Precision Postoperative Radiotherapy in Sinonasal Carcinomas after Endonasal Endoscopic Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Toward Endoscopic Resection
3. Radiotherapy
3.1. Indication and Techniques
3.2. Local Control with IMRT
3.3. Toxicities Induced by IMRT
3.4. Toxicities Induced by Proton Therapy or Charged Particle Therapy
4. Precision Radiotherapy
4.1. Definition of Irradiated Volumes and Histology-Specific Radiotherapy Tumor Volumes and Margins
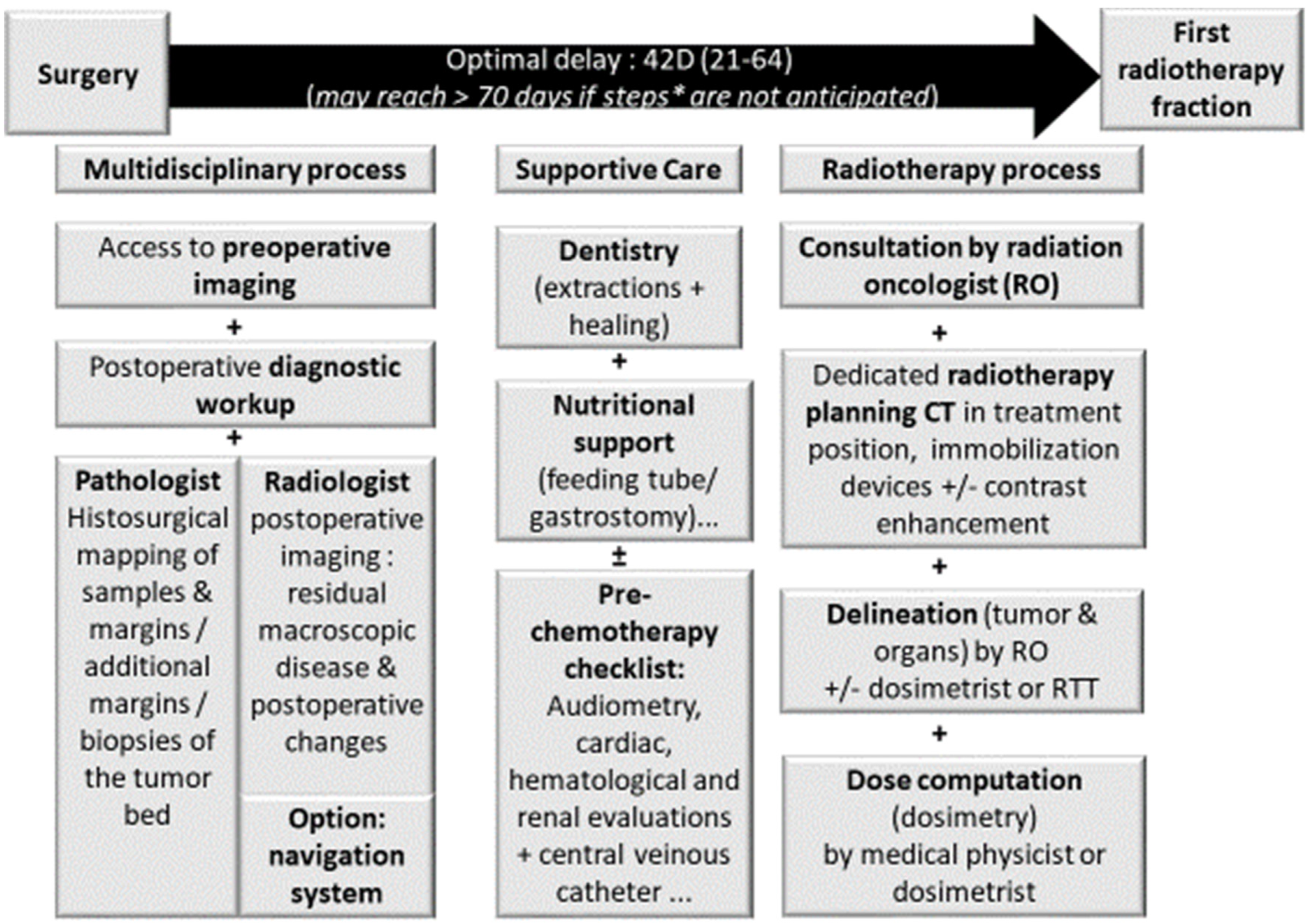
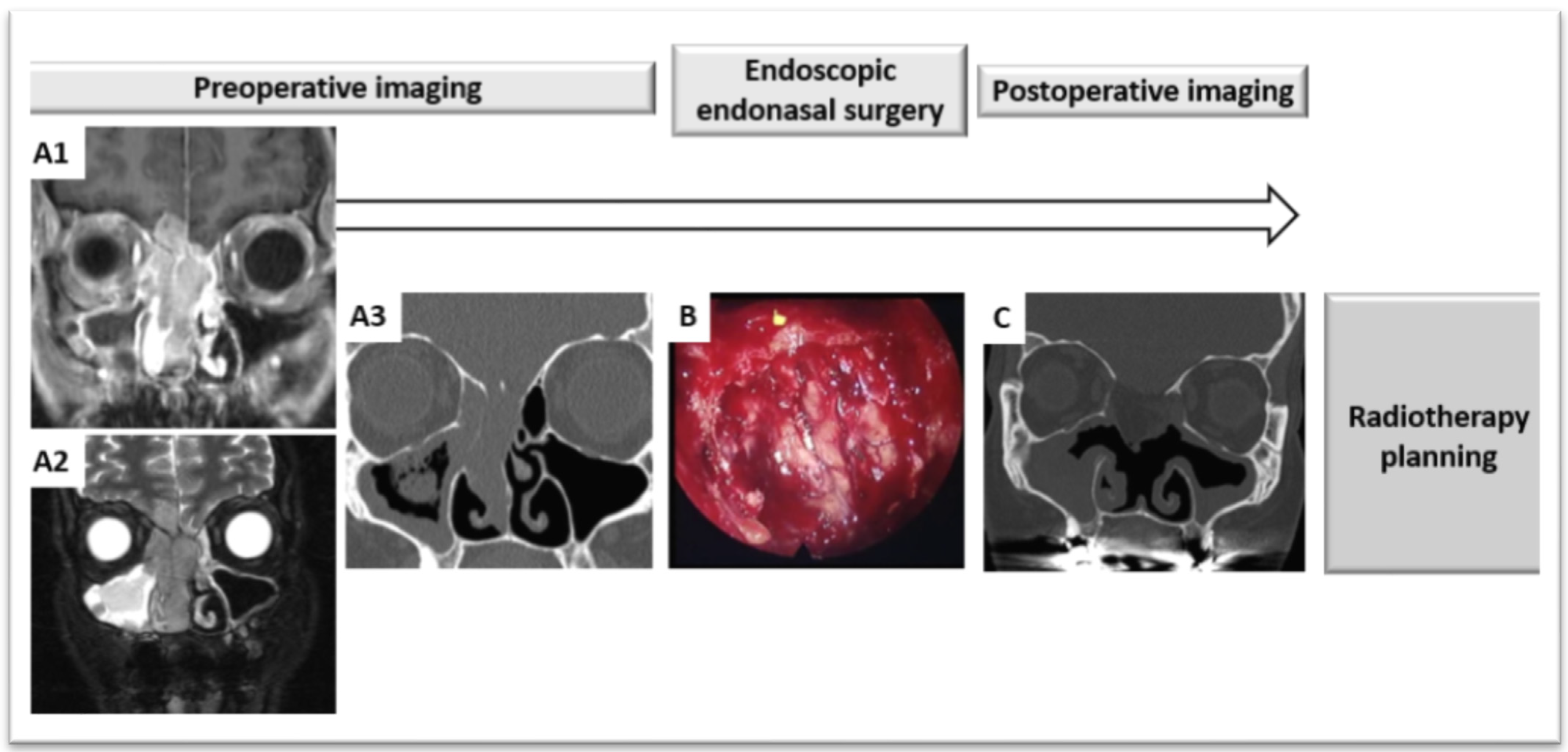
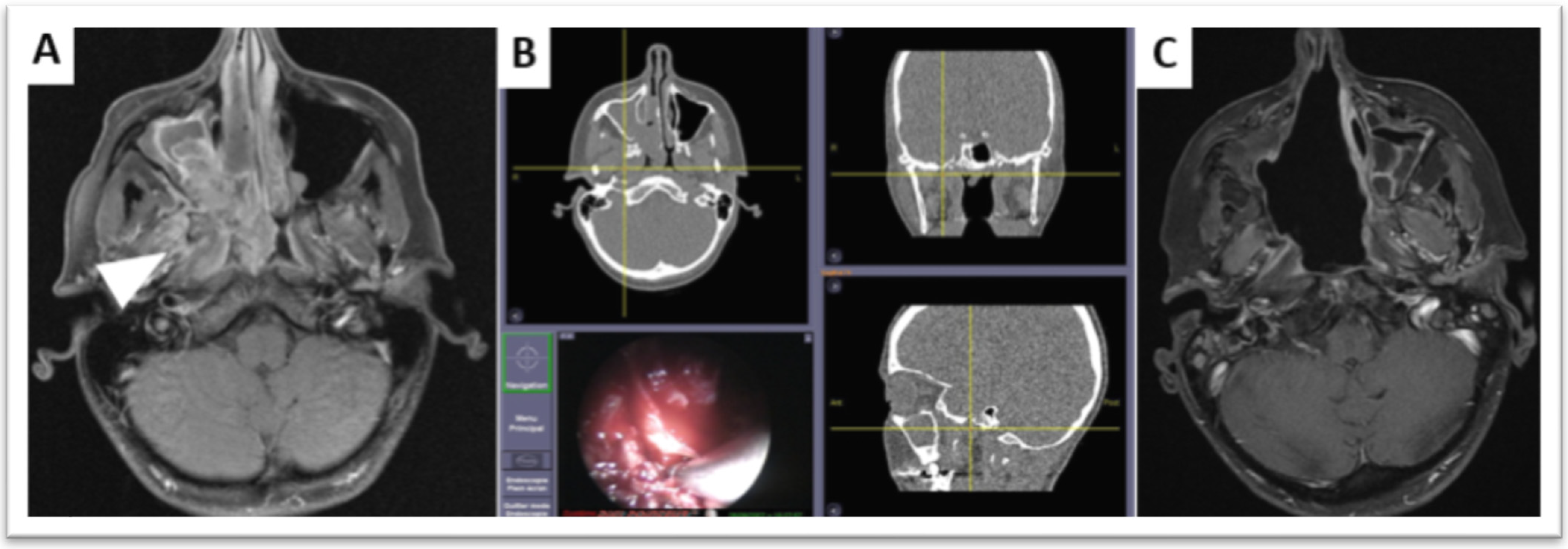
4.2. Radiotherapy Planning after Endoscopic Endonasal Surgery
5. Combined Systemic Treatments
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gore, M.R.; Zanation, A.M. Survival in Sinonasal Melanoma: A Meta-analysis. J. Neurol. Surg. B Skull Base 2012, 73, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thariat, J.; Moya Plana, A.; Verillaud, B.; Vergez, S.; Regis-Ferrand, F.; Digue, L.; Even, C.; Costes, V.; Baujat, B.; de Gabory, L.; et al. Diagnosis, prognosis and treatment of sinonasal carcinomas (excluding melanomas, sarcomas and lymphomas). Bull. Cancer 2020, 107, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Slevin, F.; Pan, S.; Mistry, H.; Denholm, M.; Shor, D.; Oong, Z.; Price, J.; Jadon, R.; Fleming, J.C.; Barnett, G.; et al. A Multicentre UK Study of Outcomes for Locally Advanced Sinonasal Squamous Cell Carcinoma Treated with Adjuvant or Definitive Intensity-modulated Radiotherapy. Clin. Oncol. 2021, 33, e450–e461. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Mortuaire, G.; Armas, G.L.; Hartl, D.; Auperin, A.; El Bedoui, S.; Chevalier, D.; Lefebvre, J.L. Sinonasal cancer: Analysis of oncological failures in 156 consecutive cases. Head Neck 2014, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Owin, N.; Elsayad, K.; Rolf, D.; Haverkamp, U.; Suwelack, D.; Tschakert, R.; Berssenbrugge, H.; Kleinheinz, J.; Rudack, C.; Eich, H.T. Radiotherapy as Part of Treatment Strategies in Nasal Cavity and Paranasal Sinus Malignancies. Anticancer Res. 2021, 41, 1587–1592. [Google Scholar] [CrossRef]
- Laskar, S.G.; Pai, P.; Sinha, S.; Budrukkar, A.; Nair, D.; Swain, M.; Mummudi, N.; Gupta, T.; Murthy, V.; Agarwal, J.P.; et al. Intensity-modulated radiation therapy for nasal cavity and paranasal sinus tumors: Experience from a single institute. Head Neck 2021, 43, 2045–2057. [Google Scholar] [CrossRef]
- Shay, A.; Ganti, A.; Raman, A.; Kuhar, H.N.; Auger, S.R.; Eggerstedt, M.; Patel, T.; Kuan, E.C.; Batra, P.S.; Tajudeen, B.A. Survival in low-grade and high-grade sinonasal adenocarcinoma: A national cancer database analysis. Laryngoscope 2019, 130, E1–E10. [Google Scholar] [CrossRef]
- Teitelbaum, J.I.; Issa, K.; Barak, I.R.; Ackall, F.Y.; Jung, S.H.; Jang, D.W.; Abi Hachem, R. Sinonasal Squamous Cell Carcinoma Outcomes: Does Treatment at a High-Volume Center Confer Survival Benefit? Otolaryngol. Head Neck Surg. 2020, 163, 986–991. [Google Scholar] [CrossRef]
- Pare, A.; Blanchard, P.; Rosellini, S.; Auperin, A.; Gorphe, P.; Casiraghi, O.; Temam, S.; Bidault, F.; Page, P.; Kolb, F.; et al. Outcomes of multimodal management for sinonasal squamous cell carcinoma. J. Craniomaxillofac. Surg. 2017, 45, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Turri-Zanoni, M.; Lambertoni, A.; Margherini, S.; Giovannardi, M.; Ferrari, M.; Rampinelli, V.; Schreiber, A.; Cherubino, M.; Antognoni, P.; Locatelli, D.; et al. Multidisciplinary treatment algorithm for the management of sinonasal cancers with orbital invasion: A retrospective study. Head Neck 2019, 41, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Patel, S.G.; Singh, B.; Kraus, D.H.; Bridger, P.G.; Cantu, G.; Cheesman, A.; De Sa, G.; Donald, P.; Fliss, D.M.; et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck 2005, 27, 575–584. [Google Scholar] [CrossRef]
- Abdelmeguid, A.S.; Bell, D.; Hanna, E.Y. Sinonasal Undifferentiated Carcinoma. Curr. Oncol. Rep. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Lilja, M.; Markkanen-Leppanen, M.; Viitasalo, S.; Saarilahti, K.; Lindford, A.; Lassus, P.; Makitie, A. Olfactory and gustatory functions after free flap reconstruction and radiotherapy for oral and pharyngeal cancer: A prospective follow-up study. Eur. Arch. Otorhinolaryngol. 2018, 275, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Volpi, L.; Bignami, M.; Lepera, D.; Karligkiotis, A.; Pistochini, A.; Ottini, G.; Grigioni, E.; Lombardi, D.; Nicolai, P.; Castelnuovo, P. Endoscopic endonasal resection of adenoid cystic carcinoma of the sinonasal tract and skull base. Laryngoscope 2018. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, M.; Haapaniemi, A.; Hagstrom, J.; Juteau, S.; Hernberg, M.; Makitie, A.A.; Vento, S.I. Similar survival outcome after endoscopic and open approaches for sinonasal mucosal melanoma. Rhinology 2019, 57, 132–138. [Google Scholar] [CrossRef]
- Kilic, S.; Kilic, S.S.; Baredes, S.; Chan Woo Park, R.; Mahmoud, O.; Suh, J.D.; Gray, S.T.; Eloy, J.A. Comparison of endoscopic and open resection of sinonasal squamous cell carcinoma: A propensity score-matched analysis of 652 patients. Int. Forum Allergy Rhinol. 2018, 8, 421–434. [Google Scholar] [CrossRef]
- Meccariello, G.; Deganello, A.; Choussy, O.; Gallo, O.; Vitali, D.; De Raucourt, D.; Georgalas, C. Endoscopic nasal versus open approach for the management of sinonasal adenocarcinoma: A pooled-analysis of 1826 patients. Head Neck 2016, 38 (Suppl. 1), E2267–E2274. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.V.; Roberts, D.; Levine, N.B.; DeMonte, F.; Hanna, E.Y.; Kupferman, M.E. Impact of Chemoradiotherapy on CSF Leak Repair after Skull Base Surgery. J. Neurol. Surg. B Skull Base 2014, 75, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Mortuaire, G.; Leroy, X.; Vandenhende-Szymanski, C.; Chevalier, D.; Thisse, A.S. Comparison of endoscopic and external resections for sinonasal instestinal-type adenocarcinoma. Eur. Arch. Otorhinolaryngol. 2016, 273, 4343–4350. [Google Scholar] [CrossRef]
- Xiao, R.; Joshi, R.R.; Husain, Q.; Cracchiolo, J.R.; Lee, N.; Tsai, J.; Yu, Y.; Chen, L.; Kang, J.J.; McBride, S.; et al. Timing of surgery and adjuvant radiation therapy for sinonasal malignancies: Effect of surgical approach. Head Neck 2019, 41, 3551–3563. [Google Scholar] [CrossRef]
- Oker, N.; Verillaud, B.; Wassef, M.; Froelich, S.; Bresson, D.; Kania, R.; Herman, P. Ethmoidal adenocarcinoma treated by exclusive endoscopic approach: Focus on learning curve and modification of management. Head Neck 2018, 40, 126–136. [Google Scholar] [CrossRef]
- Verillaud, B.; Bresson, D.; Sauvaget, E.; Mandonnet, E.; Georges, B.; Kania, R.; Herman, P. Exposure techniques in endoscopic skull base surgery: Posterior septectomy, medial maxillectomy, transmaxillary and transpterygoid approach. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 284–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicaut, E.; Bertrand, B.; Betton, J.L.; Bizon, A.; Briche, D.; Castillo, L.; Lecanu, J.B.; Lindas, P.; Lombard, B.; Malard, O.; et al. Use of a navigation system in endonasal surgery: Impact on surgical strategy and surgeon satisfaction. A prospective multicenter study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Bastier, P.L.; de Gabory, L. Design and assessment of an anatomical diagram for sinonasal malignant tumour resection. Rhinology 2016, 54, 361–367. [Google Scholar] [CrossRef]
- Antognoni, P.; Turri-Zanoni, M.; Gottardo, S.; Molteni, M.; Volpi, L.; Facco, C.; Freguia, S.; Mordacchini, C.; AlQahtani, A.; Bignami, M.; et al. Endoscopic resection followed by adjuvant radiotherapy for sinonasal intestinal-type adenocarcinoma: Retrospective analysis of 30 consecutive patients. Head Neck 2015, 37, 677–684. [Google Scholar] [CrossRef]
- Thariat, J.; Bolle, S.; Demizu, Y.; Marcy, P.Y.; Hu, Y.; Santini, J.; Bourhis, J.; Pommier, P. New techniques in radiation therapy for head and neck cancer: IMRT, CyberKnife, protons, and carbon ions. Improved effectiveness and safety? Impact on survival? Anticancer Drugs 2011, 22, 596–606. [Google Scholar] [CrossRef]
- Fierens, S.; Moya-Plana, A.; Vergez, S.; Benard, A.; Gallard, R.; Molinier-Blossier, S.; Castain, C.; Orsel, S.; Verillaud, B.; Mortuaire, G.; et al. Do practitioners assess sinonasal adenocarcinoma extension similarly? Interdisciplinary concordance in 21 cases. Clin. Otolaryngol. 2021, 46, 665–669. [Google Scholar] [CrossRef]
- Sweeney, A.R.; Walker, B.; Bhrany, A.D.; Chang, S.H.; Jian-Amadi, A. Ophthalmic Changes Following Maxillectomy With or Without Postoperative Radiation Therapy. J. Craniofac. Surg. 2019, 30, 1448–1451. [Google Scholar] [CrossRef]
- Lund, V.J.; Clarke, P.M.; Swift, A.C.; McGarry, G.W.; Kerawala, C.; Carnell, D. Nose and paranasal sinus tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S111–S118. [Google Scholar] [CrossRef]
- NCCN. National Comprehensive Cancer Network. Head Neck Cancers. 2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 19 August 2021).
- Suh, Y.G.; Lee, C.G.; Kim, H.; Choi, E.C.; Kim, S.H.; Kim, C.H.; Keum, K.C. Treatment outcomes of intensity-modulated radiotherapy versus 3D conformal radiotherapy for patients with maxillary sinus cancer in the postoperative setting. Head Neck 2016, 38 (Suppl. 1), E207–E213. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xia, P.; Akazawa, P.; Akazawa, C.; Quivey, J.M.; Verhey, L.J.; Kaplan, M.; Lee, N. Comparison of treatment plans using intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for paranasal sinus carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 158–168. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Lai, C.C.; Ho, C.Y.; Shu, C.H.; Lin, C.Z. Toward a better understanding of sinonasal mucosal melanoma: Clinical review of 23 cases. J. Chin. Med. Assoc. 2007, 70, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.H.; Wang, Z.; Wong, W.W.; Murad, M.H.; Buckey, C.R.; Mohammed, K.; Alahdab, F.; Altayar, O.; Nabhan, M.; Schild, S.E.; et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1027–1038. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Hegenbarth, P.; Timke, C.; Saleh-Ebrahimi, L.; Debus, J.; Roder, F.; Huber, P.E. Intensity modulated radiation therapy (IMRT) for sinonasal tumors: A single center long-term clinical analysis. Radiat. Oncol. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mamgani, A.; Monserez, D.; Rooij, P.; Verduijn, G.M.; Hardillo, J.A.; Levendag, P.C. Highly-conformal intensity-modulated radiotherapy reduced toxicity without jeopardizing outcome in patients with paranasal sinus cancer treated by surgery and radiotherapy or (chemo)radiation. Oral Oncol. 2012, 48, 905–911. [Google Scholar] [CrossRef]
- Fan, M.; Kang, J.J.; Lee, A.; Fan, D.; Wang, H.; Kitpanit, S.; Fox, P.; Sine, K.; Mah, D.; McBride, S.M.; et al. Outcomes and toxicities of definitive radiotherapy and reirradiation using 3-dimensional conformal or intensity-modulated (pencil beam) proton therapy for patients with nasal cavity and paranasal sinus malignancies. Cancer 2020, 126, 1905–1916. [Google Scholar] [CrossRef]
- Frederic-Moreau, T.; Piram, L.; Bellini, R.; Martin, F.; Miroir, J.; Saroul, N.; Pham Dang, N.; Lapeyre, M.; Biau, J. Postoperative volumetric modulated arc therapy for sinonasal cancer: Improved survival compared with 3D conformal radiation therapy. Head Neck 2019, 41, 448–455. [Google Scholar] [CrossRef]
- Liang, Z.G.; Kusumawidjaja, G.; Kazmi, F.; Wee, J.T.S.; Chua, M.L.K. Intensity-modulated radiotherapy for paranasal sinuses and base of skull tumors. Oral Oncol. 2018, 86, 61–68. [Google Scholar] [CrossRef]
- Dirix, P.; Vanstraelen, B.; Jorissen, M.; Vander Poorten, V.; Nuyts, S. Intensity-modulated radiotherapy for sinonasal cancer: Improved outcome compared to conventional radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 998–1004. [Google Scholar] [CrossRef]
- Chi, A.; Nguyen, N.P.; Tse, W.; Sobremonte, G.; Concannon, P.; Zhu, A. Intensity modulated radiotherapy for sinonasal malignancies with a focus on optic pathway preservation. J. Hematol. Oncol. 2013, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thariat, J.; Racadot, S.; Pointreau, Y.; Boisselier, P.; Grange, J.D.; Graff, P.; Weber, D.C. Intensity-modulated radiotherapy of head and neck cancers: Dose effects on the ocular, orbital and eyelid structures. Cancer Radiother. 2016, 20, 467–474. [Google Scholar] [CrossRef]
- Combs, S.E.; Konkel, S.; Schulz-Ertner, D.; Munter, M.W.; Debus, J.; Huber, P.E.; Thilmann, C. Intensity modulated radiotherapy (IMRT) in patients with carcinomas of the paranasal sinuses: Clinical benefit for complex shaped target volumes. Radiat. Oncol. 2006, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Combs, S.E.; Konkel, S.; Thilmann, C.; Debus, J.; Schulz-Ertner, D. Local high-dose radiotherapy and sparing of normal tissue using intensity-modulated radiotherapy (IMRT) for mucosal melanoma of the nasal cavity and paranasal sinuses. Strahlenther. Onkol. 2007, 183, 63–68. [Google Scholar] [CrossRef]
- Chen, A.M.; Daly, M.E.; El-Sayed, I.; Garcia, J.; Lee, N.Y.; Bucci, M.K.; Kaplan, M.J. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 338–343. [Google Scholar] [CrossRef]
- Daly, M.E.; Chen, A.M.; Bucci, M.K.; El-Sayed, I.; Xia, P.; Kaplan, M.J.; Eisele, D.W. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 151–157. [Google Scholar] [CrossRef]
- Wiegner, E.A.; Daly, M.E.; Murphy, J.D.; Abelson, J.; Chapman, C.H.; Chung, M.; Yu, Y.; Colevas, A.D.; Kaplan, M.J.; Fischbein, N.; et al. Intensity-modulated radiotherapy for tumors of the nasal cavity and paranasal sinuses: Clinical outcomes and patterns of failure. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Batth, S.S.; Sreeraman, R.; Dienes, E.; Beckett, L.A.; Daly, M.E.; Cui, J.; Mathai, M.; Purdy, J.A.; Chen, A.M. Clinical-dosimetric relationship between lacrimal gland dose and ocular toxicity after intensity-modulated radiotherapy for sinonasal tumours. Br. J. Radiol. 2013, 86, 20130459. [Google Scholar] [CrossRef] [Green Version]
- Duthoy, W.; Boterberg, T.; Claus, F.; Ost, P.; Vakaet, L.; Bral, S.; Duprez, F.; Van Landuyt, M.; Vermeersch, H.; De Neve, W. Postoperative intensity-modulated radiotherapy in sinonasal carcinoma: Clinical results in 39 patients. Cancer 2005, 104, 71–82. [Google Scholar] [CrossRef]
- Sharma, M.B.; Jensen, K.; Urbak, S.F.; Funding, M.; Johansen, J.; Bechtold, D.; Amidi, A.; Eskildsen, S.F.; Jorgensen, J.O.L.; Grau, C. A multidimensional cohort study of late toxicity after intensity modulated radiotherapy for sinonasal cancer. Radiother. Oncol. 2020, 151, 58–65. [Google Scholar] [CrossRef]
- Huang, T.L.; Chien, C.Y.; Tsai, W.L.; Liao, K.C.; Chou, S.Y.; Lin, H.C.; Dean Luo, S.; Lee, T.F.; Lee, C.H.; Fang, F.M. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 2016, 38 (Suppl. 1), E1026–E1032. [Google Scholar] [CrossRef] [PubMed]
- Dautruche, A.; Bolle, S.; Feuvret, L.; Le Tourneau, C.; Jouffroy, T.; Goudjil, F.; Zefkili, S.; Nauraye, C.; Rodriguez, J.; Herman, P.; et al. Three-year results after radiotherapy for locally advanced sinonasal adenoid cystic carcinoma, using highly conformational radiotherapy techniques proton therapy and/or Tomotherapy. Cancer Radiother. 2018, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Snyers, A.; Janssens, G.O.; Twickler, M.B.; Hermus, A.R.; Takes, R.P.; Kappelle, A.C.; Merkx, M.A.; Dirix, P.; Kaanders, J.H. Malignant tumors of the nasal cavity and paranasal sinuses: Long-term outcome and morbidity with emphasis on hypothalamic-pituitary deficiency. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Di Pede, P.; Guglielmo, M.; Granata, R.; Alfieri, S.; Iacovelli, N.A.; Orlandi, E.; Guzzo, M.; Bianchi, R.; Ferella, L.; et al. Prevalence of Fatigue in Head and Neck Cancer Survivors. Ann. Otol. Rhinol. Laryngol. 2019, 128, 413–419. [Google Scholar] [CrossRef]
- Ferris, M.J.; Zhong, J.; Switchenko, J.M.; Higgins, K.A.; Cassidy, R.J., 3rd; McDonald, M.W.; Eaton, B.R.; Patel, K.R.; Steuer, C.E.; Baddour, H.M., Jr.; et al. Brainstem dose is associated with patient-reported acute fatigue in head and neck cancer radiation therapy. Radiother. Oncol. 2018, 126, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, S.N.; Tran, E.; Ho, C.; Berthelet, E.; Wu, J.; DeVries, K.; LaPointe, V.; Bowman, A.; Lagman, M.; Olson, R. Patient-reported outcome measures in patients undergoing radiotherapy for head and neck cancer. Support. Care Cancer 2021, 29, 2537–2547. [Google Scholar] [CrossRef]
- Gulliford, S.L.; Miah, A.B.; Brennan, S.; McQuaid, D.; Clark, C.H.; Partridge, M.; Harrington, K.J.; Morden, J.P.; Hall, E.; Nutting, C.M. Dosimetric explanations of fatigue in head and neck radiotherapy: An analysis from the parsport Phase III trial. Radiother. Oncol. 2012, 104, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.M.; Dawson, C.; Skoretz, S.A. Late Dysphagia Following Radiotherapy After Nasopharyngeal Carcinoma: A Case Series. Am. J. Speech Lang. Pathol. 2020, 29, 319–326. [Google Scholar] [CrossRef]
- Hakansson, K.; Smulders, B.; Specht, L.; Zhu, M.; Friborg, J.; Rasmussen, J.H.; Bentzen, S.M.; Vogelius, I.R. Radiation dose-painting with protons vs. photons for head-and-neck cancer. Acta Oncol. 2020, 59, 525–533. [Google Scholar] [CrossRef]
- Yu, N.Y.; Gamez, M.E.; Hartsell, W.F.; Tsai, H.K.; Laramore, G.E.; Larson, G.L.; Simone, C.B., 2nd; Rossi, C.; Katz, S.R.; Buras, M.R.; et al. A Multi-Institutional Experience of Proton Beam Therapy for Sinonasal Tumors. Adv. Radiat. Oncol. 2019, 4, 689–698. [Google Scholar] [CrossRef]
- Orlandi, E.; Iacovelli, N.A.; Cavallo, A.; Resteghini, C.; Gandola, L.; Licitra, L.; Bossi, P. Could the extreme conformality achieved with proton therapy in paranasal sinuses cancers accidentally results in a high rate of leptomeningeal progression? Head Neck 2019, 41, 3733–3735. [Google Scholar] [CrossRef]
- Toyomasu, Y.; Demizu, Y.; Matsuo, Y.; Sulaiman, N.S.; Mima, M.; Nagano, F.; Terashima, K.; Tokumaru, S.; Hayakawa, T.; Daimon, T.; et al. Outcomes of Patients With Sinonasal Squamous Cell Carcinoma Treated With Particle Therapy Using Protons or Carbon Ions. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Kato, T.; Ono, T.; Oyama, S.; Komori, S.; Arai, K.; Abe, Y.; Harada, T.; Nakamura, T.; Wada, H.; et al. Effect of anatomical change on dose distribution during radiotherapy for maxillary sinus carcinoma: Passive scattering proton therapy versus volumetric-modulated arc therapy. Br. J. Radiol. 2019, 92, 20180273. [Google Scholar] [CrossRef]
- Nenoff, L.; Matter, M.; Hedlund Lindmar, J.; Weber, D.C.; Lomax, A.J.; Albertini, F. Daily adaptive proton therapy—The key to innovative planning approaches for paranasal cancer treatments. Acta Oncol. 2019, 58, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Dagan, R.; Bryant, C.; Li, Z.; Yeung, D.; Justice, J.; Dzieglewiski, P.; Werning, J.; Fernandes, R.; Pirgousis, P.; Lanza, D.C.; et al. Outcomes of Sinonasal Cancer Treated With Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 377–385. [Google Scholar] [CrossRef]
- Ferella, L.; Cavallo, A.; Miceli, R.; Iacovelli, N.A.; Giandini, T.; Pignoli, E.; Calareso, G.; Bossi, P.; Resteghini, C.; Gravina, G.L.; et al. Prognostic role of primary tumor, nodal neck, and retropharyngeal GTVs for unresectable sinonasal cancers treated with IMRT and chemotherapy. Tumori J. 2020, 106, 39–46. [Google Scholar] [CrossRef]
- Leung, W.S.; Wu, V.W.C.; Liu, C.Y.W.; Cheng, A.C.K. A dosimetric comparison of the use of equally spaced beam (ESB), beam angle optimization (BAO), and volumetric modulated arc therapy (VMAT) in head and neck cancers treated by intensity modulated radiotherapy. J. Appl. Clin. Med. Phys. 2019, 20, 121–130. [Google Scholar] [CrossRef]
- Zhong-Hua, N.; Jing-Ting, J.; Xiao-Dong, L.; Jin-Ming, M.; Jun-Chong, M.; Jian-Xue, J.; Ming, G.; Qi-Lin, L.; Wen-Dong, G.; Lu-Jun, C.; et al. Coplanar VMAT vs. noncoplanar VMAT in the treatment of sinonasal cancer. Strahlenther. Onkol. 2015, 191, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, S.W.; Kwak, J.; Cho, I.; Yoon, S.M.; Kim, J.H.; Park, J.H.; Choi, E.K.; Song, S.Y.; Kim, Y.S.; et al. A dosimetric comparison of volumetric modulated arc therapy (VMAT) and non-coplanar intensity modulated radiotherapy (IMRT) for nasal cavity and paranasal sinus cancer. Radiat. Oncol. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.Y.; Zhang, J.Y.; Li, M.; Cheung, M.L.; Li, Y.K.; Zheng, J.; Huang, B.T.; Zhang, W.Z. A simple optimization approach for improving target dose homogeneity in intensity-modulated radiotherapy for sinonasal cancer. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Madani, I.; Duprez, F.; Boterberg, T.; Van de Wiele, C.; Bonte, K.; Deron, P.; De Gersem, W.; Coghe, M.; De Neve, W. Maximum tolerated dose in a phase I trial on adaptive dose painting by numbers for head and neck cancer. Radiother. Oncol. 2011, 101, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, L.A.; Berwouts, D.; Madani, I.; De Gersem, W.; Vercauteren, T.; Duprez, F.; De Neve, W. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother. Oncol. 2014, 111, 348–353. [Google Scholar] [CrossRef]
- Olteanu, L.A.M.; Duprez, F.; De Neve, W.; Berwouts, D.; Vercauteren, T.; Bauters, W.; Deron, P.; Huvenne, W.; Bonte, K.; Goethals, I.; et al. Late mucosal ulcers in dose-escalated adaptive dose-painting treatments for head-and-neck cancer. Acta Oncol. 2018, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Berwouts, D.; Madani, I.; Duprez, F.; Olteanu, A.L.; Vercauteren, T.; Boterberg, T.; Deron, P.; Bonte, K.; Huvenne, W.; De Neve, W.; et al. Long-term outcome of (18) F-fluorodeoxyglucose-positron emission tomography-guided dose painting for head and neck cancer: Matched case-control study. Head Neck 2017, 39, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Bayatiani, M.R.; Hamidi, S.; Kargaran, M. Investigating the Effect of Air Cavities of Sinuses on the Radiotherapy Dose Distribution Using Monte Carlo Method. J. Biomed. Phys. Eng. 2019, 9, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Rev. Mutat. Res. 2016, 770, 369–386. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; de Vincentiis, M.; Valentini, V.; Musio, D.; Mezi, S.; Lo Mele, L.; Terenzi, V.; D’Aguanno, V.; Cassoni, A.; Di Brino, M.; et al. Follow-up program in head and neck cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.N.; Lee, J.T.; Wang, M.B.; Suh, J.D. Treatment delays in surgically managed sinonasal cancer and association with survival. Laryngoscope 2020, 130, 2–11. [Google Scholar] [CrossRef]
- Leleu, T.; Bastit, V.; Dore, M.; Kammerer, E.; Florescu, C.; Alfonsi, M.; Troussier, I.; Bensadoun, R.J.; Biau, J.; Blais, E.; et al. Histosurgical mapping of endoscopic endonasal surgery of sinonasal tumours to improve radiotherapy guidance. Cancer Radiother. 2021. [Google Scholar] [CrossRef]
- Sander, I.M.; Liepert, T.T.; Doney, E.L.; Leevy, W.M.; Liepert, D.R. Patient Education for Endoscopic Sinus Surgery: Preliminary Experience Using 3D-Printed Clinical Imaging Data. J. Funct. Biomater. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licitra, L.; Resteghini, C.; Bossi, P. The Evolving Role of Systemic Therapy in the Primary Treatment of Sinonasal Cancer. Adv. Otorhinolaryngol. 2020, 84, 78–86. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Wang, K.; Wu, R.; Zhang, Y.; Huang, X.; Zhang, S.; Xiao, J.; Yi, J.; Gao, L.; et al. The value of preoperative radiotherapy in the treatment of locally advanced nasal cavity and paranasal sinus squamous cell carcinoma: A single institutional experience. Oral. Oncol. 2020, 101, 104512. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Chin, C.J.; Xu, W.; Che, J.; Huang, S.H.; Monteiro, E.; Alghonaim, Y.; Ringash, J.; Witterick, I.J. Impact of neoadjuvant radiation on margins for non-squamous cell carcinoma sinonasal malignancies. Laryngoscope 2018, 128, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Perrone, F.; Bossi, P.; Cortelazzi, B.; Locati, L.; Quattrone, P.; Pierotti, M.A.; Pilotti, S.; Licitra, L. TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma. J. Clin. Oncol. 2010, 28, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Abdelmeguid, A.S.; Watcherporn, T.; Takahashi, H.; Tam, S.; Bell, D.; Ferrarotto, R.; Glisson, B.; Kupferman, M.E.; Roberts, D.B.; et al. Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma. J. Clin. Oncol. 2019, 37, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Fitzek, M.M.; Thornton, A.F.; Varvares, M.; Ancukiewicz, M.; McIntyre, J.; Adams, J.; Rosenthal, S.; Joseph, M.; Amrein, P. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer 2002, 94, 2623–2634. [Google Scholar] [CrossRef]
- Modesto, A.; Blanchard, P.; Tao, Y.G.; Rives, M.; Janot, F.; Serrano, E.; Benlyazid, A.; Guigay, J.; Ferrand, F.R.; Delord, J.P.; et al. Multimodal treatment and long-term outcome of patients with esthesioneuroblastoma. Oral. Oncol. 2013, 49, 830–834. [Google Scholar] [CrossRef]
- Hermsen, M.A.; Riobello, C.; Garcia-Marin, R.; Cabal, V.N.; Suarez-Fernandez, L.; Lopez, F.; Llorente, J.L. Translational genomics of sinonasal cancers. Semin. Cancer Biol. 2020, 61, 101–109. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thariat, J.; Carsuzaa, F.; Marcy, P.Y.; Verillaud, B.; de Gabory, L.; Ferrand, F.R. Precision Postoperative Radiotherapy in Sinonasal Carcinomas after Endonasal Endoscopic Surgery. Cancers 2021, 13, 4802. https://doi.org/10.3390/cancers13194802
Thariat J, Carsuzaa F, Marcy PY, Verillaud B, de Gabory L, Ferrand FR. Precision Postoperative Radiotherapy in Sinonasal Carcinomas after Endonasal Endoscopic Surgery. Cancers. 2021; 13(19):4802. https://doi.org/10.3390/cancers13194802
Chicago/Turabian StyleThariat, Juliette, Florent Carsuzaa, Pierre Yves Marcy, Benjamin Verillaud, Ludovic de Gabory, and Francois Regis Ferrand. 2021. "Precision Postoperative Radiotherapy in Sinonasal Carcinomas after Endonasal Endoscopic Surgery" Cancers 13, no. 19: 4802. https://doi.org/10.3390/cancers13194802





