Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
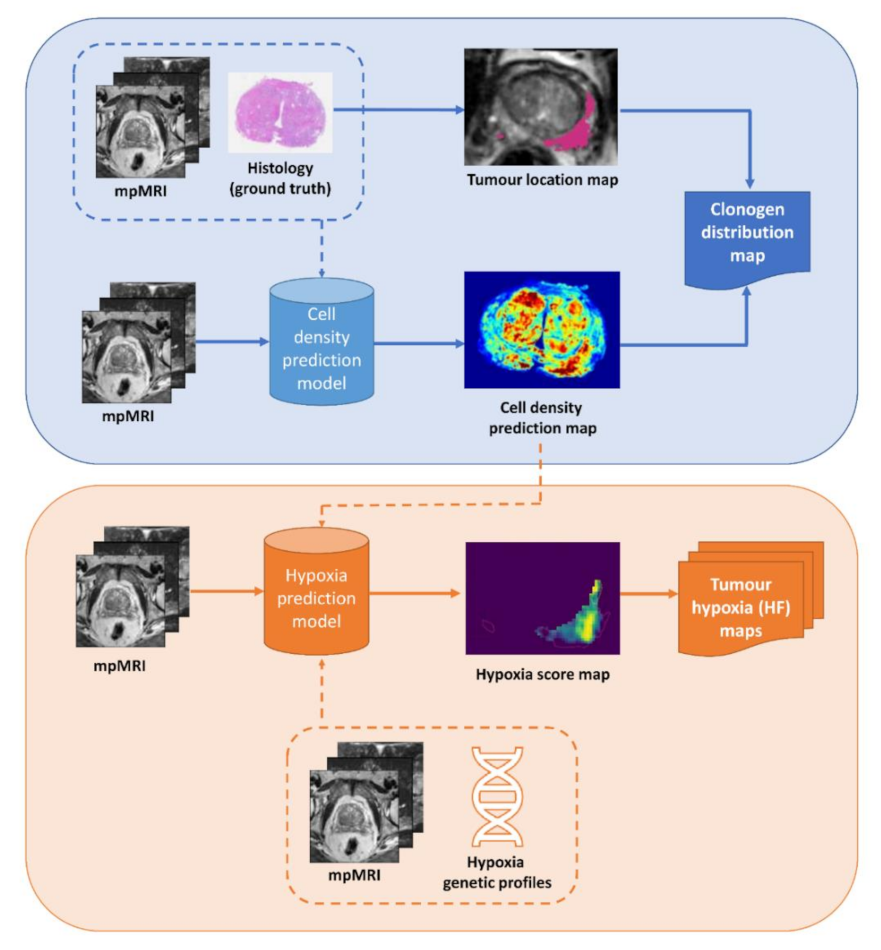
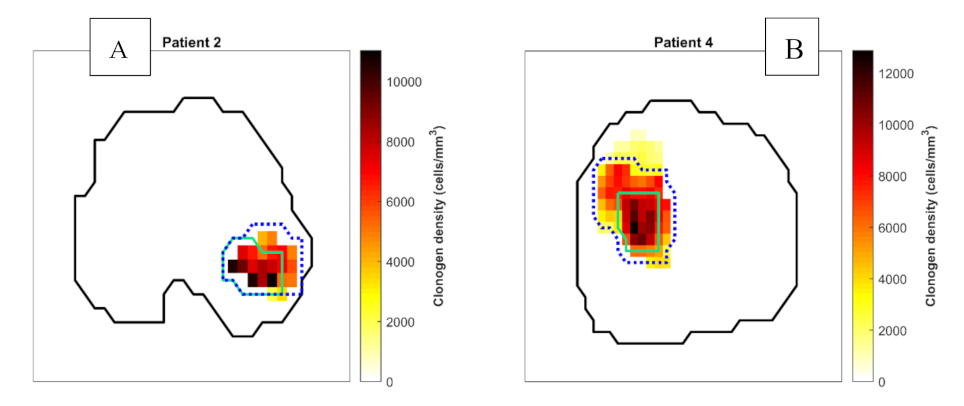
2.1. Tumour Biology Maps
2.2. TCP Model
2.3. Treatment Planning
2.3.1. Method 1: Uniform-Dose Planning
2.3.2. Method 2: Focal Tumour DE
2.3.3. Method 3: Focal Tumour + Hypoxia DE
2.3.4. Method 4: Biologically Targeted Radiotherapy Approach
2.4. Aim 1: Effect of Hypoxia on <TCP> of Dose-Based Planning Methods
2.5. Aim 2: Comparison of Normal Tissue Effects in Focal DE and Biologically Optimised Plans for Targeting Tumour Hypoxia
2.6. Sensitivity to Oxygen Enhancement Ratio (OER)
2.7. Statistical Analysis
3. Results
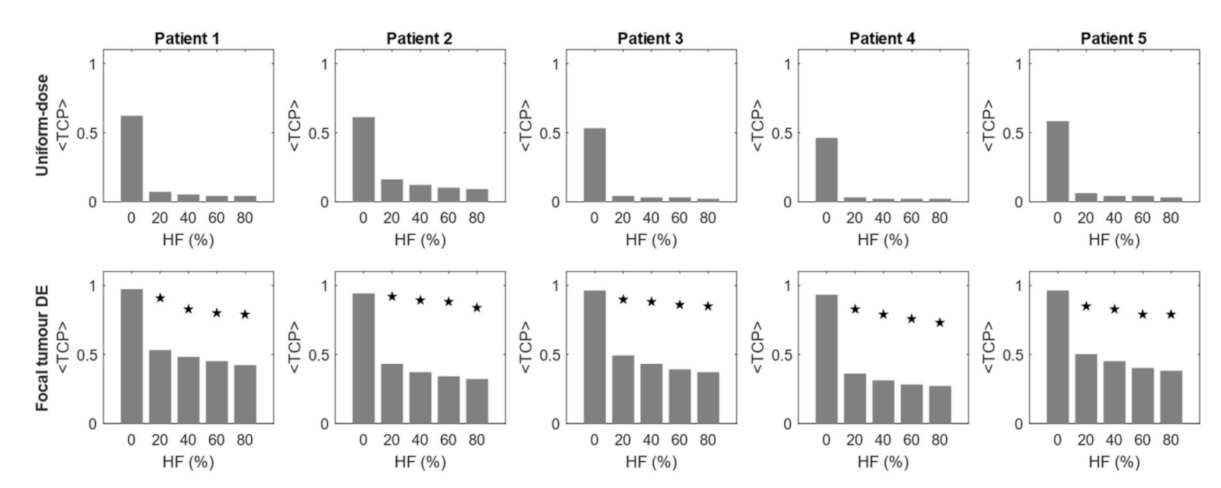
3.1. Effect of Hypoxia on <TCP> of Dose-Based Planning Methods
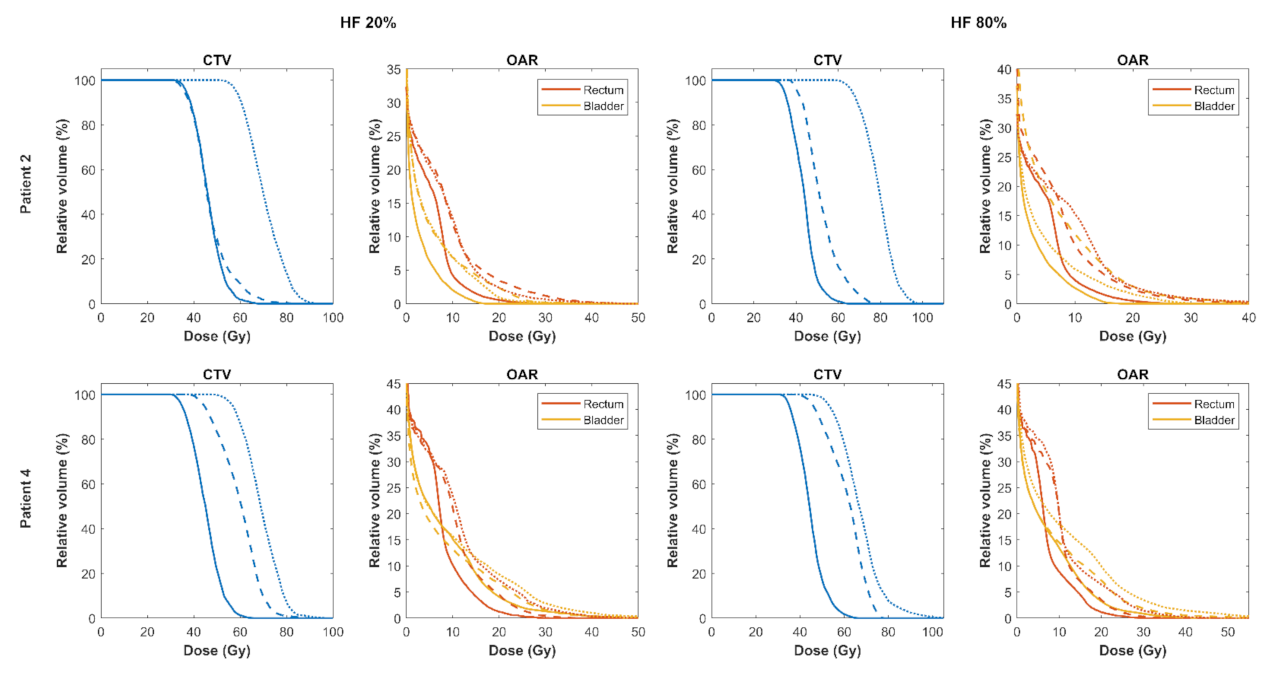
3.2. Comparison of Focal DE and Biologically Optimised Plans for Targeting Tumour Hypoxia
3.3. Sensitivity of Robust Biological Optimisation to OER
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milosevic, M.; Warde, P.; Ménard, C.; Chung, P.; Toi, A.; Ishkanian, A.; McLean, M.; Pintilie, M.; Sykes, J.; Gospodarowicz, M.; et al. Tumor Hypoxia Predicts Biochemical Failure following Radiotherapy for Clinically Localized Prostate Cancer. Clin. Cancer Res. 2012, 18, 2108–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popple, R.A.; Ove, R.; Shen, S. Tumor control probability for selective boosting of hypoxic subvolumes, including the effect of reoxygenation. Int. J. Radiat. Oncol. 2002, 54, 921–927. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; Zijp, J.V.D.V.V.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; Zyp, J.V.D.V.V.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, G.M.; Peters, L.J. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int. J. Radiat Oncol. Biol. Phys. 1999, 44, 747–748. [Google Scholar] [PubMed]
- Her, E.J.; Haworth, A.; Rowshanfarzad, P.; Ebert, M.A. Progress towards Patient-Specific, Spatially-Continuous Radiobiological Dose Prescription and Planning in Prostate Cancer IMRT: An Overview. Cancers 2020, 12, 854. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Reynolds, H.M.; Parameswaran, B.; Wraith, D.; Finnegan, M.E.; Williams, S.; Haworth, A. Multiparametric MRI and radi-omics in prostate cancer: A review. Australas Phys. Eng. Sci. Med. 2019, 42, 3–25. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Williams, S.; Jackson, P.; Mitchell, C.; Hofman, M.; Hicks, R.J.; Murphy, D.G.; Haworth, A. Voxel-wise correlation of positron emission tomography/computed tomography with multiparametric magnetic resonance imaging and histology of the prostate using a sophisticated registration framework. BJU Int. 2019, 123, 1020–1030. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Williams, S.G.; Zhang, A.; Chakravorty, R.; Rawlinson, D.; Ong, C.S.; Esteva, M.; Mitchell, C.; Parameswaran, B.; Finnegan, M.; et al. Development of a registration framework to validate MRI with histology for prostate focal therapy. Med. Phys. 2015, 42, 7078–7089. [Google Scholar] [CrossRef]
- Sun, Y.; Williams, S.; Byrne, D.; Keam, S.; Reynolds, H.M.; Mitchell, C.; Wraith, D.; Murphy, D.; Haworth, A. As-sociation analysis between quantitative MRI features and hypoxia-related genetic profiles in prostate cancer. A Pilot Study. Br. J. Radiol. 2019, 92, 20190373. [Google Scholar]
- Sun, Y.; Reynolds, H.M.; Wraith, D.; Williams, S.; Finnegan, M.E.; Mitchell, C.; Murphy, D.; Haworth, A. Voxel-wise prostate cell den-sity prediction using multiparametric magnetic resonance imaging and machine learning. Acta Oncol. (Madr). 2018, 57, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Guerrero, M.; Li, X. How low is the α/β ratio for prostate cancer? Int. J. Radiat. Oncol. 2003, 55, 194–203. [Google Scholar] [CrossRef]
- Magliaro, C.; Mattei, G.; Iacoangeli, F.; Corti, A.; Piemonte, V.; Ahluwalia, A. Oxygen Consumption Characteristics in 3D Con-structs Depend on Cell Density. Front. Bioeng Biotechnol. 2019, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Haworth, A.; Ebert, M.; Waterhouse, D.; Joseph, D.; Duchesne, G. Assessment of i-125 prostate implants by tumor bioeffect. Int. J. Radiat. Oncol. 2004, 59, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Li, X.A.; Mayr, N.A. Dose escalation to combat hypoxia in prostate cancer: A radiobiological study on clinical data. Br. J. Radiol. 2006, 79, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J. Fractionation and late rectal toxicity. Int. J. Radiat. Oncol. 2004, 60, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rogers, K. Radiobiological Impact of Acuros XB Dose Calculation Algorithm on Low-Risk Prostate Cancer Treatment Plans Created by RapidArc Technique. Austral-Asian J. Cancer 2012, 11, 261–269. [Google Scholar]
- Stewart, F.A.; Randhawa, V.S.; Michael, B.D. Multifraction irradiation of mouse bladders. Radiother. Oncol. 1984, 2, 131–140. [Google Scholar] [CrossRef]
- Alasti, H.; Cho, Y.-B.; Catton, C.; Berlin, A.; Chung, P.; Bayley, A.; Vandermeer, A.; Kong, V.; Jaffray, D. Evaluation of high dose volu-metric CT to reduce inter-observer delineation variability and PTV margins for prostate cancer radiotherapy. Radiother On-Col. 2017, 125, 118–123. [Google Scholar] [CrossRef]
- Kron, T.; Thomas, J.; Fox, C.; Thompson, A.; Owen, R.; Herschtal, A.; Haworth, A.; Tai, K.-H.; Foroudi, F. Intra-fraction prostate dis-placement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother. Oncol. 2010, 95, 191–197. [Google Scholar] [CrossRef]
- Quon, H.; Loblaw, D.; Cheung, P.; Holden, L.; Tang, C.; Pang, G.; Morton, G.; Mamedov, A.; Deabreu, A. Intra-fraction Motion during Extreme Hypofractionated Radiotherapy of the Prostate using Pre- and Post-treatment Imaging. Clin. Oncol. 2012, 24, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, E.; Mairani, A.; Ziegenhein, P.; Jäkel, O.; Bangert, M. matRad-a multi-modality open source 3D treatment planning toolkit. In XXVI Brazilian Congress on Biomedical Engineering; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; Volume 51, pp. 1608–1611. [Google Scholar] [CrossRef]
- Wieser, H.-P.; Cisternas, E.; Wahl, N.; Ulrich, S.; Stadler, A.; Mescher, H.; Müller, L.-R.; Klinge, T.; Gabryś, H.; Burigo, L.; et al. Development of the open-source dose calculation and optimization toolkit matRad. Med. Phys. 2017, 44, 2556–2568. [Google Scholar] [CrossRef] [PubMed]
- Unkelbach, J.; Alber, M.; Bangert, M.; Bokrantz, R.; Chan, T.C.Y.; Deasy, J.O.; Fredriksson, A.; Gorissen, B.L.; van Herk, M.; Liu, W.; et al. Robust radiotherapy planning. Phys. Med. Biol. 2018, 63, e22TR02. [Google Scholar] [CrossRef] [Green Version]
- de Boer, H.C.; Heijmen, B.J. A protocol for the reduction of systematic patient setup errors with minimal portal imaging work-load. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1350–1365. [Google Scholar] [CrossRef]
- Gordon, J.J.; Siebers, J.V. Evaluation of dosimetric margins in prostate IMRT treatment plans. Med. Phys. 2008, 35, 569–575. [Google Scholar] [CrossRef]
- Witte, M.G.; Sonke, J.-J.; Siebers, J.; Deasy, J.O.; Van Herk, M. Beyond the margin recipe: The probability of correct target dosage and tumor control in the presence of a dose limiting structure. Phys. Med. Biol. 2017, 62, 7874–7888. [Google Scholar] [CrossRef]
- Dean, C.J.; Sykes, J.R.; Cooper, R.A.; Hatfield, P.; Carey, B.; Swift, S.; Bacon, S.E.; Thwaites, D.; Sebag-Montefiore, D.; Morgan, A.M. An evaluation of four CT–MRI co-registration techniques for radiotherapy treatment planning of prone rectal cancer patients. Br. J. Radiol. 2012, 85, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.D.; Dinh, C.V.; Steggerda, M.J.; ter Beek, L.C.; Smolic, M.; van Buuren, L.D.; Pos, F.J.; van der Heide, U.A. Prostate fiducial marker detection with the use of multi-parametric magnetic resonance imaging. Phys. Imaging Radiat. Oncol. 2017, 1, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.; Damyanovich, A.; Haycocks, T.; Haider, M.; Bayley, A.; Catton, C. Magnetic resonance imaging in the radiation treatment planning of localized prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother. Oncol. 2003, 66, 217–224. [Google Scholar] [CrossRef]
- Sun, Y.; Reynolds, H.; Wraith, D.; Williams, S.; Finnegan, M.E.; Mitchell, C.; Murphy, D.; Ebert, M.; Haworth, A. Predicting prostate tumour location from multiparametric MRI using Gaussian kernel support vector machines: A preliminary study. Australas. Phys. Eng. Sci. Med. 2017, 40, 39–49. [Google Scholar] [CrossRef]
- Her, E.J.; Reynolds, H.M.; Mears, C.; Williams, S.; Moorehouse, C.; Millar, J.; Ebert, M.; Haworth, A. Radiobiological parameters in a tumour control probability model for prostate cancer LDR brachytherapy. Phys. Med. Biol. 2018, 63, e135011. [Google Scholar] [CrossRef]
- Witte, M.G.; van der Geer, J.; Schneider, C.; Lebesque, J.V.; Alber, M.; van Herk, M. IMRT optimization including random and sys-tematic geometric errors based on the expectation of TCP and NTCP: Optimization of expected TCP and NTCP. Med. Phys. 2007, 34, 3544–3555. [Google Scholar] [CrossRef]
- Niemierko, A. A generalized concept of equivalent uniform dose (EUD). Med. Phys. 1999, 26, e1100. [Google Scholar]
- Her, E.J.; Ebert, M.A.; Kennedy, A.; Reynolds, H.M.; Sun, Y.; Williams, S.; Haworth, A. Standard versus hypofractionated intensi-ty-modulated radiotherapy for prostate cancer: Assessing the impact on dose modulation and normal tissue effects when us-ing patient-specific cancer biology. Phys. Med. Biol. 2021, 66, e045007. [Google Scholar] [CrossRef]
- Haworth, A.; Mears, C.; Betts, J.; Reynolds, H.; Tack, G.; Leo, K.; Williams, S.; Ebert, M. A radiobiology-based inverse treatment planning method for optimisation of permanent l-125 prostate implants in focal brachytherapy. Phys. Med. Biol. 2015, 61, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Barendsen, G.W.; Koot, C.J.; van Kersen, G.R.; Bewley, D.K.; Field, S.B.; Parnell, C.J. The Effect of Oxygen on Impairment of the Prolif-erative Capacity of Human Cells in Culture by Ionizing Radiations of Different LET. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1966, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Broerse, J.J. Survival of Cultured Human Cells after Irradiation with Fast Neutrons of Different Energies in Hypoxic and Oxy-genated Conditions. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1968, 13, 559–572. [Google Scholar] [CrossRef]
- Carlson, D.J.; Keall, P.J.; Loo, B.W.; Chen, Z.J.; Brown, J.M. Hypofractionation results in reduced tumor cell kill compared to conven-tional fractionation for tumors with regions of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, I.; Peschke, P.; Karger, C.P. A voxel-based multiscale model to simulate the radiation response of hypoxic tumors. Med. Phys. 2014, 42, 90–102. [Google Scholar] [CrossRef]
- Jeong, J.; Shoghi, K.I.; Deasy, J.O. Modelling the interplay between hypoxia and proliferation in radiotherapy tumour response. Phys. Med. Biol. 2013, 58, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Ruggieri, R.; Naccarato, S.; Nahum, A. Severe hypofractionation: Non-homogeneous tumour dose delivery can counteract tumour hypoxia. Acta Oncol. 2010, 49, 1304–1314. [Google Scholar] [CrossRef]
- Thorwarth, D.; Eschmann, S.-M.; Paulsen, F.; Alber, M. A Model of Reoxygenation Dynamics of Head-And-Neck Tumors Based on Serial 18F-Fluoromisonidazole Positron Emission Tomography Investigations. Int. J. Radiat. Oncol. 2007, 68, 515–521. [Google Scholar] [CrossRef]
- Toma-Daşu, I.; Daşu, A.; Brahme, A. Dose prescription and optimisation based on tumour hypoxia. Acta Oncol. (Madr). 2009, 48, 1181–1192. [Google Scholar] [CrossRef]
- Ackerstaff, E.; Artemov, D.; Gillies, R.J.; Bhujwalla, Z.M. Hypoxia and the Presence of Human Vascular Endothelial Cells Affect Prostate Cancer Cell Invasion and Metabolism. Neoplasia 2007, 9, 1138–1151. [Google Scholar] [CrossRef] [Green Version]
- Alqawi, O.; Wang, H.P.; Espiritu, M.; Singh, G. Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free. Radic. Res. 2007, 41, 788–797. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McCarthy, H.O.; Devlin, A.; Ming, L.; Robson, T.; McKeown, S.R.; Worthington, J. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int. J. Cancer 2008, 123, 760–768. [Google Scholar] [CrossRef]
- Dai, Y.; Bae, K.; Siemann, D.W. Impact of Hypoxia on the Metastatic Potential of Human Prostate Cancer Cells. Int. J. Radiat. Oncol. 2011, 81, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafar, M.A.; Anastasiadis, A.G.; Chen, M.-W.; Burchardt, M.; Olsson, L.E.; Xie, H.; Benson, M.C.; Buttyan, R. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate 2003, 54, 58–67. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, S.; Sakamoto, S.; Kyprianou, N. Maspin modulates prostate cancer cell apoptotic and angiogenic response to hypoxia via targeting AKT. Oncogene 2008, 27, 7171–7179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terraneo, L.; Bianciardi, P.; Caretti, A.; Ronchi, R.; Samaja, M. Chronic systemic hypoxia promotes LNCaP prostate cancer growth in vivo. Prostate 2010, 70, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Nomura, T.; Sato, F.; Mimata, H. Chronic hypoxia induces androgen-independent and invasive behavior in LNCaP human prostate cancer cells. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Supiot, S.; Rousseau, C.; Dore, M.; Chèze-Le-Rest, C.; Kandel-Aznar, C.; Potiron, V.; Guerif, S.; Paris, F.; Ferrer, L.; Campion, L.; et al. Reoxygenation during radiotherapy in intermediate-risk prostate cancer. Radiother. Oncol. 2019, 133, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Her, E.J.; Haworth, A.; Reynolds, H.M.; Sun, Y.; Kennedy, A.; Panettieri, V.; Bangert, M.; Williams, S.; Ebert, M. Voxel-level biological optimisation of prostate IMRT using patient-specific tumour location and clonogen density derived from mpMRI. Radiat. Oncol. 2020, 15, 172. [Google Scholar] [CrossRef]
- Paczkowski, M.; Kretzschmar, W.W.; Markelc, B.; Liu, S.K.; Kunz-Schughart, L.A.; Harris, A.L.; Partridge, M.; Byrne, H.M.; Kannan, P. Reciprocal interactions between tumour cell populations enhance growth and reduce radiation sensitivity in prostate cancer. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]


| Patient | Age (Years) | Prostate Volume (cm3) | PSA (ng/mL) | Gleason Score of the Dominant Nodule | Pathological StagSe |
|---|---|---|---|---|---|
| 1 | 58 | 28.9 | 9 | 9 (5 + 4) | pT3b N0 |
| 2 | 64 | 30.6 | 6.1 | 7 (4 + 3) | pT3a |
| 3 | 68 | 28.1 | 11 | 7 (4 + 3) | pT3b N1 |
| 4 | 68 | 57.0 | 42 | 7 (4 + 3) | pT3a |
| 5 | 72 | 27.6 | 2.2 | 7 (4 + 3) | pT3a |
| Patient | Total Cell Number in CTV | |
|---|---|---|
| Original (Normal + Clonogen) | Scaled (Clonogen) | |
| 1 | 2.07 × 108 | 7.27 × 106 |
| 2 | 1.65 × 108 | 5.79 × 106 |
| 3 | 4.46 × 108 | 1.57 × 107 |
| 4 | 1.14 × 109 | 4.00 × 107 |
| 5 | 2.85 × 108 | 1.00 × 107 |
| Tumour Control Probability | ||
|---|---|---|
| TCP of voxel for sample | [1] | |
| TCP of voxel i over population distribution | [2] | |
| sample for hypoxic tissue | [3] | |
| ratio for hypoxic tissue | [4] | |
| TCP across whole target volume | [5] | |
| Expectation value of target TCP in presence of geometric uncertainty | [6] | |
| Expectation value of target TCP for sample in presence of geometric uncertainty | [7] | |
| Normal Tissue Response | ||
| Expectation value of EUD in presence of geometric uncertainty | [8] | |
| Equivalent dose in 2 Gy fractions for voxel | [9] | |
| Expectation value of NTCP in presence of geometric uncertainty | [10] | |
| NTCP model parameter | [11] | |
| Symbol | Parameter | Value | Reference |
|---|---|---|---|
| Tumour dose-proportional radiosensitivity coefficient | Sampled from lognormal population distribution | N/A | |
| Mean value of distribution | 0.15 Gy−1 | [12] | |
| Standard deviation of distribution | 0.04 Gy−1 | [12] | |
| Fractionation-correction parameter for tumour or OAR | 3.1 Gy (tumour) 5.4 Gy (rectum) 8.0 Gy (bladder) | [12] [16] [17,18] | |
| Tumour clonogen density (voxel-specific) | Variable (population median 107 cells per whole prostate volume) | [12] | |
| Voxel volume (resolution-dependent) | 2 × 2 × 2.5 mm3 | N/A | |
| Number of treatment fractions | 5 | N/A | |
| Dose per fraction per voxel | Variable | N/A | |
| Overall treatment time | Approximated as days | N/A | |
| Potential tumour doubling time | 42 days | [12] | |
| Number of samples from population distribution | 36 | N/A | |
| Weight factors normalising the distribution such that | Variable | N/A | |
| Oxygen enhancement ratio | 1.4 (1.2–1.8) | [12] | |
| Number of voxels comprising structure (prostate or OAR) | Variable | N/A | |
| Effective systematic geometric error | Variable | [19,20,21] | |
| Gaussian distribution of effective systematic error | Variable | N/A | |
| EUD model scaling parameter | See Table 5 | N/A | |
| OAR uniform dose which will lead to complications in 50% of the population | See Table 5 | N/A | |
| NTCP model slope | See Table 5 | N/A |
| Planning Method | VOI | Constraints |
|---|---|---|
| (1) Uniform-dose | CTV | V35Gy ≥ 99% |
| PTV | V33.25Gy ≥ 99% | |
| Rectum | V28Gy ≤ 15% V32Gy ≤ 20% | |
| Bladder | V28Gy ≤ 15% V32Gy ≤ 20% | |
| HOF | V28Gy ≤ 5% | |
| (2) Focal DE to tumour * | GTV | V35Gy ≥ 99%; aimed up to 50 Gy ⁑ V52Gy ≤ 0.1cc |
| (3) Focal DE to tumour and hypoxic volume * | GTV | V35Gy ≥ 99%; aimed up to 50 Gy ⁑ |
| HTV | V35Gy ≥ 99%; aimed up to 60 Gy ⁑ V62Gy ≤ 0.1cc | |
| (4) Biological optimisation | CTV | Maximise <TCP> V35Gy≥ 99% |
| Rectum | Minimise <NTCP> TD50 = 78.4 Gy, m = 0.108, a = 6 | |
| Bladder | Minimise <NTCP> TD50 = 80 Gy, m = 0.11, a = 6 | |
| HOF | V28Gy ≤ 5% |
| Uncertainties (mm) | Dose-Based Planning | Biological Optimisation | ||
|---|---|---|---|---|
| Mean | Margin | |||
| AP | −0.86 | 3.6 | 9.0 | 5.3 |
| LR | −0.03 | 2.6 | 6.5 | 4.6 |
| SI | 0.21 | 3.2 | 8.0 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Her, E.J.; Haworth, A.; Sun, Y.; Williams, S.; Reynolds, H.M.; Kennedy, A.; Ebert, M.A. Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging. Cancers 2021, 13, 4897. https://doi.org/10.3390/cancers13194897
Her EJ, Haworth A, Sun Y, Williams S, Reynolds HM, Kennedy A, Ebert MA. Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging. Cancers. 2021; 13(19):4897. https://doi.org/10.3390/cancers13194897
Chicago/Turabian StyleHer, Emily J., Annette Haworth, Yu Sun, Scott Williams, Hayley M. Reynolds, Angel Kennedy, and Martin A. Ebert. 2021. "Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging" Cancers 13, no. 19: 4897. https://doi.org/10.3390/cancers13194897






