Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Cancer Cell Cultures
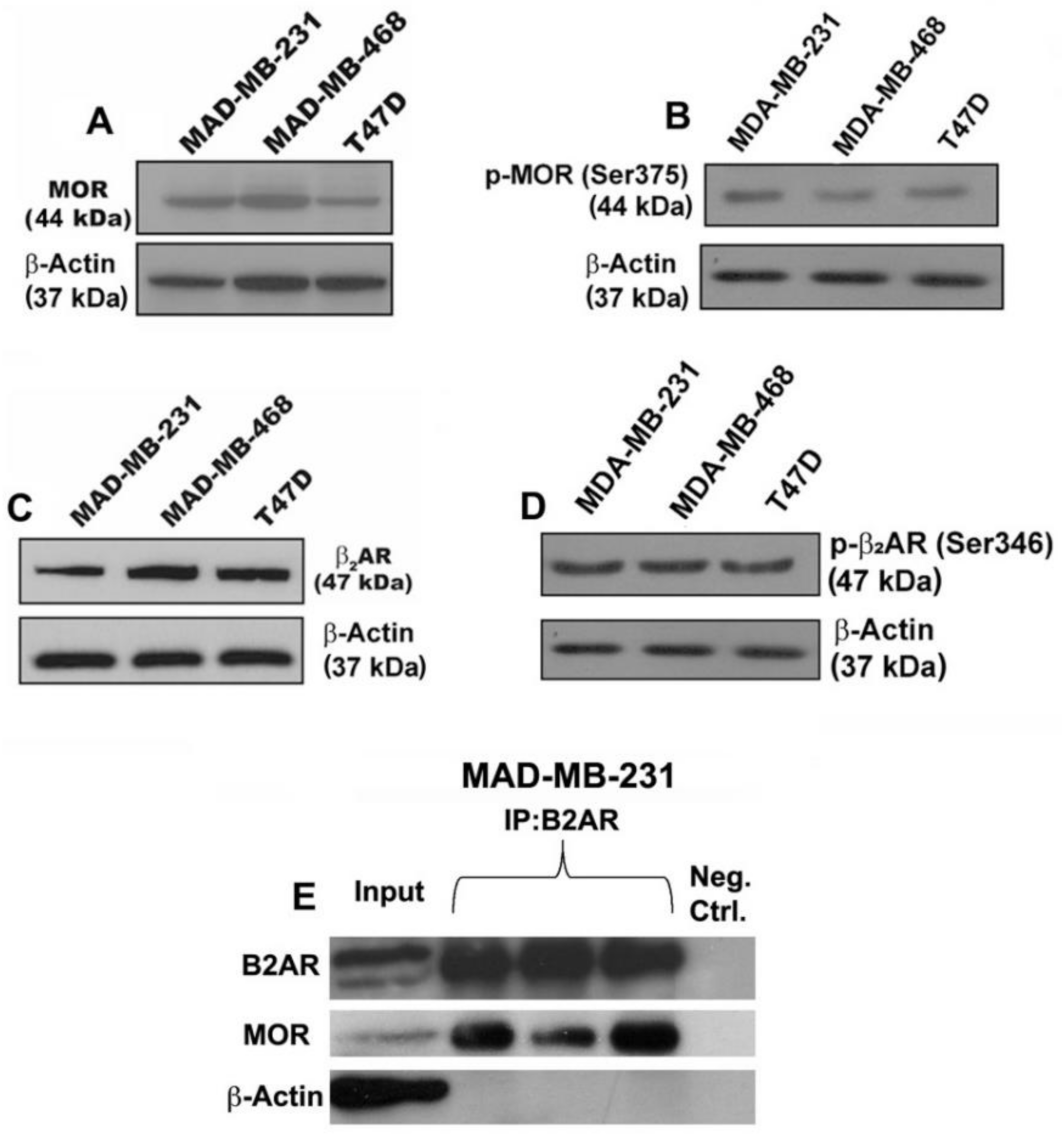
2.2. Immunoprecipitation and Immunoblotting Analysis of Beta-Adrenergic and Opioid Receptors
2.3. Cell Viability, Colony Formation, Migration, and Invasion Assays
2.4. Cell Cycle Analysis
2.5. Animal Maintenance
2.6. Subcutaneous Xenograft Experiments
2.7. Histopathology and Immunohistochemistry
2.8. Immunoblotting Detection of Proteins from Immune Cells
2.9. Enrichment of NK Cells from Spleen and PBMCs and the NK Cell Cytolytic Assay
2.10. Flow Cytometry Analysis of Immune Cells
2.11. Cytokine Multiplex Immunoassay
2.12. Statistical Analysis
3. Results
3.1. Verification That Breast Cancer Cells Contain Beta-Adrenergic and Opioidergic Receptors
3.2. Effects of Beta-Adrenergic and Opioidergic Drugs Alone or in Combination on Cancer Cell Viability, Clonogenicity, Migration, and Invasion
3.3. Effects on Cell Cycle Changes in Cancer Cells
3.4. Effects on Apoptotic Regulatory Protein Levels and Signaling Pathway in Cancer Cells
3.5. Effect on the Growth of Cell Line-Derived Tumor Xenograft
3.6. Effect on Cell Mitosis, Cell Proliferation, Cellular Apoptosis, and Epithelial–Mesenchymal Transition in Tumor Xenograft
3.7. Effects of Drugs on Immune Cell Functions in the Tumor Xenograft Host
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 3 August 2021).
- Hwang, K.T.; Kim, J.; Jung, J.; Chang, J.H.; Chai, Y.J.; Oh, S.W.; Oh, S.; Kim, Y.A.; Park, S.B.; Hwang, K.R. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: A population-based study using SEER database. Clin. Cancer Res. 2019, 25, 1970–1979. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.; Fleming, J.; Robinson, W.R.; Kirk, E.L.; Perou, C.M.; Troester, M.A. Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res. Treat. 2015, 152, 437–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune infiltrates in the breast cancer microenvironment detection, characterization and clinical implication. Breast Cancer 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.I.; Tonelli, L.; Sternberg, E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002, 20, 125–163. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Cretney, E.; Kershaw, M.H.; Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 2004, 202, 275–293. [Google Scholar] [CrossRef]
- Herberman, R.B. Possible role of natural killer cells and other effector cells in immune surveillance against cancer. J. Investig. Dermatol. 1984, 83, 137s–140s. [Google Scholar] [CrossRef] [Green Version]
- Lavandero, S.; Donoso, E.; Sapag-Hagar, M. Beta-adrenergic receptors in rat mammary gland. Biochem. Pharmacol. 1985, 34, 2034–2036. [Google Scholar] [CrossRef]
- Vandewalle, B.; Revillion, F.; Lefebvre, J. Functional beta-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 1990, 116, 303–306. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [Green Version]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population-based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef] [Green Version]
- Barron, T.I.; Sharp, L.; Visvanathan, K. Beta-adrenergic blocking drugs in breast cancer: A perspective review. Ther. Adv. Med. Oncol. 2012, 4, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Lutz, P.E.; Kieffer, B.L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013, 36, 195–206. [Google Scholar] [CrossRef] [Green Version]
- De Kloet, E.R.; Reul, J.M. Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 1987, 12, 83–105. [Google Scholar] [CrossRef]
- Kreek, M.J. Drug addictions: Molecular and cellular endpoints. Ann. N. Y. Acad. Sci. 2001, 937, 1749–6632. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Enoch, M.A.; Acheson, A.; Cohoon, A.J.; Sorocco, K.H.; Hodgkinson, C.A.; Vincent, A.S.; Glahn, D.C.; Goldman, D. Cortisol stress response in men and women modulated differentially by the mu-opioid receptor gene polymorphism OPRM1 A118G. Neuropsychopharmacology 2015, 40, 2546–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieślińska, A.; Sienkiewicz-Szłapka, E.; Kostyra, E.; Fiedorowicz, E.; Snarska, J.; Wroński, K.; Tenderenda, M.; Jarmołowska, B.; Matysiewicz, M. μ-Opioid receptor gene (OPRM1) polymorphism in patients with breast cancer. Tumour Biol. 2015, 36, 4655–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin-Fenton, D. Opioids and breast cancer recurrence. Curr. Opin. Support. Palliat. Care 2019, 13, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.D.; Fernandes, B.J.D. Low Doses Naltrexone: The Potential Benefit Effects for its Use in Patients with Cancer. Curr. Drug Res. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.A.; Trapaidze, N.; Gomes, I.; Nivarthi, R.; Devi, L.A. Oligomerization of opioid receptors with beta 2-adrenergic receptors: A role in trafficking and mitogen-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 2001, 98, 343–348. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.K.; Sengupta, A.; Zhang, C.; Boyadjieva, N.; Murugan, S. Opiate antagonist prevents μ- and δ-opiate receptor dimerization to facilitate ability of agonist to control ethanol-altered natural killer cell functions and mammary tumor growth. J. Biol. Chem. 2012, 287, 16734–16747. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, e51046. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Murugan, S.; Boyadjieva, N.; Jabbar, S.; Shrivastava, P.; Sarkar, D.K. Beta-endorphin cell therapy for cancer prevention. Cancer Prev. Res. 2015, 8, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanstructured lipid carrier on MDAMBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, L.C.; Waterhouse, N.J. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Mezzanotte, L.; Fazzina, R.; Michelini, E.; Tonelli, R.; Pession, A.; Branchini, B.; Roda, A. In vivo bioluminescence imaging of murine xenograft cancer models with a red-shifted thermostable luciferase. Mol. Imaging Biol. 2010, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, N.M.; Sestelo, M.; Meira-Machado, L. A method for determining groups in multiple survival curves. Stat. Med. 2019, 38, 866–877. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, L.; Polák, S.; Varga, I. Ki67, PCNA and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta. Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Felipe Lima, J.; Nofech-Mozes, S.; Bayani, J.; Bartlett, J.M. EMT in breast carcinoma—A review. J. Clin. Med. 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hougen, H.P. The athymic nude rat. Immunobiological characteristics with special reference to establishment of non-antigen-specific T-cell reactivity and induction of antigen-specific immunity. APMIS Suppl. 1991, 21, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Fasoulakis, Z.; Kolios, G.; Papamanolis, V.; Kontomanolis, E.N. Interleukins associated with breast cancer. Cureus 2018, 10, e3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef] [Green Version]
- Montoya, A.; Amaya, C.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, R.P.; Pepe, S.; Spurgeon, H.A.; Capogrossi, M.C.; Lakatta, E.G. Opioid peptide receptor stimulation reverses beta-adrenergic effects in rat heart cells. Am. J. Physiol. 1997, 272, H797–H805. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Cascella, M.; Rea, D.; Palma, G.; Del Vecchio, V.; Forte, C.A.; Del Prato, F.; Arra, C.; Cuomo, A. The effects of naloxone on human breast cancer progression: In vitro and in vivo studies on MDA.MB231 cells. Onco. Targets Ther. 2018, 11, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Boyadjieva, N.I.; Chaturvedi, K.; Poplawski, M.M.; Sarkar, D.K. Opioid antagonist naltrexone disrupts feedback interaction between mu and delta opioid receptors in splenocytes to prevent alcohol inhibition of NK cell function. J. Immunol. 2004, 173, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed. Pharmacother. 2018, 104, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hollmén, M.; Karaman, S.; Schwager, S.; Lisibach, A.; Christiansen, A.J.; Maksimow, M.; Varga, Z.; Jalkanen, S.; Detmar, M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 2015, 5, e1115177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 70, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partecke, L.I.; Speerforck, S.; Käding, A.; Seubert, F.; Kühn, S.; Lorenz, E.; Schwandke, S.; Sendler, M.; Keßler, W.; Trung, D.N.; et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonized by beta-adrenergic receptor blockade. Pancreatology 2016, 16, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Parkitny, L.; Younger, J. Reduced pro-inflammatory cytokines after eight weeks of low-dose naltrexone for fibromyalgia. Biomedicines 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [Green Version]
- Sica, A. Role of tumour-associated macrophages in cancer-related inflammation. Exp. Oncol. 2010, 32, 153–158. [Google Scholar] [PubMed]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative beta-blockade with propranolol reduces biomarkers of metastasis in breast cancer: A phase II randomized trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.J.; Powell, N.D.; Reader, B.F.; Bhave, N.S.; Roloson, A.L.; Carson, W.E., 3rd; Sheridan, J.F. Beta-adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain Behav. Immun. 2012, 26, 1226–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugan, S.; Rousseau, B.; Sarkar, D.K. Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer. Cancers 2021, 13, 4858. https://doi.org/10.3390/cancers13194858
Murugan S, Rousseau B, Sarkar DK. Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer. Cancers. 2021; 13(19):4858. https://doi.org/10.3390/cancers13194858
Chicago/Turabian StyleMurugan, Sengottuvelan, Bénédicte Rousseau, and Dipak K. Sarkar. 2021. "Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer" Cancers 13, no. 19: 4858. https://doi.org/10.3390/cancers13194858
APA StyleMurugan, S., Rousseau, B., & Sarkar, D. K. (2021). Beta 2 Adrenergic Receptor Antagonist Propranolol and Opioidergic Receptor Antagonist Naltrexone Produce Synergistic Effects on Breast Cancer Growth Prevention by Acting on Cancer Cells and Immune Environment in a Preclinical Model of Breast Cancer. Cancers, 13(19), 4858. https://doi.org/10.3390/cancers13194858





