Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
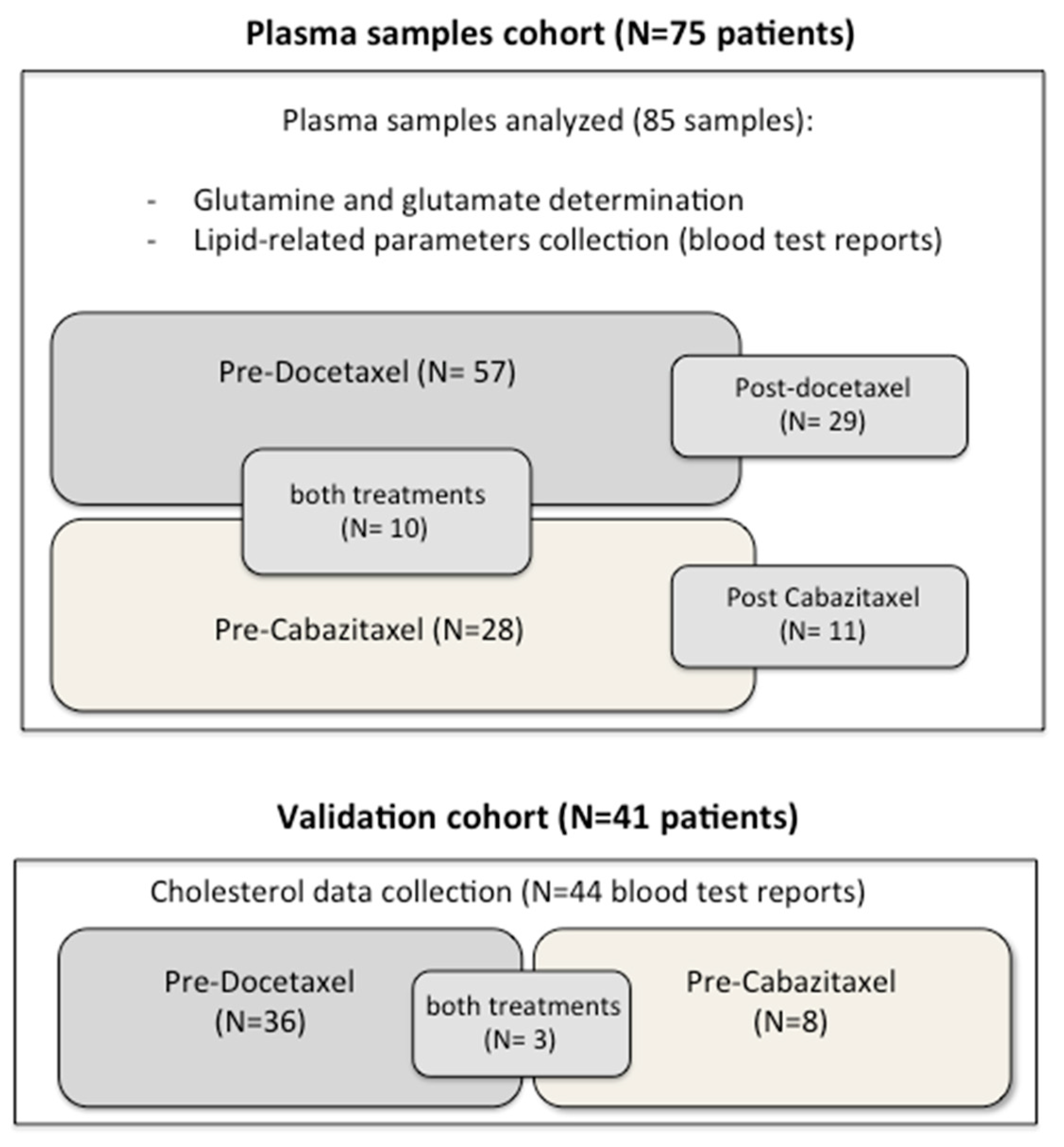
2.1. Study Design
2.2. Samples Collection and Glutamine Determination
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
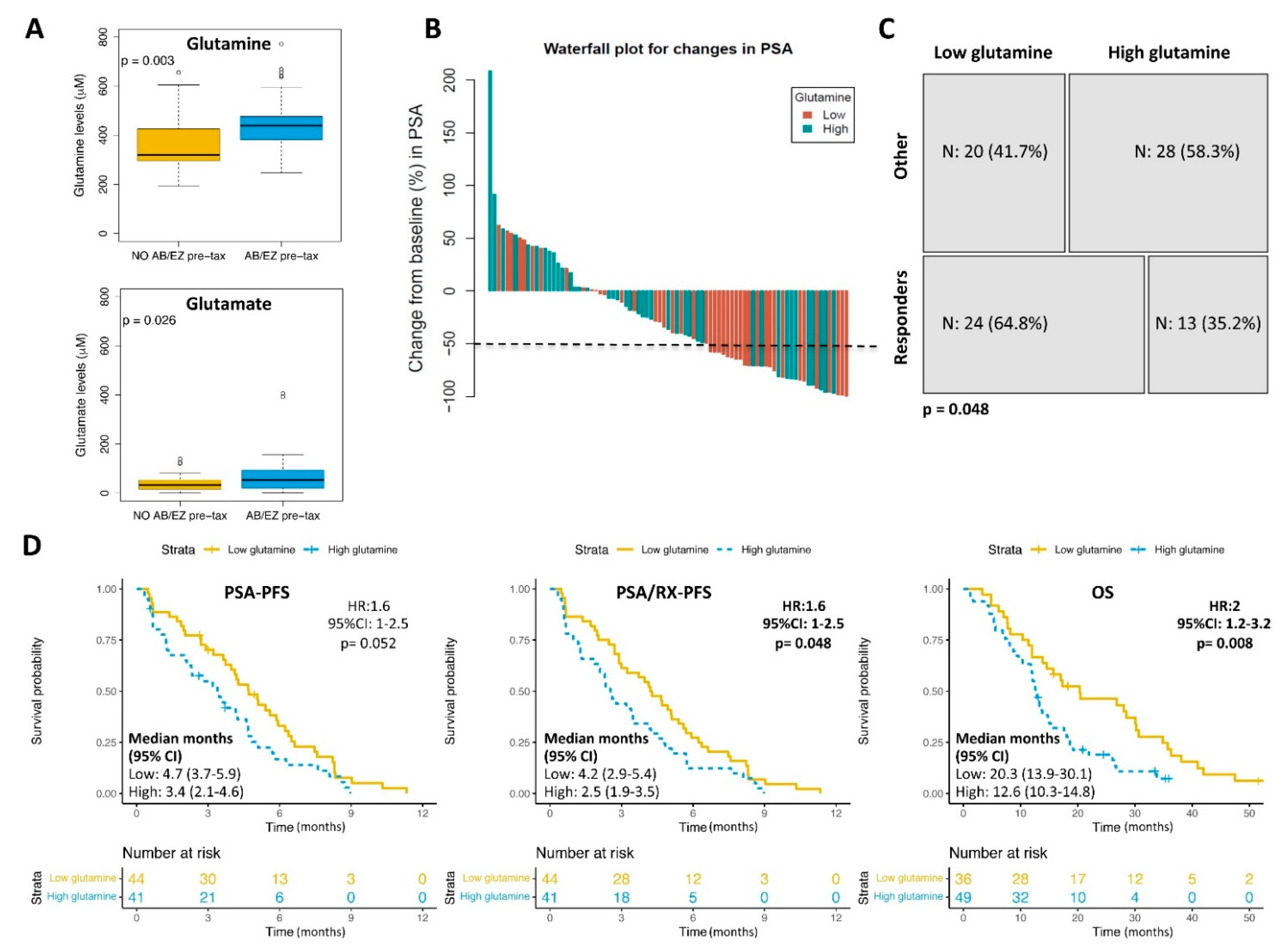
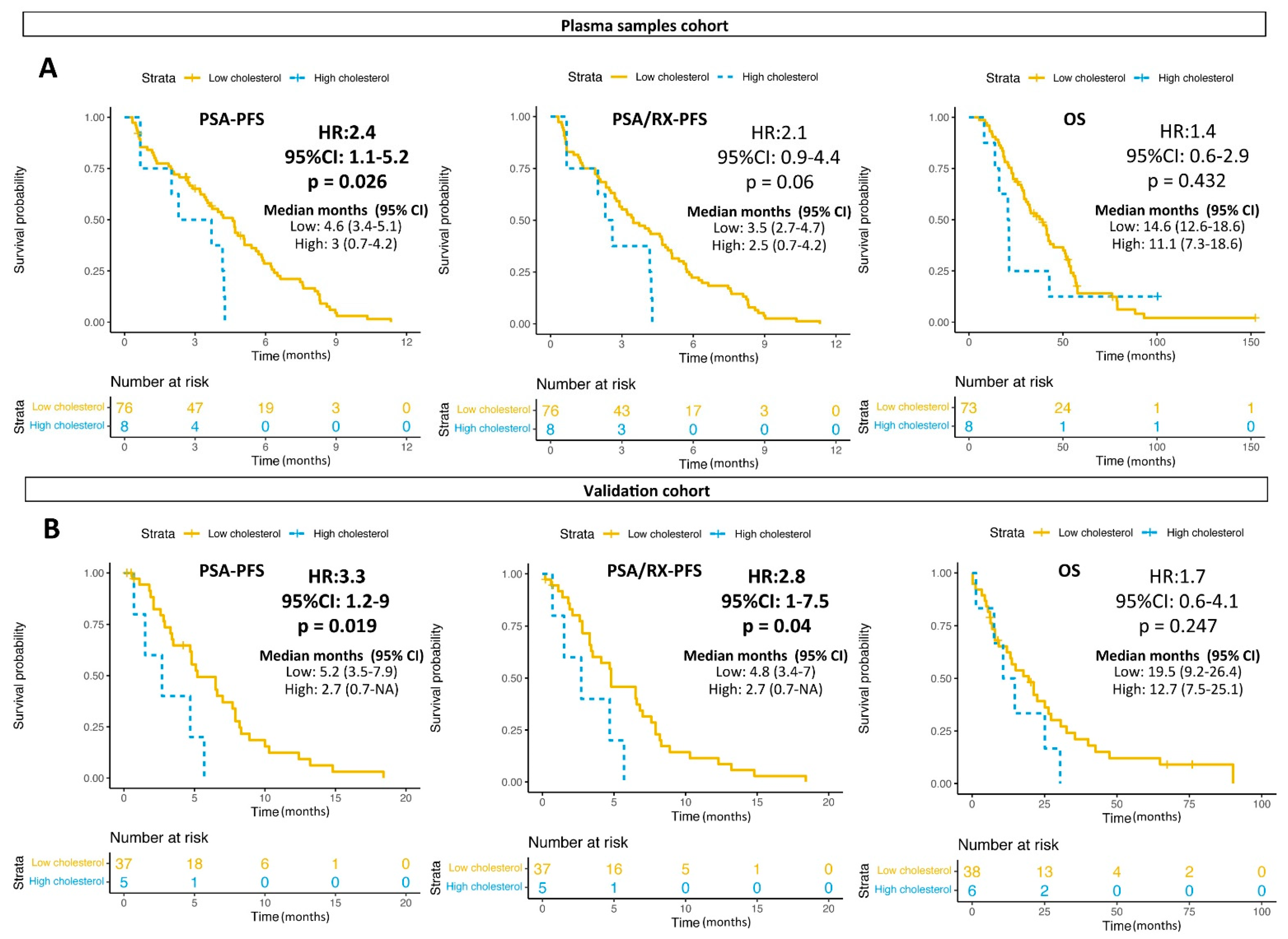
3.2. Glutamine Plasma Levels and Clinical Outcomes
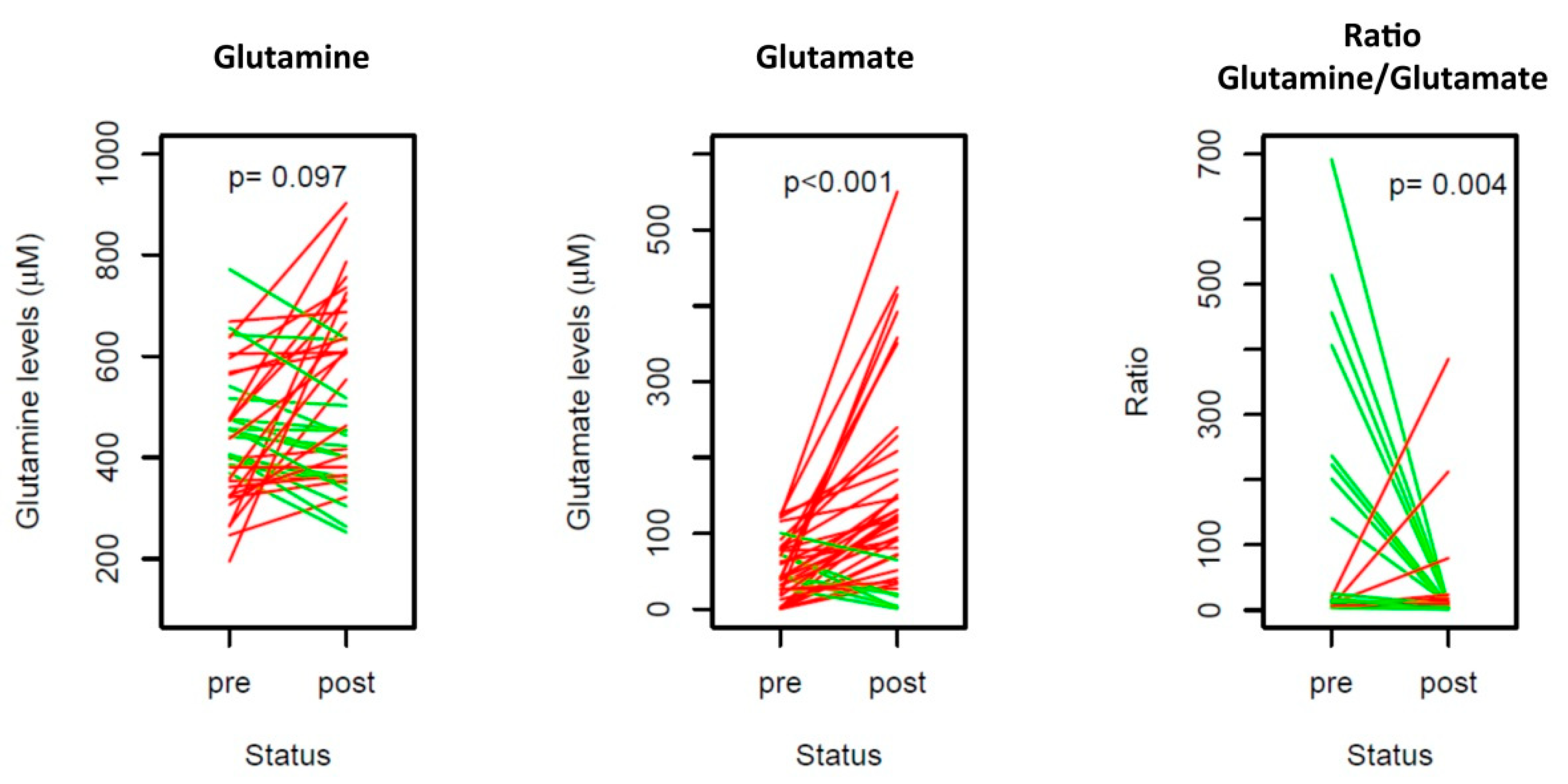
3.3. Glutamine Analysis in the Sequential Samples
3.4. Glutamine and Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Giunchi, F.; Fiorentino, M.; Loda, M. The Metabolic Landscape of Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 28–36. [Google Scholar] [CrossRef]
- Kimmelman, A.C. Metabolic Dependencies in RAS-Driven Cancers. Clin. Cancer Res. 2015, 21, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Wang, Q.; Bailey, C.; Ng, C.; Tiffen, J.; Thoeng, A.; Minhas, V.; Lehman, M.L.; Hendy, S.C.; Buchanan, G.; Nelson, C.C.; et al. Androgen Receptor and Nutrient Signaling Pathways Coordinate the Demand for Increased Amino Acid Transport during Prostate Cancer Progression. Cancer Res. 2011, 71, 7525–7536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bott, A.; Peng, I.-C.; Fan, Y.; Faubert, B.; Zhao, L.; Li, J.; Neidler, S.; Sun, Y.; Jaber, N.; Krokowski, D.; et al. Oncogenic Myc Induces Expression of Glutamine Synthetase through Promoter Demethylation. Cell Metab. 2015, 22, 1068–1077. [Google Scholar] [CrossRef] [Green Version]
- Koochekpour, S.; Majumdar, S.; Azabdaftari, G.; Attwood, K.; Scioneaux, R.; Subramani, D.; Manhardt, C.; Lorusso, G.D.; Willard, S.S.; Thompson, H.; et al. Serum Glutamate Levels Correlate with Gleason Score and Glutamate Blockade Decreases Proliferation, Migration, and Invasion and Induces Apoptosis in Prostate Cancer Cells. Clin. Cancer Res. 2012, 18, 5888–5901. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Wu, X.; Hu, J.; Hu, M.; Xu, H.; Jiang, H.; Ding, Q. Maternal High-Fat Diet Promotes the Development and Progression of Prostate Cancer in Transgenic Adenocarcinoma Mouse Prostate Offspring. Cell. Physiol. Biochem. 2018, 47, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Heemers, H.; Van de Sande, T.; De Schrijver, E.; Brusselmans, K.; Heyns, W.; Verhoeven, G. Androgens, lipogenesis and prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 273–279. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.J.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Hothorn, T.; Lausen, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Ippolito, L.; Marini, A.; Cavallini, L.; Morandi, A.; Pietrovito, L.; Pintus, G.; Giannoni, E.; Schrader, T.; Puhr, M.; Chiarugi, P.; et al. Metabolic shift toward oxidative phosphorylation in docetaxel resistant prostate cancer cells. Oncotarget 2016, 7, 61890–61904. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Xu, L.; Yin, Y.; Li, Y.; Chen, X.; Chang, Y.; Zhang, H.; Liu, J.; Beasley, J.; McCaw, P.; Zhang, H.; et al. A glutaminase isoform switch drives therapeutic resistance and disease progression of prostate cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2012748118. [Google Scholar] [CrossRef]
- Sun, J.; Bok, R.; Santos, J.D.; Upadhyay, D.; Santos, R.D.; Agarwal, S.; Van Criekinge, M.; Vigneron, D.; Aggarwal, R.; Peehl, D.; et al. Resistance to Androgen Deprivation Leads to Altered Metabolism in Human and Murine Prostate Cancer Cell and Tumor Models. Metabolites 2021, 11, 139. [Google Scholar] [CrossRef]
- Bai, S.; Cao, S.; Jin, L.; Kobelski, M.; Schouest, B.; Wang, X.; Ungerleider, N.; Baddoo, M.; Zhang, W.; Corey, E.; et al. A positive role of c-Myc in regulating androgen receptor and its splice variants in prostate cancer. Oncogene 2019, 38, 4977–4989. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; Macdonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Yuneva, M.; Zamboni, N.; Oefner, P.; Sachidanandam, R.; Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 2007, 178, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curi, R. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Kim, S.-H.; Im, C.Y.; Hwang, H.-J. Recent Development of Small Molecule Glutaminase Inhibitors. Curr. Top. Med. Chem. 2018, 18, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K.; Park, K.-G. Targeting Glutamine Metabolism for Cancer Treatment. Biomol. Ther. 2018, 26, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Guan, Y.F.; Hancock, S.E.; Wahi, K.; van Geldermalsen, M.; Zhang, B.K.; Pang, A.; Nagarajah, R.; Mak, B.; Freidman, N.; et al. Inhibition of guanosine monophosphate synthetase (GMPS) blocks glutamine metabolism and prostate cancer growth. J. Pathol. 2021, 254, 135–146. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.-M.; Oh, M.-H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J.; Tam, A.; et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366, 1013–1021. [Google Scholar] [CrossRef]
- Pelton, K.; Freeman, M.R.; Solomon, K.R. Cholesterol and prostate cancer. Curr. Opin. Pharmacol. 2012, 12, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; et al. Fatty Acid Synthase: A Metabolic Enzyme and Candidate Oncogene in Prostate Cancer. J. Natl. Cancer Inst. 2009, 101, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Sonpavde, G.; Pond, G.; Lucarelli, G.; Rossetti, S.; Facchini, G.; Scagliarini, S.; Cartenì, G.; Federico, P.; Daniele, B.; et al. Statin Use and Survival in Patients with Metastatic Castration-resistant Prostate Cancer Treated with Abiraterone Acetate. Eur. Urol. Focus 2018, 4, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Engel-Nitz, N.; Behl, A.; Blauer-Peterson, C.; Dawson, N. PD37-04 Statins and Oral Treatments in Patients with Metastatic Castration Resistant Prostate Cancer (MCRPC): Real-World Outcomes. J. Urol. 2017, 197, e675. [Google Scholar] [CrossRef]
- Tramontina, A.C.; Wartchow, K.M.; Rodrigues, L.; Biasibetti, R.; Quincozes-Santos, A.; Bobermin, L.; Tramontina, F.; Gonçalves, C.-A. The neuroprotective effect of two statins: Simvastatin and pravastatin on a streptozotocin-induced model of Alzheimer’s disease in rats. J. Neural Transm. 2011, 118, 1641–1649. [Google Scholar] [CrossRef]
- Vandresen-Filho, S.; Martins, W.C.; Bertoldo, D.B.; Mancini, G.; Herculano, B.A.; de Bem, A.F.; Tasca, C.I. Atorvastatin prevents cell damage via modulation of oxidative stress, glutamate uptake and glutamine synthetase activity in hippocampal slices subjected to oxygen/glucose deprivation. Neurochem. Int. 2013, 62, 948–955. [Google Scholar] [CrossRef]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Davidson, S.M.; Wirth, G.J.; Fiske, B.; Mayers, J.; Schwab, M.; Bellinger, G.; Csibi, A.; et al. Metformin Decreases Glucose Oxidation and Increases the Dependency of Prostate Cancer Cells on Reductive Glutamine Metabolism. Cancer Res. 2013, 73, 4429–4438. [Google Scholar] [CrossRef] [Green Version]

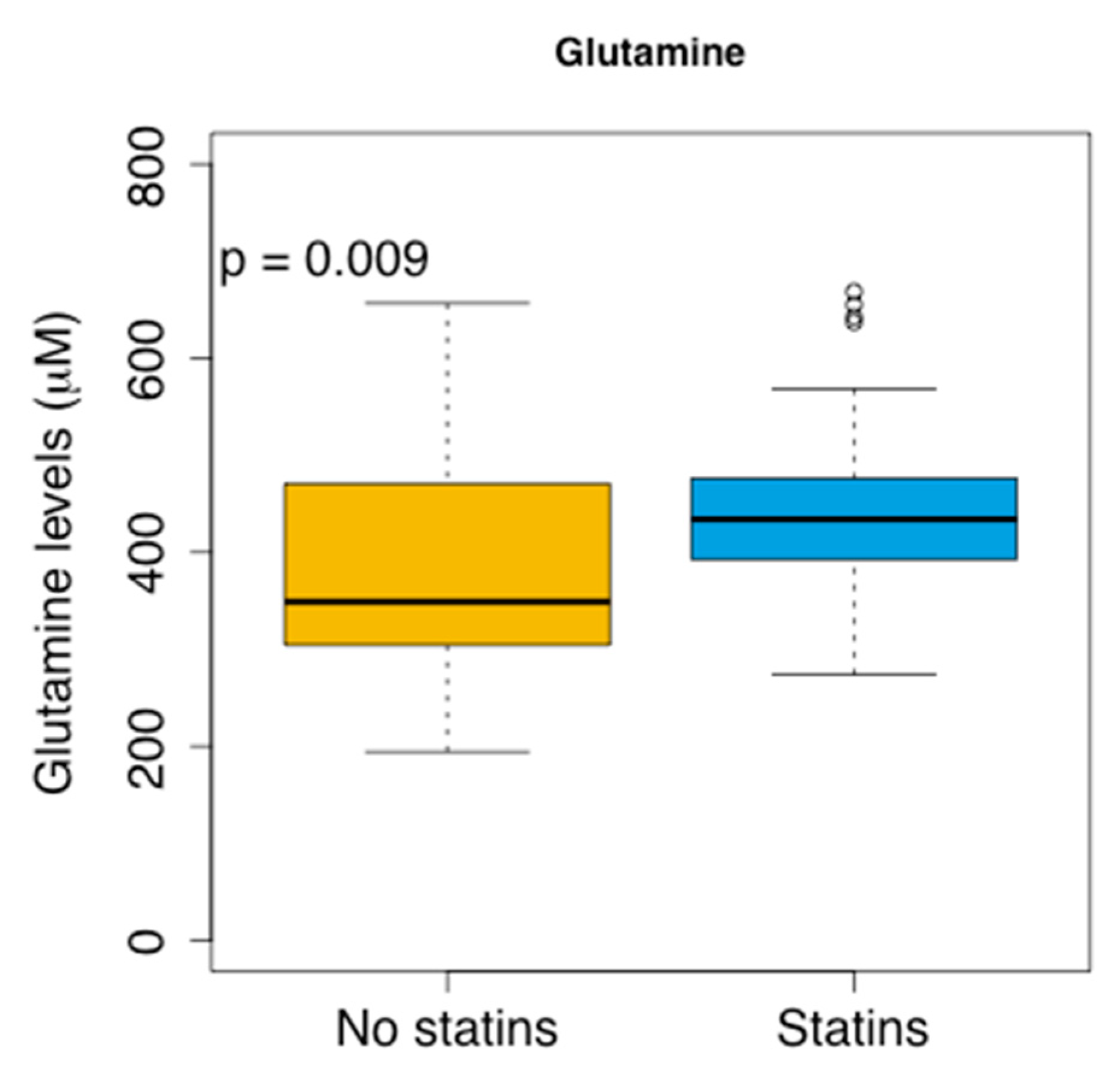
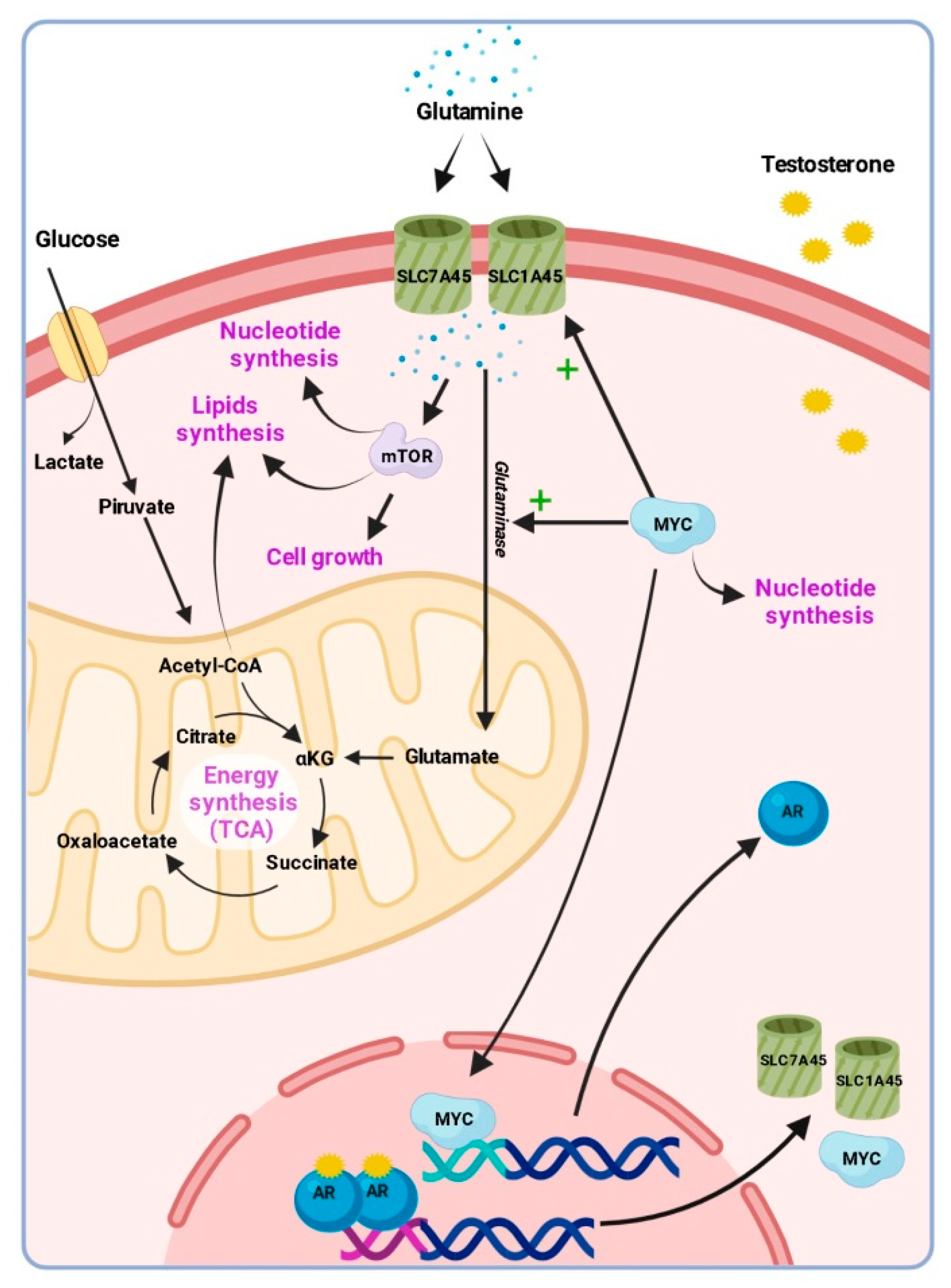
| Characteristics | Plasma Samples Cohort | Cholesterol Validation Cohort |
|---|---|---|
| Number of patients (samples) | 75 (85) | 41 (44) |
| Age at treatment (years) | ||
| Median (range) | 70.4 (55.8–83.5) | 71.6 (37.3–85.9) |
| Time since diagnosis to treat (years) | ||
| Median (range) | 4.5 (0.34–21.5) | 4.7 (0.33–14.01) |
| Stage at diagnosis, N (%) | ||
| ≤3 | 33 (44) | 15 (36.6) |
| ≥4 | 36 (48) | 25 (60.9) |
| NA | 6 (8) | 1 (2.4) |
| Gleason sum at diagnosis, N (%) | ||
| ≤7 | 28 (37.3) | 14 (34.1) |
| ≥8 | 45 (60) | 23 (9.8) |
| NA | 2 (2.7) | 4 (9.8) |
| Presence of bone metastases, N (%) | ||
| Yes | 69 (92) | 38 (92.7) |
| No | 6 (8) | 6 (14.6) |
| Presence of visceral metastases, N (%) | ||
| Yes | 16 (21.3) | 15 (36.6) |
| No | 59 (78.7) | 26 (63.4) |
| ECOG performance status score, N (%) | ||
| 0 | 12 (16) | 9 (21.9) |
| 1 or 2 | 61 (81.3) | 31 (75.6) |
| NA | 2 (2.7) | 1 (2.4) |
| Baseline Prostate-specific antigen (ng/mL) | ||
| Median (range) | 42.17 (0.04–2398.7) | 42.41 (0.04–1284) |
| Baseline hemoglobin concentration (g/L) | ||
| Median (range) | 124.1 (87–154) | 121.5 (15.3–151) |
| Baseline alkaline phosphatase (U/L) | ||
| Median (range) | 180 (54–1953) | 191.5 (75–2311) |
| Baseline lactate dehydrogenase (U/L) | ||
| Median (range) | 378 (151–2381) | 381.5 (163–1979) |
| Use of abiraterone/enzalutamide, N (%) | ||
| Pre-chemotherapy | 42 (56) | 25 (60.9) |
| Never or Post-chemotherapy | 33 (44) | 16 (39) |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| PSA-PFS | ||||||||
| Stage at diagnosis * | 1.04 | 0.65 | 1.65 | 0.880 | - | - | - | - |
| Gleason at diagnosis * | 1.43 | 0.90 | 2.30 | 0.134 | - | - | - | - |
| ECOG * | 1.60 | 0.81 | 3.16 | 0.179 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.006 | 1.00 | 1.00 | 1.00 | 0.012 |
| Hb ** | 0.98 | 0.96 | 1.00 | 0.016 | 0.98 | 0.97 | 1.00 | 0.026 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.010 | 1.00 | 1.00 | 1.00 | 0.036 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.862 | - | - | - | - |
| Visceral metastases * | 0.90 | 0.52 | 1.58 | 0.724 | - | - | - | - |
| Bone metastases * | 0.56 | 0.24 | 1.31 | 0.182 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.59 | 1.00 | 2.52 | 0.048 | 1.67 | 0.98 | 2.84 | 0.058 |
| Glutamine levels * | 1.57 | 1.00 | 2.46 | 0.052 | 1.28 | 0.77 | 2.15 | 0.344 |
| PSA/RX-PFS | ||||||||
| Stage at diagnosis * | 1.09 | 0.69 | 1.70 | 0.709 | - | - | - | - |
| Gleason at diagnosis * | 1.57 | 0.99 | 2.47 | 0.053 | 1.78 | 1.01 | 2.91 | 0.022 |
| ECOG * | 1.61 | 0.84 | 3.07 | 0.152 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.009 | 1.00 | 1.00 | 1.00 | 0.108 |
| Hb ** | 0.98 | 0.96 | 0.99 | 0.007 | 0.98 | 0.96 | 0.99 | 0.006 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.002 | 1.00 | 1.00 | 1.00 | 0.009 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.608 | - | - | - | - |
| Visceral metastases * | 0.96 | 0.57 | 1.63 | 0.894 | - | - | - | - |
| Bone metastases * | 0.56 | 0.25 | 1.22 | 0.145 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.48 | 0.95 | 2.29 | 0.081 | 1.42 | 0.83 | 2.43 | 0.198 |
| Glutamine levels * | 1.55 | 1.00 | 2.40 | 0.049 | 1.47 | 0.88 | 2.48 | 0.144 |
| OS | ||||||||
| Stage at diagnosis * | 0.98 | 0.61 | 1.58 | 0.932 | - | - | - | - |
| Gleason at diagnosis * | 1.52 | 0.94 | 2.47 | 0.087 | 1.69 | 1.00 | 2.85 | 0.049 |
| ECOG * | 1.60 | 0.82 | 3.13 | 0.170 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.000 | 1.00 | 1.00 | 1.00 | 0.011 |
| Hb ** | 0.97 | 0.95 | 0.99 | 0.001 | 0.97 | 0.95 | 0.99 | 0.001 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.091 | 1.00 | 1.00 | 1.00 | 0.241 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.775 | - | - | - | - |
| Visceral metastases * | 1.01 | 0.60 | 1.72 | 0.964 | - | - | - | - |
| Bone metastases * | 0.84 | 0.38 | 1.83 | 0.657 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.69 | 1.05 | 2.72 | 0.032 | 1.86 | 1.02 | 3.41 | 0.043 |
| Glutamine levels * | 1.95 | 1.19 | 3.21 | 0.009 | 1.49 | 0.83 | 2.69 | 0.183 |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| PSA-PFS | ||||||||
| Stage at diagnosis * | 1.04 | 0.65 | 1.65 | 0.880 | - | - | - | - |
| Gleason at diagnosis * | 1.43 | 0.90 | 2.30 | 0.134 | - | - | - | - |
| ECOG * | 1.60 | 0.81 | 3.16 | 0.179 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.006 | 1.00 | 1.00 | 1.00 | 0.017 |
| Hb ** | 0.98 | 0.96 | 1.00 | 0.016 | 0.98 | 0.97 | 1.00 | 0.052 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.010 | 1.00 | 1.00 | 1.00 | 0.024 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.862 | - | - | - | - |
| Visceral metastases * | 0.90 | 0.52 | 1.58 | 0.724 | - | - | - | - |
| Bone metastases * | 0.56 | 0.24 | 1.31 | 0.182 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.59 | 1.00 | 2.52 | 0.048 | 1.91 | 1.12 | 3.19 | 0.009 |
| Cholesterol levels * | 2.39 | 1.11 | 5.15 | 0.026 | 2.10 | 0.92 | 4.78 | 0.078 |
| PSA/RX-PFS | ||||||||
| Stage at diagnosis * | 1.09 | 0.69 | 1.70 | 0.709 | - | - | - | - |
| Gleason at diagnosis * | 1.57 | 0.99 | 2.47 | 0.053 | 1.61 | 0.97 | 2.70 | 0.068 |
| ECOG * | 1.61 | 0.84 | 3.07 | 0.152 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.009 | 1.00 | 1.00 | 1.00 | 0.121 |
| Hb ** | 0.98 | 0.96 | 0.99 | 0.007 | 0.98 | 0.96 | 1.00 | 0.013 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.002 | 1.00 | 1.00 | 1.00 | 0.005 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.608 | - | - | - | - |
| Visceral metastases * | 0.96 | 0.57 | 1.63 | 0.894 | - | - | - | - |
| Bone metastases * | 0.56 | 0.25 | 1.22 | 0.145 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.48 | 0.95 | 2.29 | 0.081 | 1.75 | 1.09 | 2.82 | 0.021 |
| Cholesterol levels * | 2.06 | 0.96 | 4.39 | 0.062 | 1.42 | 0.59 | 3.38 | 0.426 |
| OS | ||||||||
| Stage at diagnosis * | 0.98 | 0.61 | 1.58 | 0.932 | - | - | - | - |
| Gleason at diagnosis * | 1.52 | 0.94 | 2.47 | 0.087 | 1.65 | 0.98 | 2.78 | 0.058 |
| ECOG * | 1.60 | 0.82 | 3.13 | 0.170 | - | - | - | - |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.000 | 1.00 | 1.00 | 1.00 | 0.011 |
| Hb ** | 0.97 | 0.95 | 0.99 | 0.001 | 0.96 | 0.94 | 0.98 | 0.000 |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.091 | 1.00 | 1.00 | 1.00 | 0.229 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.775 | - | - | - | - |
| Visceral metastases * | 1.01 | 0.60 | 1.72 | 0.964 | - | - | - | - |
| Bone metastases * | 0.84 | 0.38 | 1.83 | 0.657 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.69 | 1.05 | 2.72 | 0.032 | 2.28 | 1.34 | 3.87 | 0.002 |
| Cholesterol levels * | 1.37 | 0.63 | 3.00 | 0.432 | - | - | - | - |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| PSA-PFS | ||||||||
| Stage at diagnosis * | 0.91 | 0.62 | 1.33 | 0.634 | - | - | - | - |
| Gleason at diagnosis * | 1.41 | 0.95 | 2.09 | 0.085 | 1.11 | 0.72 | 1.70 | 0.643 |
| ECOG * | 1.76 | 1.04 | 2.98 | 0.035 | 1.59 | 0.92 | 2.74 | 0.096 |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.001 | 1.00 | 1.00 | 1.00 | 0.004 |
| Hb ** | 1.00 | 0.99 | 1.01 | 0.943 | - | - | - | - |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.003 | 1.00 | 1.00 | 1.00 | 0.040 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.212 | - | - | - | - |
| Visceral metastases * | 0.77 | 0.51 | 1.16 | 0.209 | - | - | - | - |
| Bone metastases * | 0.89 | 0.47 | 1.67 | 0.715 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.14 | 0.78 | 1.66 | 0.507 | - | - | - | - |
| Cholesterol levels * | 2.54 | 1.39 | 4.65 | 0.002 | 2.45 | 1.22 | 4.92 | 0.012 |
| PSA/RX-PFS | ||||||||
| Stage at diagnosis * | 0.93 | 0.64 | 1.34 | 0.688 | - | - | - | - |
| Gleason at diagnosis * | 1.58 | 1.08 | 2.31 | 0.020 | 1.31 | 0.86 | 1.99 | 0.210 |
| ECOG * | 1.84 | 1.10 | 3.06 | 0.019 | 1.63 | 0.96 | 2.77 | 0.069 |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.001 | 1.00 | 1.00 | 1.00 | 0.004 |
| Hb ** | 1.00 | 0.99 | 1.01 | 0.884 | - | - | - | - |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.001 | 1.00 | 1.00 | 1.00 | 0.010 |
| AP ** | 1.00 | 1.00 | 1.00 | 0.424 | - | - | - | - |
| Visceral metastases * | 0.83 | 0.56 | 1.24 | 0.363 | - | - | - | - |
| Bone metastases * | 0.89 | 0.49 | 1.63 | 0.703 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.06 | 0.734 | 1.52 | 0.772 | - | - | - | - |
| Cholesterol levels * | 2.16 | 1.19 | 3.93 | 0.011 | 2.12 | 1.06 | 4.22 | 0.034 |
| OS | ||||||||
| Stage at diagnosis * | 1.03 | 0.71 | 1.51 | 0.874 | - | - | - | - |
| Gleason at diagnosis * | 1.48 | 1.00 | 2.20 | 0.053 | 1.41 | 0.92 | 2.15 | 0.114 |
| ECOG * | 1.77 | 1.04 | 3.01 | 0.037 | 1.76 | 1.00 | 3.13 | 0.052 |
| LDH ** | 1.00 | 1.00 | 1.00 | 0.000 | 1.00 | 1.00 | 1.00 | 0.000 |
| Hb ** | 1.00 | 1.00 | 1.01 | 0.444 | - | - | - | - |
| PSA ** | 1.00 | 1.00 | 1.00 | 0.118 | - | - | - | - |
| AP ** | 1.00 | 1.00 | 1.00 | 0.248 | - | - | - | - |
| Visceral metastases * | 0.88 | 0.58 | 1.33 | 0.541 | - | - | - | - |
| Bone metastases * | 1.01 | 0.56 | 1.84 | 0.970 | - | - | - | - |
| AB/EZ prior to taxanes * | 1.21 | 0.83 | 1.77 | 0.315 | - | - | - | - |
| Cholesterol levels * | 1.49 | 0.83 | 2.67 | 0.181 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Aguilera, M.; Pereira, M.V.; Jiménez, N.; Reig, Ò.; Cuartero, A.; Victoria, I.; Aversa, C.; Ferrer-Mileo, L.; Prat, A.; Mellado, B. Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes. Cancers 2021, 13, 4960. https://doi.org/10.3390/cancers13194960
Marín-Aguilera M, Pereira MV, Jiménez N, Reig Ò, Cuartero A, Victoria I, Aversa C, Ferrer-Mileo L, Prat A, Mellado B. Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes. Cancers. 2021; 13(19):4960. https://doi.org/10.3390/cancers13194960
Chicago/Turabian StyleMarín-Aguilera, Mercedes, María V. Pereira, Natalia Jiménez, Òscar Reig, Anna Cuartero, Iván Victoria, Caterina Aversa, Laura Ferrer-Mileo, Aleix Prat, and Begoña Mellado. 2021. "Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes" Cancers 13, no. 19: 4960. https://doi.org/10.3390/cancers13194960
APA StyleMarín-Aguilera, M., Pereira, M. V., Jiménez, N., Reig, Ò., Cuartero, A., Victoria, I., Aversa, C., Ferrer-Mileo, L., Prat, A., & Mellado, B. (2021). Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes. Cancers, 13(19), 4960. https://doi.org/10.3390/cancers13194960





