High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Acquisition of Clinical and Pathological Data
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for DKK1 Levels
2.4. Detection of Circulating Tumor Cells
2.5. Detection of Disseminated Tumor Cells
2.6. Statistical Analysis
3. Results
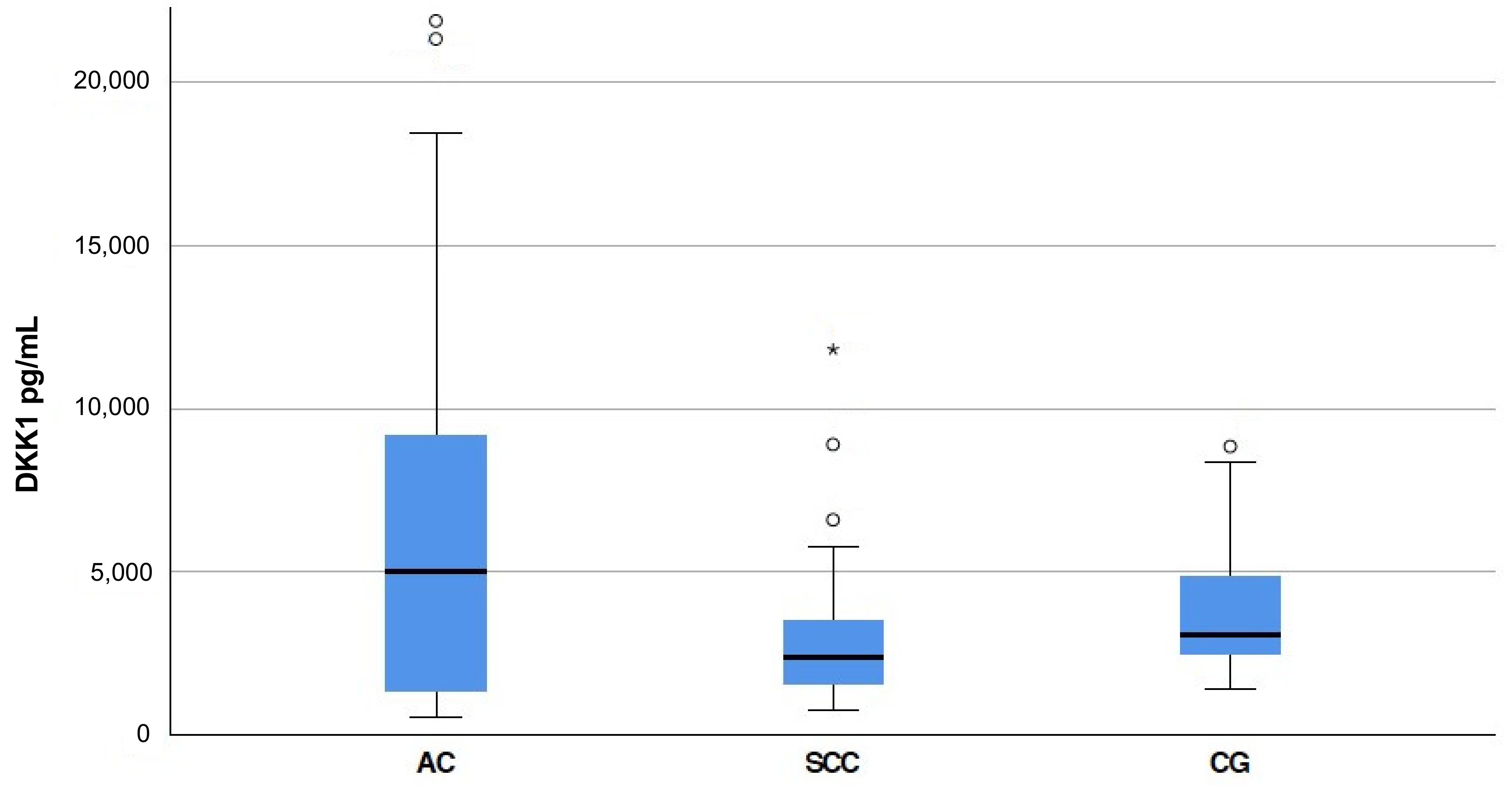
3.1. S-DKK1 Levels in Esophageal Cancer Patients
3.2. Recurrence of Cancer Occurs Earlier in Patients with High S-DKK1 and Detectable CTCs
3.3. High Levels of S-DKK1 and the Presence of CTCs in Blood Are Associated with Lower Overall Survival in Esophageal Cancer Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Shao, Y.C.; Wei, Y.; Liu, J.F.; Xu, X.Y. The role of Dickkopf family in cancers: From Bench to Bedside. Am. J. Cancer Res. 2017, 7, 1754–1768. [Google Scholar] [PubMed]
- Jung, Y.-S.; Park, J.-I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Sakane, H.; Yamamoto, H.; Kikuchi, A. LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J. Cell Sci. 2010, 123, 360. [Google Scholar] [CrossRef] [Green Version]
- Kagey, M.H.; He, X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol. 2017, 174, 4637–4650. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tang, W.; Xie, L.; Wang, J.; Deng, Y.; Peng, Q.; Zhai, L.; Li, S.; Qin, X. Prognostic significance of dickkopf-1 overexpression in solid tumors: A meta-analysis. Tumour Biol. 2014, 35, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Yamabuki, T.; Takano, A.; Hayama, S.; Ishikawa, N.; Kato, T.; Miyamoto, M.; Ito, T.; Ito, H.; Miyagi, Y.; Nakayama, H.; et al. Dikkopf-1 as a Novel Serologic and Prognostic Biomarker for Lung and Esophageal Carcinomas. Cancer Res. 2007, 67, 2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [Green Version]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, B.A.; Jueckstock, J.; Andergassen, U.; Salmen, J.; Schochter, F.; Fink, V.; Alunni-Fabbroni, M.; Rezai, M.; Beck, T.; Beckmann, M.W.; et al. Evaluation of two different analytical methods for circulating tumor cell detection in peripheral blood of patients with primary breast cancer. BioMed Res. Int. 2014, 2014, 491459. [Google Scholar] [CrossRef] [PubMed]
- Vashist, Y.K.; Effenberger, K.E.; Vettorazzi, E.; Riethdorf, S.; Yekebas, E.F.; Izbicki, J.R.; Pantel, K. Disseminated tumor cells in bone marrow and the natural course of resected esophageal cancer. Ann. Surg. 2012, 255, 1105–1112. [Google Scholar] [CrossRef]
- Effenberger, K.E.; Schroeder, C.; Eulenburg, C.; Reeh, M.; Tachezy, M.; Riethdorf, S.; Vashist, Y.K.; Izbicki, J.R.; Pantel, K.; Bockhorn, M. Disseminated tumor cells in pancreatic cancer-an independent prognosticator of disease progression and survival. Int. J. Cancer 2012, 131, E475–E483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehm, T.; Braun, S.; Muller, V.; Janni, W.; Gebauer, G.; Marth, C.; Schindlbeck, C.; Wallwiener, D.; Borgen, E.; Naume, B.; et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 2006, 107, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Borgen, E.; Naume, B.; Nesland, J.M.; Kvalheim, G.; Beiske, K.; Fodstad, O.; Diel, I.; Solomayer, E.F.; Theocharous, P.; Coombes, R.C.; et al. Standardization of the immunocytochemical detection of cancer cells in BM and blood: I. establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy 1999, 1, 377–388. [Google Scholar] [CrossRef]
- Mazon, M.; Masi, D.; Carreau, M. Modulating Dickkopf-1: A Strategy to Monitor or Treat Cancer? Cancers 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158. [Google Scholar] [CrossRef]
- Reeh, M.; Effenberger, K.E.; Koenig, A.M.; Riethdorf, S.; Eichstadt, D.; Vettorazzi, E.; Uzunoglu, F.G.; Vashist, Y.K.; Izbicki, J.R.; Pantel, K.; et al. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann. Surg. 2015, 261, 1124–1130. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.T.; Cui, X.; Chen, Q.; Li, Y.F.; Cui, Y.H.; Wang, Y.; Jiang, J. Circulating tumor cell status monitors the treatment responses in breast cancer patients: A meta-analysis. Sci. Rep. 2017, 7, 43464. [Google Scholar] [CrossRef] [Green Version]
- Smit, D.J.; Cayrefourcq, L.; Haider, M.T.; Hinz, N.; Pantel, K.; Alix-Panabieres, C.; Jucker, M. High Sensitivity of Circulating Tumor Cells Derived from a Colorectal Cancer Patient for Dual Inhibition with AKT and mTOR Inhibitors. Cells 2020, 9, 2129. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Smit, D.J.; Pantel, K.; Jücker, M. Circulating tumor cells as a promising target for individualized drug susceptibility tests in cancer therapy. Biochem. Pharm. 2021, 188, 114589. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Kuske, A.; Joosse, S.A.; Yigit, G.; Sflomos, G.; Thaler, S.; Smit, D.J.; Werner, S.; Borgmann, K.; Gartner, S.; et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 2020, 12, e11908. [Google Scholar] [CrossRef]
- Gaur, P.; Kim, M.; Dunkin, B. Esophageal cancer: Recent advances in screening, targeted therapy, and management. J. Carcinog. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Liu, Y.; Jin, X.; Xu, Z.; Yu, Q.; Li, K. Diagnostic value of multiple tumor markers for patients with esophageal carcinoma. PloS One 2015, 10, e0116951. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-M.; Hong, P.; Xu, W.W.; He, Q.-Y.; Li, B. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target. Ther. 2020, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Xiang, B.; Muthuswamy, S.K. Controlled Activation of ErbB1/ErbB2 Heterodimers Promote Invasion of Three-Dimensional Organized Epithelia in an ErbB1-Dependent Manner: Implications for Progression of ErbB2-Overexpressing Tumors. Cancer Res. 2006, 66, 5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyros, O.; Lamprecht, A.K.; Nie, L.; Thieme, R.; Götzel, K.; Gasparri, M.; Haasler, G.; Rafiee, P.; Shaker, R.; Gockel, I. Dickkopf-1 (DKK1) promotes tumor growth via Akt-phosphorylation and independently of Wnt-axis in Barrett’s associated esophageal adenocarcinoma. Am. J. Cancer Res. 2019, 9, 330–346. [Google Scholar] [PubMed]
- Niu, J.; Li, X.-M.; Wang, X.; Liang, C.; Zhang, Y.-D.; Li, H.-Y.; Liu, F.-Y.; Sun, H.; Xie, S.-Q.; Fang, D. DKK1 inhibits breast cancer cell migration and invasion through suppression of β-catenin/MMP7 signaling pathway. Cancer Cell Int. 2019, 19, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.X.; Zhou, X.; Sui, X.; He, C.C.; Cai, M.J.; Ma, J.L.; Zhang, Y.Y.; Zhou, C.Y.; Ma, C.X.; Varela-Ramirez, A.; et al. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget 2015, 6, 19907–19917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tian, X.; Gao, L.; Jiang, X.; Fu, R.; Zhang, T.; Ren, T.; Hu, P.; Wu, Y.; Zhao, P.; et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. 2019, 8, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Hölscher, A.H.; Haustermans, K.; Wittekind, C. Multimodal treatment of esophageal cancer. Langenbecks Arch. Surg. 2013, 398, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Karstens, K.-F.; Stüben, B.O.; Reeh, M. Oesophageal Adenocarcinomas: Where Do We Stand Today? Cancers 2021, 13, 109. [Google Scholar]
- Hofbauer, L.C.; Bozec, A.; Rauner, M.; Jakob, F.; Perner, S.; Pantel, K. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021, 18, 488–505. [Google Scholar] [CrossRef]
- Abdel-Latif, M.M.; Duggan, S.; Reynolds, J.V.; Kelleher, D. Inflammation and esophageal carcinogenesis. Curr. Opin. Pharm. 2009, 9, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.W.; Karakasheva, T.A.; Hicks, P.D.; Bass, A.J.; Rustgi, A.K. The tumor microenvironment in esophageal cancer. Oncogene 2016, 35, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Lyros, O.; Rafiee, P.; Nie, L.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlavoix, T.; Seelentag, W.; Yan, P.; Bachmann, A.; Bosman, F.T. Altered expression of CD44 and DKK1 in the progression of Barrett’s esophagus to esophageal adenocarcinoma. Virchows Arch. 2009, 454, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| N (%) | Low S-DKK1 <5800 pg/mL N (%) | High S-DKK1 ≥5800 pg/mL N (%) | p Value (a) Two-Sided Fisher Exact Test (b) Two-Sided Pearson Chi Quadrat Test | ||
|---|---|---|---|---|---|
| Gender | 0.208 (a) | ||||
| Female | 26 (28.6%) | 21 (80.8%) | 5 (19.2%) | ||
| Male | 65 (71.4%) | 42 (64.6%) | 23 (35.4%) | ||
| Age | 0.820 (a) | ||||
| <65 | 42 (46.2%) | 30 (71.4%) | 12 (28.6%) | ||
| ≥65 | 49 (53.8%) | 33(67.3%) | 16 (32.7%) | ||
| Tumor Entity | 0.015 (a) | ||||
| Squamous cell carcinoma | 30 (33%) | 26 (86.7%) | 4 (13.3%) | ||
| Adenocarcinoma | 61 (67%) | 37 (60.7%) | 24 (39.3%) | ||
| Tumor Stage | 0.047 (b) | ||||
| T0 | 2 (2.2%) | 2 (100%) | 0 (0%) | ||
| T1 | 21 (23.1%) | 17 (81%) | 4 (19%) | ||
| T2 | 14 (15.4%) | 13 (92.9%) | 1 (7.1%) | ||
| T3 | 48 (52.7%) | 28 (58.3%) | 20 (41.7%) | ||
| T4 | 6 (6.6%) | 3 (50%) | 3 (50%) | ||
| Lymphatic Node Metastasis | 0.198 (b) | ||||
| N0 | 34 (37.4%) | 27 (79.4%) | 7 (20.6%) | ||
| N1 | 28 (30.8%) | 16 (57.1%) | 12 (42.9%) | ||
| N2 | 17 (18.7%) | 13 (76.5%) | 4 (23.5%) | ||
| N3 | 12 (13.2%) | 7 (58.3%) | 5 (41.7%) | ||
| Distant Metastasis | 0.550 (a) | ||||
| M0 | 88 (96.7%) | 60 (68.2%) | 28 (31.8%) | ||
| M1 | 3 (3.3%) | 3 (100%) | 0 (0%) | ||
| Lymphatic Vessel Infiltration | 0.093 (a) | ||||
| L0 | 32 (35.2%) | 26 (81.3%) | 6 (18.8%) | ||
| L1 | 56 (51.5%) | 35 (62.5%) | 21 (37.5%) | ||
| Venous Vessel Infiltration | 0.108 (a) | ||||
| V0 | 67 (73.6%) | 50 (74.6%) | 17 (25.4%) | ||
| V1 | 22 (24.2%) | 12 (54.5%) | 10 (45.5%) | ||
| Grading | 0.553 (b) | ||||
| G1 | 6 (6.6%) | 4 (66.7%) | 2 (33.3%) | ||
| G2 | 36 (39.6%) | 28 (77.8%) | 8 (22.2%) | ||
| G3 | 45 (49.5%) | 29 (64.4%) | 16 (25.6%) | ||
| G4 | 2 (2.2%) | 1 (50%) | 1 (50%) | ||
| Resection Status | 0.542 (a) | ||||
| R0 | 75 (82.4%) | 53 (70.7%) | 22 (20.3%) | ||
| R1 | 15 (16.5%) | 9 (60%) | 6 (40%) | ||
| Neoadjuvant Treatment | 0.031 (a) | ||||
| No | 75 (82.4%) | 48 (64%) | 27 (36%) | ||
| Yes | 15 (16.5%) | 14 (93.3%) | 1 (6.7%) | ||
| Recurrence | <0.001 (a) | ||||
| No | 36 (39.6%) | 32 (88.9%) | 4 (11.1%) | ||
| Yes | 54 (59.3%) | 30 (55.6%) | 24 (44.4%) | ||
| CTC | |||||
| No | 48 (73.8%) | 39 (81.3%) | 9 (18.8%) | <0.001 (a) | |
| Yes | 17 (26.2%) | 6 (35.3%) | 11 (64.7%) | ||
| DTC | |||||
| No | 49 (53.80%) | 39 (79.6%) | 10 (20.4%) | 0.003 (a) | |
| Yes | 14 (15.4%) | 5 (35.7%) | 9 (64.3%) |
| N (%) | Low S-DKK1 <5800 pg/mL N (%) | High S-DKK1 ≥5800 pg/mL N (%) | p Value (a) Two-Sided Fisher Exact Test (b) Two-Sided Pearson Chi Quadrat Test | ||
|---|---|---|---|---|---|
| Gender | 1 (a) | ||||
| Female | 15 (50%) | 13 (86.7%) | 2 (13.3%) | ||
| Male | 15 (50%) | 13 (86.7%) | 2 (13.3%) | ||
| Age | 0.632 (a) | ||||
| <65 | 12 (40%) | 11 (91.7%) | 1 (8.3%) | ||
| ≥65 | 18 (60%) | 15 (83.3%) | 3 (16.7%) | ||
| Tumor Stage | 0.483 (b) | ||||
| T0 | 2 (6.7%) | 2 (100%) | 0 (0%) | ||
| T1 | 7 (23.3%) | 7 (100%) | 0 (0%) | ||
| T2 | 8 (26.7%) | 7 (87.5%) | 1 (12.5%) | ||
| T3 | 13 (43.3%) | 10 (76.9%) | 3 (23.1%) | ||
| Lymphatic Node Metastasis | 0.725 (b) | ||||
| N0 | 14 (46.7%) | 12 (85.7%) | 2 (14.3%) | ||
| N1 | 10 (33.33%) | 8 (80%) | 2 (20%) | ||
| N2 | 3 (10%) | 3 (100%) | 0 (0%) | ||
| N3 | 3 (10%) | 3 (100%) | 0 (0%) | ||
| Distant Metastasis | 1 (a) | ||||
| M0 | 29 (96.7%) | 25 (86.2%) | 4 (13.8%) | ||
| M1 | 1 (3.3%) | 1 (100%) | 0 (0%) | ||
| Lymphatic Vessel Infiltration | 1 (a) | ||||
| L0 | 16 (53.3%) | 14 (87.5%) | 2 (12.5%) | ||
| L1 | 14 (46.7%) | 12 (85.7%) | 2 (14.3%) | ||
| Venous Vessel Infiltration | 0.454 (a) | ||||
| V0 | 26 (86.7%) | 23 (88.5%) | 3 (11.5%) | ||
| V1 | 4 (13.3%) | 3 (75%) | 1 (25%) | ||
| Grading | 0.753 (b) | ||||
| G1 | 2 (6.7%) | 2 (100%) | 0 (0%) | ||
| G2 | 17 (56.7%) | 15 (88.2%) | 2 (11.8%) | ||
| G3 | 11 (36.7%) | 9 (81.8%) | 2 (18.2%) | ||
| Resection Status | 0.454 (a) | ||||
| R0 | 26 (86.7%) | 23 (88.5%) | 3 (11.5%) | ||
| R1 | 4 (13.3%) | 3 (75%) | 1 (25%) | ||
| Neoadjuvant Treatment | 0.557 (a) | ||||
| No | 24 (80%) | 20 (83.3%) | 4 (16.7%) | ||
| Yes | 6 (20%) | 6 (100%) | 0 (0%) | ||
| Recurrence | 0.037 (a) | ||||
| No | 16 (53.3%) | 16 (100%) | 0 (0%) | ||
| Yes | 14 (46.7%) | 10 (71.4%) | 4 (28.6%) | ||
| CTC | |||||
| No | 12 (66.7%) | 11 (91.7%) | 1 (8.3%) | 0.083 (a) | |
| Yes | 6 (33.3%) | 3 (50%) | 3 (50%) | ||
| DTC | |||||
| No | 15 (88.2%) | 13 (86.7%) | 2 (13.3%) | 0.331 (a) | |
| Yes | 2 (11.8%) | 1 (50%) | 1 (50%) |
| N (%) | Low S-DKK1 < 5800 pg/mL N (%) | High S-DKK1 ≥ 5800 pg/mL N (%) | p Value (a) Two-Sided Fisher Exact Test (b) Two-Sided Pearson Chi Quadrat Test | ||
|---|---|---|---|---|---|
| Gender | 0.502 (a) | ||||
| Female | 11 (18%) | 8 (72.7%) | 3 (27.3%) | ||
| Male | 50 (82%) | 29 (58%) | 21 (42%) | ||
| Age | 0.795 (a) | ||||
| <65 | 30 (49.2%) | 19 (63.3%) | 11 (36.7%) | ||
| ≥65 | 31 (50.8%) | 18 (58.1%) | 13 (41.9%) | ||
| Tumor Stage | 0.107 (b) | ||||
| T1 | 14 (23%) | 10 (71.4%) | 4 (28.6%) | ||
| T2 | 6 (9.8%) | 6 (100%) | 0 (0%) | ||
| T3 | 35 (57.4%) | 18 (51.4%) | 17 (48.6%) | ||
| T4 | 6 (9.8%) | 3 (50%) | 3 (50%) | ||
| Lymphatic Node Metastasis | 0.146 (b) | ||||
| N0 | 20 (32.8%) | 15 (75%) | 5 (25%) | ||
| N1 | 18 (29.5%) | 8 (44.4%) | 10 (55.6%) | ||
| N2 | 14 (23%) | 10 (71.4%) | 4 (28.6%) | ||
| N3 | 9 (14.8%) | 4 (44.4%) | 5 (55.6%) | ||
| Distant Metastasis | 0.515 (a) | ||||
| M0 | 59 (96.7%) | 35 (59.3%) | 24 (40.7%) | ||
| M1 | 2 (3.3%) | 2 (100%) | 0 (0%) | ||
| Lymphatic Vessel Infiltration | 0.232 (a) | ||||
| L0 | 16 (27.6%) | 12 (75%) | 4 (25%) | ||
| L1 | 42 (72.4%) | 23 (54.8%) | 19 (45.2%) | ||
| Venous Vessel Infiltration | 0.265 (a) | ||||
| V0 | 41 (69.5%) | 27 (65.9%) | 14 (34.1%) | ||
| V1 | 18 (30.5%) | 9 (50%) | 9 (50%) | ||
| Grading | 0.846 (b) | ||||
| G1 | 4 (6.8%) | 2 (50%) | 2 (50%) | ||
| G2 | 19 (32.2%) | 13 (68.4%) | 6 (31.6%) | ||
| G3 | 34 (57.6%) | 20 (58.8%) | 14 (41.2%) | ||
| G4 | 2 (3.4%) | 1 (50%) | 1 (50%) | ||
| Resection Status | 0.741 (a) | ||||
| R0 | 49 (81.7%) | 30 (61.2%) | 19 (38.8%) | ||
| R1 | 11 (18.3%) | 6 (54.5%) | 5 (45.5%) | ||
| Neoadjuvant Treatment | 0.072 (a) | ||||
| No | 51 (85%) | 28 (54.9%) | 23 (45.1%) | ||
| Yes | 9 (15%) | 8 (88.9%) | 1 (11.1%) | ||
| Recurrence | 0.029 (a) | ||||
| No | 20 (33.3%) | 16 (80%) | 4 (20%) | ||
| Yes | 40 (66.7%) | 20 (50%) | 20 (50%) | ||
| CTC | |||||
| No | 36 (76.6%) | 28 (77.8%) | 8 (22.2%) | 0.004 (a) | |
| Yes | 11 (23.4%) | 3 (27.3%) | 8 (72.7%) | ||
| DTC | |||||
| No | 34 (73.9%) | 26 (76.5%) | 8 (23.5%) | 0.013 (a) | |
| Yes | 12 (26.1%) | 4 (33.3%) | 8 (66.7%) |
| N | Time to Relapse (Median) in Months | (95% CI) ± Standard Error | Logrank Test (Mantel-Cox) p-Value | ||
|---|---|---|---|---|---|
| S-DKK1 | <0.0005 | ||||
| Low | 62 | 22 | (15.43–28.57) ± 3.35 | ||
| High | 28 | 12 | (8.2–15.8) ± 1.94 | ||
| CTC | 0.003 | ||||
| No | 48 | 22 | (12.43–31.57) ± 4.883 | ||
| Yes | 17 | 11 | (9.01–12.99) ± 1.01 | ||
| DTC | 0.059 | ||||
| No | 49 | 18 | (8.3–27.70) ± 4.95 | ||
| Yes | 14 | 11 | (7.33–14.67) ± 1.87 |
| N | Overall Survival (Median) in Months | (95% CI) ± Standard Error | Logrank (Mantel-Cox) p-Value | ||
|---|---|---|---|---|---|
| DKK1 | 0.003 | ||||
| low | 63 | 26 | (21.30–30.70) ± 2.40 | ||
| high | 28 | 14 | (8.65–19.36) ± 2.73 | ||
| CTC | 0.010 | ||||
| No | 48 | 26 | (21.47–30.53) ± 2.31 | ||
| Yes | 17 | 13 | (6.59–19.41) ± 3.27 | ||
| DTC | 0.116 | ||||
| No | 49 | 24 | (17.58–30.42) ± 3.28 | ||
| Yes | 14 | 13 | (5.67–20.33) ± 3.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, J.G.; Smit, D.J.; Viol, F.; Schrader, J.; Ghadban, T.; Pantel, K.; Izbicki, J.R.; Reeh, M. High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients. Cancers 2021, 13, 4980. https://doi.org/10.3390/cancers13194980
Ramirez JG, Smit DJ, Viol F, Schrader J, Ghadban T, Pantel K, Izbicki JR, Reeh M. High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients. Cancers. 2021; 13(19):4980. https://doi.org/10.3390/cancers13194980
Chicago/Turabian StyleRamirez, José Giron, Daniel J. Smit, Fabrice Viol, Jörg Schrader, Tarik Ghadban, Klaus Pantel, Jakob R. Izbicki, and Matthias Reeh. 2021. "High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients" Cancers 13, no. 19: 4980. https://doi.org/10.3390/cancers13194980







