.; Xing, W.; Zhao, R.S.; Tan, Y.; Wu, X.F.; Ao, L.Q.; Li, Z.; Yao, M.W.; Yuan, M.; Guo, W.; et al. HDAC2 inhibits EMT-mediated cancer metastasis by downregulating the long noncoding RNA H19 in colorectal cancer. J. Exp. Clin. Cancer Res. 2020, 39, 270. [Google Scholar] [CrossRef]
- Weichert, W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009, 280, 168–176. [Google Scholar] [CrossRef]
- Giaginis, C.; Alexandrou, P.; Delladetsima, I.; Giannopoulou, I.; Patsouris, E.; Theocharis, S. Clinical significance of histone deacetylase (HDAC)-1, HDAC-2, HDAC-4, and HDAC-6 expression in human malignant and benign thyroid lesions. Tumour Biol. 2014, 35, 61–71. [Google Scholar] [CrossRef]
- Mutze, K.; Langer, R.; Becker, K.; Ott, K.; Novotny, A.; Luber, B.; Hapfelmeier, A.; Göttlicher, M.; Höfler, H.; Keller, G. Histone deacetylase (HDAC) 1 and 2 expression and chemotherapy in gastric cancer. Ann. Surg. Oncol. 2010, 17, 3336–3343. [Google Scholar] [CrossRef] [PubMed]
- Mottet, D.; Castronovo, V. Histone deacetylases: Target enzymes for cancertherapy. Clin. Exp. Metastasis 2008, 25, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Viatour, P.; Legrand-Poels, S.; van Lint, C.; Warnier, M.; Merville, M.P.; Gielen, J.; Piette, J.; Bours, V.; Chariot, A. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J. Biol. Chem. 2003, 278, 46541–46548. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jones, C. The bovine herpesvirus 1 immediate-early protein (bICP0) associates with histone deacetylase 1 to activate transcription. J. Virol. 2001, 75, 9571–9578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Gu, J.; Feng, Z.; Yang, Y.; Zhu, N.; Lu, W.; Qi, F. Both HDAC5 and HDAC6 are required for the proliferation and metastasis of melanoma cells. J. Transl. Med. 2016, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef] [PubMed]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 1983, 96, 502–509. [Google Scholar] [CrossRef]
- Sisley, K.; Rennie, I.G.; Parsons, M.A.; Jacques, R.; Hammond, D.W.; Bell, S.M.; Potter, A.M.; Rees, R.C. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer 1997, 19, 22–28. [Google Scholar] [CrossRef]
- Suraweera, A.; O’Byrne, K.J.; Richard, D.J. Combination Therapy with Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Front. Oncol. 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, J.J.; Li, X.; Chou, C.J. Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. Adv. Cancer Res. 2018, 138, 183–211. [Google Scholar]
- Masaoutis, C.; Kokkali, S.; Theocharis, S. Immunotherapy in uveal melanoma: Novel strategies and opportunities for personalized treatment. Expert Opin. Investig. Drugs 2021, 30, 555–569. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Median | Range |
|---|---|---|
| Age | 65 | 14–94 years |
| Number of Mitoses per 40 HPFs | 3 | 0–25 |
| Tumor size | 15 | range 7–25 mm |
| Number | % | |
| Gender | ||
| Male | 31/75 | 41% |
| Female | 44/75 | 59% |
| Posterior pole involvement | 18/75 | 26% |
| Ciliary body involvement | 32/75 | 43% |
| Iris involvement * | 6/75 | 8% |
| Irido-corneal angle involvement | 7/75 | 9% |
| Presence of retinal detachment | 32/75 | 43% |
| Presence of vitreous hemorrhage | 10/75 | 13% |
| Intrasclera involvement | 64/75 | 85% |
| Extrasclera involvement | 8/75 | 12% |
| Histological cell type | ||
| Epithelioid cell | 18/75 | 24% |
| Mixed cell | 36/75 | 48% |
| Spindle cell | 21/75 | 28% |
| Loss of chromosome 3 | 13/57 | 23% |
| Gain 8q | 25/30 | 83% |
| Presence of metastasis | 40/75 | 53% |
| T-category (AJCC) | ||
| Τ1 | 1/75 | 1% |
| Τ2 | 11/75 | 15% |
| Τ3 | 25/75 | 33% |
| Τ4 | 38/75 | 51% |
| Event | ||
| Death of disease | 43/75, within 9–99 months | 57% |
| Censored | 32/75, follow-up 5–115 months | 43% |
| Presence of relapse ** | 7/75, within 13–109 months | 9% |
| Parameter | Immunoreactivity Score (IRS) | |||
|---|---|---|---|---|
| Absent | Mild | Moderate | Strong | |
| HDAC-1 | ||||
| Nuclear | 51 (74%) | 7 (10%) | 11 (16%) | - |
| Cytoplasmic | 46 (67%) | 18 (26%) | 5 (7%) | - |
| HDAC-2 | ||||
| Nuclear | 25 (34%) | 19 (26%) | 23 (31%) | 9 (9%) |
| Cytoplasmic | 67 (91%) | 3 (4%) | 4 (5%) | - |
| HDAC-4 cytoplasmic | 38 (55%) | 13 (19%) | 17 (25%) | 1 (1%) |
| HDAC-6 cytoplasmic | 37 (51%) | 9 (12%) | 25 (36%) | 1 (1%) |
| Model A | Hazard Ratio (HR) | p | 95% Confidence Interval | |
| HDAC-2 nuclear IRS | 0.286 | 0.001 | 0.133 | 0.618 |
| Tumor size | 1.133 | 0.02 | 1.023 | 1.255 |
| Number of mitoses | 1.053 | 0.07 | 0.997 | 1.113 |
| Mixed cell type vs. Epithelioid type | 0.605 | 0.22 | 0.271 | 1.351 |
| Spindle cell type vs. Epithelioid type | 0.169 | 0.003 | 0.052 | 0.545 |
| Presence of metastasis | 3.949 | 0.002 | 1.682 | 9.271 |
| Model B | Hazard Ratio (HR) | p | 95% Confidence Interval | |
| HDAC-2 heterogeneous staining widespread/clusters vs. negative/isolated cells | 0.303 | 0.001 | 0.145 | 0.631 |
| Tumor size | 1.078 | 0.16 | 0.972 | 1.194 |
| Number of mitoses | 1.051 | 0.07 | 0.995 | 1.110 |
| Mixed cell type vs. Epithelioid type | 0.567 | 0.17 | 0.250 | 1.284 |
| Spindle cell type vs. Epithelioid type | 0.215 | 0.007 | 0.070 | 0.662 |
| Presence of metastasis | 3.610 | 0.003 | 1.555 | 8.381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levidou, G.; Gajdzis, P.; Cassoux, N.; Donizy, P.; Masaoutis, C.; Gajdzis, M.; Gardrat, S.; Pergaris, A.; Danas, E.; Klijanienko, J.; et al. Histone Deacetylase (HDAC)-1, -2, -4, and -6 in Uveal Melanomas: Associations with Clinicopathological Parameters and Patients’ Survival. Cancers 2021, 13, 4763. https://doi.org/10.3390/cancers13194763
Levidou G, Gajdzis P, Cassoux N, Donizy P, Masaoutis C, Gajdzis M, Gardrat S, Pergaris A, Danas E, Klijanienko J, et al. Histone Deacetylase (HDAC)-1, -2, -4, and -6 in Uveal Melanomas: Associations with Clinicopathological Parameters and Patients’ Survival. Cancers. 2021; 13(19):4763. https://doi.org/10.3390/cancers13194763
Chicago/Turabian StyleLevidou, Georgia, Pawel Gajdzis, Nathalie Cassoux, Piotr Donizy, Christos Masaoutis, Malgorzata Gajdzis, Sophie Gardrat, Alexandros Pergaris, Eougken Danas, Jerzy Klijanienko, and et al. 2021. "Histone Deacetylase (HDAC)-1, -2, -4, and -6 in Uveal Melanomas: Associations with Clinicopathological Parameters and Patients’ Survival" Cancers 13, no. 19: 4763. https://doi.org/10.3390/cancers13194763
APA StyleLevidou, G., Gajdzis, P., Cassoux, N., Donizy, P., Masaoutis, C., Gajdzis, M., Gardrat, S., Pergaris, A., Danas, E., Klijanienko, J., & Theocharis, S. (2021). Histone Deacetylase (HDAC)-1, -2, -4, and -6 in Uveal Melanomas: Associations with Clinicopathological Parameters and Patients’ Survival. Cancers, 13(19), 4763. https://doi.org/10.3390/cancers13194763








