Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Non-Functioning Secreting Pituitary Adenoma
3.2. GH-Secreting Pituitary Adenoma
3.3. ACTH-Secreting Pituitary Adenoma
3.4. PRL-Secreting Pituitary Adenoma
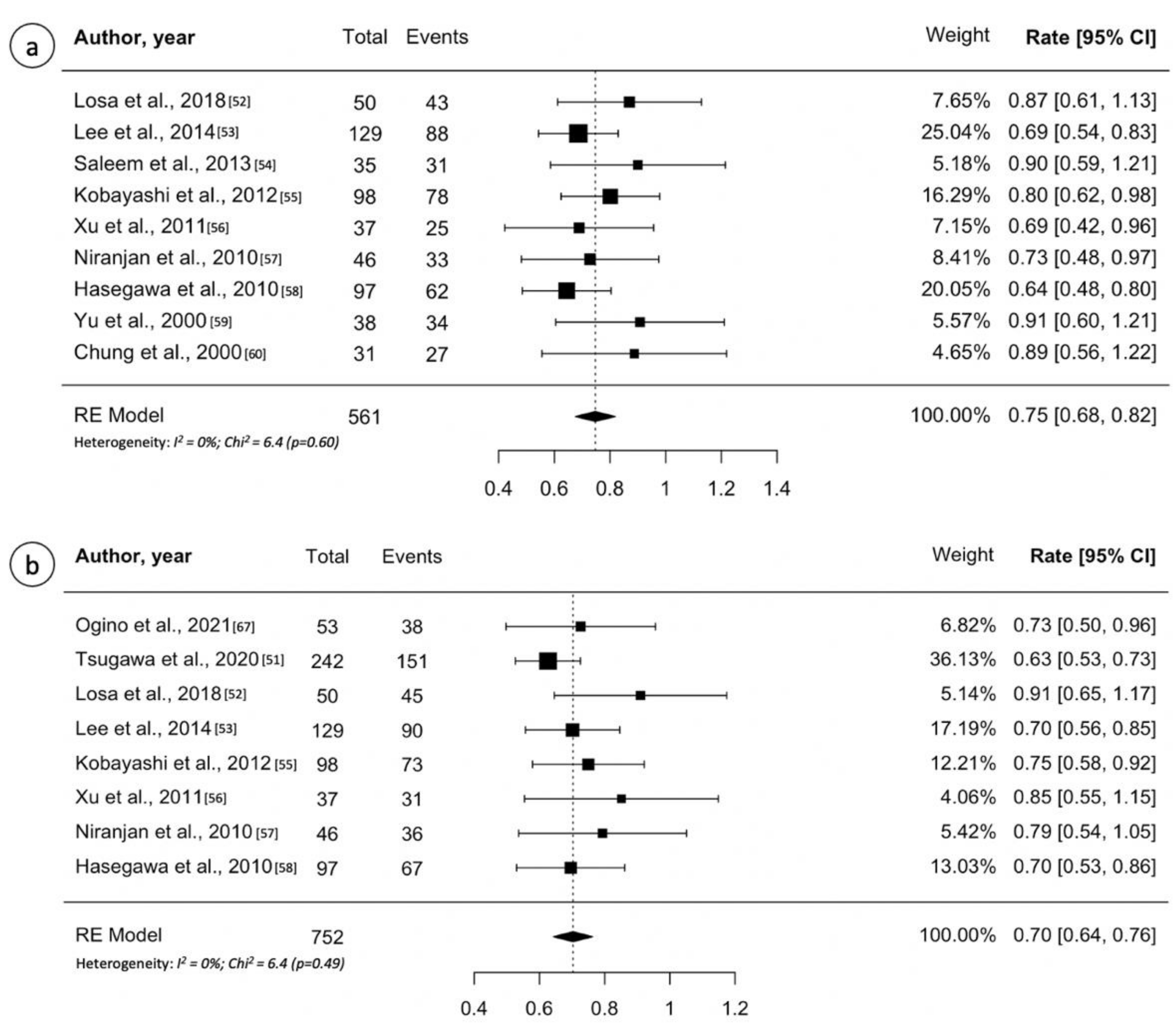
3.5. Craniopharyngioma
4. Discussion
4.1. Gamma Knife Outcome for Non-Functioning Pituitary Adenoma
4.2. Gamma Knife Outcome for Secreting Pituitary Adenoma
4.3. Gamma Knife Outcome for Craniopharyngioma
4.4. Complications
4.4.1. Hypopituitarism
4.4.2. Optic Neuropathy
4.4.3. Other Rare Toxicities
4.5. Multisession Gamma Knife Radiosurgery for Pituitary Tumors
4.6. Other Pituitary Tumors
4.7. Methodological Considerations and Limitations
5. Key Takeaways
- A margin dose of 12–15 Gy is used for nonfunctioning pituitary adenomas;
- Higher margin doses (up to 20–30 Gy) are used for functional adenomas;
- GK SRS is safe and provides tumor control in >90% patients with recurrent or residual nonfunctioning pituitary adenomas;
- Risks of visual dysfunction, or neurological deficit appear to be quite low;
- Delayed Endocrinopathy can be expected in 30–40% patients;
- The endocrine remission response to SRS is best with ACTH producing tumors, followed by GH producing tumors, with prolactinoma having the poorest response.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Mortini, P.; Barzaghi, L.R.; Albano, L.; Panni, P.; Losa, M. Microsurgical therapy of pituitary adenomas. Endocrine 2017, 59, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chatzellis, E.; Alexandraki, K.I.; Androulakis, I.I.; Kaltsas, G. Aggressive Pituitary Tumors. Neuroendocrinology 2015, 101, 87–104. [Google Scholar] [CrossRef]
- Lee, C.-C.; Sheehan, J.P. Advances in Gamma Knife radiosurgery for pituitary tumors. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 331–338. [Google Scholar] [CrossRef]
- Albano, L.; Losa, M.; Flickinger, J.; Mortini, P.; Minniti, G. Radiotherapy of Parasellar Tumours. Neuroendocrinology 2020, 110, 848–858. [Google Scholar] [CrossRef]
- Kotecha, R.; Sahgal, A.; Rubens, M.; De Salles, A.; Fariselli, L.; Pollock, B.E.; Levivier, M.; Ma, L.; Paddick, T.; Regis, J.; et al. Stereotactic radiosurgery for non-functioning pituitary adenomas: Meta-analysis and International Stereotactic Radiosurgery Society practice opinion. Neuro. Oncol. 2020, 22, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Didwania, P.; Lehrer, E.J.; Sheehan, D.; Sheehan, K.; Trifiletti, D.M.; Trifiletti, D.M.; Sheehan, J.P. Stereotactic radiosurgery for acromegaly: An in-ternational systematic review and meta-analysis of clinical outcomes. J. Neurooncol. 2020, 148, 401–418. [Google Scholar] [CrossRef]
- Heringer, L.C.; De Lima, M.M.; Rotta, J.M.; Botelho, R.V. Effect of Stereotactic Radiosurgery on Residual or Relapsed Pituitary Adenoma: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 136, 374–381.e4. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.; Li, X.; Wu, L.; Quan, T.; Peng, C.; Fu, J.; Yang, X.; Yu, J. Long-term results of Gamma Knife Radiosurgery for Postsurgical residual or recurrent nonfunctioning Pituitary Adenomas. Int. J. Med. Sci. 2020, 17, 1532–1540. [Google Scholar] [CrossRef]
- Sun, S.; Liu, A.; Zhang, Y. Long-Term Follow-Up Studies of Gamma Knife Radiosurgery for Postsurgical Nonfunctioning Pitu-itary Adenomas. World Neurosurg. 2019, 124, e715–e723. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, C.S.; Link, M.J.; Brown, P.D.; Young, W.F., Jr.; Pollock, B.E. Hypopituitarism After Single-Fraction Pituitary Adenoma Ra-diosurgery: Dosimetric Analysis Based on Patients Treated Using Contemporary Techniques. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Mohammed, N.; Bir, S.C.; Savardekar, A.R.; Patra, D.P.; Bollam, P.; Nanda, A. Long-Term Outcome of Nonfunctioning and Hormonal Active Pituitary Adenoma After Gamma Knife Radiosurgery. World Neurosurg. 2018, 114, e824–e832. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Xu, Z.; Lee, C.-C.; Wu, C.-C.; Chytka, T.; Silva, D.; Sharma, M.; Radwan, H.; Grills, I.S.; Nguyen, B.; et al. Prognostic significance of corticotroph staining in radiosurgery for non-functioning pituitary adenomas: A multicenter study. J. Neuro-Oncology 2017, 135, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sadik, Z.H.A.; Voormolen, E.H.J.; Depauw, P.; Burhani, B.; Nieuwlaat, W.A.; Verheul, J.; Leenstra, S.; Fleischeuer, R.; Hanssens, P.E.J. Treatment of Nonfunctional Pituitary Adenoma Postoperative Remnants: Adjuvant or Delayed Gamma Knife Radiosurgery? World Neurosurg. 2017, 100, 361–368. [Google Scholar] [CrossRef]
- Losa, M.; Spatola, G.; Albano, L.; Gandolfi, A.; del Vecchio, A.; Bolognesi, A.; Mortini, P. Frequency, pattern, and outcome of recurrences after gamma knife radiosurgery for pituitary adenomas. Endocrine 2016, 56, 595–602. [Google Scholar] [CrossRef]
- Bir, S.C.; Murray, R.D.; Ambekar, S.; Bollam, P.; Nanda, A. Clinical and Radiologic Outcome of Gamma Knife Radiosurgery on Nonfunctioning Pituitary Adenomas. J. Neurol. Surg. Part. B Skull Base 2015, 76, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Kano, H.; Yang, H.-C.; Xu, Z.; Yen, C.-P.; Chung, W.-Y.; Pan, D.H.-C.; Lunsford, L.D.; Sheehan, J.P. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas. J. Neurosurg. 2014, 120, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Zeiler, F.A.; Bigder, M.; Kaufmann, A.; McDonald, P.J.; Fewer, D.; Butler, J.; Schroeder, G.; West, M. Gamma knife in the treatment of pituitary adenomas: Results of a single center. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2013, 40, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, J.P.; Starke, R.M.; Mathieu, D.; Young, B.; Sneed, P.K.; Chiang, V.L.; Lee, J.Y.K.; Kano, H.; Park, K.-J.; Niranjan, A.; et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study. J. Neurosurg. 2013, 119, 446–456. [Google Scholar] [CrossRef]
- Park, K.-J.; Kano, H.; Parry, P.V.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D.; Kondziolka, D. Long-term Outcomes After Gamma Knife Stereotactic Radiosurgery for Nonfunctional Pituitary Adenomas. Neurosurgery 2011, 69, 1188–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, M.; Chernov, M.; Tamura, N.; Nagai, M.; Yomo, S.; Ochiai, T.; Amano, K.; Izawa, M.; Hori, T.; Muragaki, Y.; et al. Gamma Knife robotic microradiosurgery of pituitary adenomas invading the cavernous sinus: Treatment concept and results in 89 cases. J. Neuro-Oncology 2010, 98, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Cochran, J.; Natt, N.; Brown, P.D.; Erickson, D.; Link, M.J.; Garces, Y.I.; Foote, R.L.; Stafford, S.L.; Schomber, P.J. Gamma knife radiosurgery for patients with nonfunc-tioning pituitary adenomas: Results from a 15-year experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Liščák, R.; Vladyka, V.; Marek, J.; Šimonová, G.; Vymazal, J. Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir. 2007, 149, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Yamanaka, K.; Yoshioka, K. Radiosurgery for Nonfunctioning Pituitary Adenomas. Neurosurgery 2005, 56, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, Z.; Yu, C.; Giannotta, S.L.; Zee, C.-S.; Apuzzo, M.L. Gamma Knife Radiosurgery for Pituitary Adenoma: Early Results. Neurosurgery 2003, 53, 51–61. [Google Scholar] [CrossRef]
- Wowra, B.; Stummer, W. Efficacy of gamma knife radiosurgery for nonfunctioning pituitary adenomas: A quantitative follow up with magnetic resonance imaging—based volumetric analysis. J. Neurosurg. 2002, 97, 429–432. [Google Scholar] [CrossRef]
- Balossier, A.; Tuleasca, C.; Cortet-Rudelli, C.; Soto-Ares, G.; Levivier, M.; Assaker, R.; Reyns, N. Gamma Knife radiosurgery for acro-megaly: Evaluating the role of the biological effective dose associated with endocrine remission in a series of 42 consecutive cases. Clin. Endocrinol. (Oxf) 2021, 94, 424–433. [Google Scholar] [CrossRef]
- Uygur, M.M.; Deyneli, O.; Yavuz, D.G. Long-term endocrinological outcomes of gamma knife radiosurgery in acromegaly pa-tients. Growth Horm IGF Res. 2020, 55, 101335. [Google Scholar] [CrossRef]
- Kong, D.S.; Kim, Y.H.; Kim, Y.H.; Hur, K.Y.; Kim, J.H.; Kim, M.S.; Paek, S.H.; Kwon, D.-H.; Kim, D.-K.; Lee, J.-I. Long-Term Efficacy and Tolerability of Gamma Knife Radio-surgery for Growth Hormone-Secreting Adenoma: A Retrospective Multicenter Study (MERGE-001). World Neurosurg. 2019, 122, e1291–e1299. [Google Scholar] [CrossRef]
- Ding, D.; Mehta, G.U.; Patibandla, M.R.; Lee, C.C.; Liscak, R.; Kano, H.; Pai, F.-Y.; Kosak, M.; Sisterson, N.D.; Martinez-Alvarez, R.; et al. Stereotactic Radiosurgery for Acromegaly: An Interna-tional Multicenter Retrospective Cohort Study. Neurosurgery 2019, 84, 717–725. [Google Scholar] [CrossRef]
- Lee, C.-C.; Vance, M.L.; Xu, Z.; Yen, C.-P.; Schlesinger, D.; Dodson, B.; Sheehan, J. Stereotactic Radiosurgery for Acromegaly. J. Clin. Endocrinol. Metab. 2014, 99, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kano, H.; Kondziolka, D.; Park, K.J.; Iyer, A.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Gamma knife radiosurgery for clinically persistent acro-megaly. J. Neurooncol. 2012, 109, 71–79. [Google Scholar] [CrossRef]
- Franzin, A.; Spatola, G.; Losa, M.; Picozzi, P.; Mortini, P. Results of Gamma Knife Radiosurgery in Acromegaly. Int. J. Endocrinol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, J.; Sheehan, J.P.; Pouratian, N.; Laws, E.R.; Steiner, L.; Vance, M.L. Gamma Knife surgery for Cushing’s disease. J. Neurosurg. 2007, 106, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, B.E.; Jacob, J.T.; Brown, P.D.; Nippoldt, T.B. Radiosurgery of growth hormone-producing pituitary adenomas: Factors as-sociated with biochemical remission. J. Neurosurg. 2007, 106, 833–838. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Øksnes, M.; Pedersen, P.-H.; Wentzel-Larsen, T.; Rødahl, E.; Thorsen, F.; Schreiner, T.; Aanderud, S.; Lund-Johansen, M. Gamma knife stereotactic radiosurgery for acromegaly. Eur. J. Endocrinol. 2007, 157, 255–263. [Google Scholar] [CrossRef]
- Jezkova, J.; Marek, J.; Hana, V.; Krsek, M.; Weiss, V.; Vladyka, V.; Lisák, R.; Vymazal, J.; Pecen, L. Gamma knife radiosurgery for acromegaly--long-term ex-perience. Clin. Endocrinol. (Oxf). 2006, 64, 588–595. [Google Scholar] [CrossRef]
- Castinetti, F.; Taieb, D.; Kuhn, J.-M.; Chanson, P.; Tamura, M.; Jaquet, P.; Conte-Devolx, B.; Régis, J.; Dufour, H.; Brue, T. Outcome of Gamma Knife Radiosurgery in 82 Patients with Acromegaly: Correlation with Initial Hypersecretion. J. Clin. Endocrinol. Metab. 2005, 90, 4483–4488. [Google Scholar] [CrossRef] [Green Version]
- Attanasio, R.; Epaminonda, P.; Motti, E.; Giugni, E.; Ventrella, L.; Cozzi, R.; Farabola, M.; Loli, P.; Beck-Peccoz, P.; Arosio, M. Gamma-Knife Radiosurgery in Acromegaly: A 4-Year Follow-Up Study. J. Clin. Endocrinol. Metab. 2003, 88, 3105–3112. [Google Scholar] [CrossRef]
- Bunevicius, A.; Kano, H.; Lee, C.C.; Krsek, M.; Nabeel, A.M.; El-Shehaby, A.; Karim, K.A.; Martinez-Moreno, N.; Mathieu, D.; Lee, J.Y.K.; et al. Early versus late Gamma Knife radiosurgery for Cushing’s disease after prior resection: Results of an international, multicenter study. J. Neurosurg. 2020, 134, 807–815. [Google Scholar] [CrossRef]
- Bunevicius, A.; Sheehan, D.; Vance, M.L.; Schlesinger, D.; Sheehan, J.P. Outcomes of Cushing’s disease following Gamma Knife radiosurgery: Effect of a center’s growing experience and era of treatment. J. Neurosurg. 2021, 134, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Ding, D.; Patibandla, M.R.; Kano, H.; Sisterson, N.; Su, Y.-H.; Krsek, M.; Nabeel, A.M.; El-Shehaby, A.; Kareem, K.; et al. Stereotactic Radiosurgery for Cushing Disease: Results of an International, Multicenter Study. J. Clin. Endocrinol. Metab. 2017, 102, 4284–4291. [Google Scholar] [CrossRef]
- Losa, M.; Picozzi, P.; Redaelli, M.G.; Laurenzi, A.; Mortini, P. Pituitary Radiotherapy for Cushing’s Disease. Neuroendocrinology 2010, 92, 107–110. [Google Scholar] [CrossRef]
- Castinetti, F.; Nagai, M.; Dufour, H.; Kuhn, J.-M.; Morange, I.; Jaquet, P.; Conte-Devolx, B.; Regis, J.; Brue, T. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur. J. Endocrinol. 2007, 156, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Xu, Z.; Salvetti, D.J.; Schmitt, P.J.; Vance, M.L. Results of gamma knife surgery for Cushing’s disease. J. Neurosurg. 2013, 119, 1486–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, M.; Samanci, Y.; Yilmaz, M.; Sengoz, M.; Peker, S. Gamma knife radiosurgery for high-risk lactotroph adenomas: Long-term results. J. Clin. Neurosci. 2021, 86, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-C.; Lee, C.-C.; Yang, H.-C.; Mohammed, N.; Kearns, K.N.; Nabeel, A.M.; Karim, K.A.; Eldin, R.M.E.; El-Shehaby, A.M.N.; Reda, W.A.; et al. The benefit and risk of stereotactic radiosurgery for prolactinomas: An international multicenter cohort study. J. Neurosurg. 2020, 133, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Xu, Z.; Schlesinger, D.; Vance, M.L.; Sheehan, J.P. Gamma Knife radiosurgery for medically and surgically refractory prolactinomas: Long-term results. Pituitary 2015, 18, 820–830. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, N.; Wang, E.M.; Wang, B.J.; Dai, J.Z.; Cai, P.W. Gamma knife radiosurgery as a primary treatment for prolactinomas. J. Neurosurg. 2000, 93, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, T.; Kobayashi, T.; Hasegawa, T.; Iwai, Y.; Matsunaga, S.; Yamamoto, M.; Hayashi, M.; Kenai, H.; Kano, T.; Mori, H.; et al. Gamma Knife Surgery for Residual or Recurrent Craniopharyngioma After Surgical Resection: A Multi-institutional Retrospective Study in Japan. Cureus 2020, 12, e6973. [Google Scholar] [CrossRef] [Green Version]
- Losa, M.; Pieri, V.; Bailo, M.; Gagliardi, F.; Barzaghi, L.R.; Gioia, L.; del Vecchio, A.; Bolognesi, A.; Mortini, P. Single fraction and multisession Gamma Knife radiosurgery for craniopharyngioma. Pituitary 2018, 21, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Yang, H.-C.; Chen, C.-J.; Hung, Y.-C.; Wu, H.-M.; Shiau, C.-Y.; Guo, W.-Y.; Pan, D.H.-C.; Chung, W.-Y.; Liu, K.-D. Gamma Knife surgery for craniopharyngioma: Report on a 20-year experience. J. Neurosurg. 2014, 121, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.A.; Hashim, A.S.; Rashid, A.; Ali, M. Role of gamma knife radiosurgery in multimodality management of cranio-pharyngioma. Acta Neurochir. Suppl. 2013, 116, 55–60. [Google Scholar]
- Kobayashi, T.; Mori, Y.; Tsugawa, T.; Hashizume, C.; Takahashi, H. PROGNOSTIC FACTORS FOR TUMOR RECURRENCE AFTER GAMMA KNIFE RADIOSURGERY OF PARTIALLY RESECTED AND RECURRENT CRANIOPHARYNGIOMAS. Nagoya J. Med. Sci. 2012, 74, 141–147. [Google Scholar]
- Xu, Z.; Yen, C.-P.; Schlesinger, D.; Sheehan, J. Outcomes of Gamma Knife surgery for craniopharyngiomas. J. Neuro-Oncology 2010, 104, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Kano, H.; Mathieu, D.; Kondziolka, D.; Flickinger, J.; Lunsford, L.D. Radiosurgery for Craniopharyngioma. Int. J. Radiat. Oncol. 2010, 78, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kobayashi, T.; Kida, Y. Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radio-surgery: Evaluation in 100 patients with craniopharyngioma. Neurosurgery 2010, 66, 688–694, discussion 94–95. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, Z.; Li, S. Combined treatment with stereotactic intracavitary irradiation and gamma knife surgery for cranio-pharyngiomas. Stereotact. Funct. Neurosurg. 2000, 75, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.Y.; Pan, D.H.; Shiau, C.Y.; Guo, W.Y.; Wang, L.W. Gamma knife radiosurgery for craniopharyngiomas. J. Neurosurg. 2000, 93 (Suppl. 3), 47–56. [Google Scholar] [CrossRef]
- Ogino, A.; Niranjan, A.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. Optimizing stereotactic radiosurgery in patients with recurrent or residual craniopharyngiomas. J. Neuro-Oncology 2021, 154, 113–120. [Google Scholar] [CrossRef]
- Mingione, V.; Yen, C.P.; Vance, M.L.; Steiner, M.; Sheehan, J.; Laws, E.R.; Steiner, L. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J. Neurosurg. 2006, 104, 876–883. [Google Scholar] [CrossRef]
- Pomeraniec, I.J.; Kano, H.; Xu, Z.; Nguyen, B.; Siddiqui, Z.; Silva, D.; Sharma, M.; Radwan, H.; Cohen, J.A.; Dallapiazza, R.F.; et al. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: A multicenter matched-cohort study. J. Neurosurg. 2018, 129, 648–657. [Google Scholar] [CrossRef]
- Pollock, B.E.; Nippoldt, T.B.; Stafford, S.L.; Foote, R.L.; Abboud, C.F. Results of stereotactic radiosurgery in patients with hor-mone-producing pituitary adenomas: Factors associated with endocrine normalization. J. Neurosurg. 2002, 97, 525–530. [Google Scholar] [CrossRef]
- Landolt, A.M.; Haller, D.; Lomax, N.; Scheib, S.; Schubiger, O.; Siegfried, J.; Wellis, G. Octreotide may act as a radioprotective agent in acromegaly. J. Clin. Endocrinol. Metab. 2000, 85, 1287–1289. [Google Scholar] [CrossRef]
- Losa, M.; Resmini, E.; Barzaghi, L.R.; Albano, L.; Bailo, M.; Webb, S.M.; Mortini, P. Resistance to first-generation somatostatin receptor ligands does not impair the results of gamma knife radiosurgery in acromegaly. Clin. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Chang, C.-L.; Yuan, K.S.-P.; Wu, A.T.; Wu, S.-Y. Toxicity Profiles of Fractionated Radiotherapy, Contemporary Stereotactic Radiosurgery, and Transsphenoidal Surgery in Nonfunctioning Pituitary Macroadenomas. Cancers 2019, 11, 1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, D.; Xu, Z.; Mehta, G.; Ding, D.; Vance, M.L.; Kano, H.; Sisterson, N.; Yang, H.-C.; Kondziolka, D.; Lunsford, L.D.; et al. Hypopituitarism after Gamma Knife radiosurgery for pituitary adenomas: A multicenter, international study. J. Neurosurg. 2019, 131, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, M.T.; Grimm, J.; Soltys, S.G.; Yorke, E.; Moiseenko, V.; Tomé, W.A.; Sahgal, A.; Xue, J.; Ma, L.; Solberg, T.D.; et al. Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways. Int. J. Radiat. Oncol. 2021, 110, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Spatola, G.; Frosio, L.; Losa, M.; del Vecchio, A.; Piloni, M.; Mortini, P. Asymptomatic internal carotid artery occlusion after gamma knife radiosurgery for pituitary adenoma: Report of two cases and review of the literature. Rep. Pract. Oncol. Radiother. 2016, 21, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeloos, L.; Levivier, M.; Devriendt, D.; Massager, N. Internal carotid occlusion following gamma knife radiosurgery for cav-ernous sinus meningioma. Stereotact. Funct. Neurosurg. 2007, 85, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Losa, M.; Nadin, F.; Barzaghi, L.R.; Parisi, V.; Del Vecchio, A.; Bolognesi, A.; Mortini, P. Safety and efficacy of multisession gamma knife radiosurgery for residual or recurrent pituitary adenomas. Endocrine 2019, 64, 639–647. [Google Scholar] [CrossRef]
- Boström, J.P.; Meyer, A.; Pintea, B.; Gerlach, R.; Surber, G.; Lammering, G.; Hamm, K. Risk-adapted single or fractionated stereotactic high-precision radiotherapy in a pooled series of nonfunctioning pituitary adenomas: High local control and low toxicity. Strahlenther. Onkol. 2014, 190, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, J.P.; Kinfe, T.; Meyer, A.; Pintea, B.; Gerlach, R.; Surber, G.; Lammering, G.; Hamm, K. Treatment of acromegaly patients with risk-adapted single or fractionated stereotactic high-precision radiotherapy: High local control and low toxicity in a pooled series. Strahlenther. Onkol. 2015, 191, 477–485. [Google Scholar] [CrossRef]
- Phillips, J.; East, H.E.; French, S.E.; Melcescu, E.; Hamilton, R.D.; Nicholas, W.C.; Fratkin, J.F.; Parent, A.D.; Luzardo, G.; Koch, C.A. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones (Athens) 2012, 11, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-S.; Hwang, J.-H.; Hwang, S.-K.; Kim, S.; Park, S.-H. Pituitary carcinoma with fourth ventricle metastasis: Treatment by excision and Gamma-knife radiosurgery. Pituitary 2013, 17, 514–518. [Google Scholar] [CrossRef]
- Akyoldaş, G.; Hergünsel, B.; Özdemir, I.E.; Şengöz, M.; Peker, S. Gamma knife radiosurgery for pituitary spindle cell oncocytomas. Clin. Neurol. Neurosurg. 2019, 187, 105560. [Google Scholar] [CrossRef] [PubMed]





| Author | Year | No. | Median Dose (Gy) | Median FU (Months) | Overall Tumor Control (%) | PFS (5-y) | PFS (10-y) | Tumor Shrinkage (%) | New Hypopituitarism (%) | Optic Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|
| Deng et al. [11] | 2020 | 148 | 14 | 65 | 87% | 88% | 74% | 75% | 28% | 4% |
| Sun et al. [12] | 2019 | 204 | 14 | 86 | 90% | 95% | 92% | 50% | 18% | 2.5% |
| Graffeo et al. [13] | 2018 | 97 | 15 | 48 | 99% | 100% | NR | 52% | 31% | 0% |
| Narayan et al. [14] | 2018 | 87 | 15 | 48 | 91% | 91% | NR | 54% | 21% | 0% |
| Cohen-Inbar et al. [15] | 2017 | 357 | 14 | 40 | 91% | 91% | NR | 80.5% | 4% | 1% |
| Sadik et al. [16] | 2017 | 50 | 15 | 40 | 95% | 95% | NR | 24% | 22% | 0% |
| Losa et al. [17] | 2017 | 272 | 15 | 79 | 90% | 95% | 79% | NR | NR | NR |
| Bir et al. [18] | 2015 | 57 | 15 | 46 | 90% | 98% | 90% | 56% | 19% | 3.5% |
| Lee et al. [19] * | 2014 | 41 | 12 | 48 | 93% | 94% | 85% | 83% | 25% | 0% |
| Zeiler et al. [20] | 2013 | 43 | 14 | 35 | 98% | 98% | NR | 51% | NR | 2% |
| Sheehan et al. [21] | 2013 | 512 | 16 | 36 | 93% | 95% | 85% | NR | 21% | 7% |
| Park et al. [22] | 2011 | 125 | 13 | 62 | 90% | 94% | 76% | 53% | 24% | 2% |
| Hayashi et al. [23] | 2010 | 43 | 18 | 36 | 100% | 100% | NR | 64% | 0%* | 0%* |
| Pollock et al. [24] | 2008 | 62 | 16 | 64 | 95% | 95% | NR | 60% | 32% | 0% |
| Liscak et al. [25] | 2007 | 79 | 20 | 60 | 100% | NR | NR | NR | 14% | 0% |
| Iwai et al. [26] | 2005 | 31 | 14 | 60 | 87% | 93% | NR | 58% | 7% | 0% |
| Petrovich et al. [27] | 2002 | 56 | 15 | 36 | 100% | NR | NR | NR | 4% | 0% |
| Wowra et al. [28] | 2002 | 30 | 16 | 58 | 93% | 93% | NR | NR | 14% | 0% |
| Author | Year | No. | Median Dose (Gy) | Median FU (Months) | Remission Rate (%) | Recurrence (%) | Hormonal Criteria | RFS (5-y) | RFS (10-y) | Tumor Shrinkage (%) | New Hypopituitarism (%) | Optic Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balossier et al. [29] | 2020 | 42 | 28 | 60.5 | 52% | NR | IGF-1 | 57% at 7 years | NR | 36% | 19% | 5% |
| Uygur et al. [30] | 2020 | 110 | 23^ | 78 | 16% | NR | IGF-1; GH < 1 µg/L | NR | NR | 94% | 5% | NR |
| Kong et al. [31] | 2019 | 138 | 25 | 85^ | 34% | NR | IGF-1; GH ≤ 2.5 µg/L | 20% | 45% | NR | 9% | NR |
| Ding et al. [32] | 2018 | 371 | 24^ | 79^ | 54% | 9% | IGF-1 | 51% | 69% | 65% | 26% | 3.5% |
| Lee et al. [33] | 2014 | 136 | 25 | 61.5 | 65% | 8% | IGF-1; OGT-GH < 1 µg/L | 73% at 6 years | NR | 47% | 32% | 3% |
| Liu et al. [34] | 2012 | 40 | 21 | 72 | 48% | NR | IGF-1; GH < 2.5 µg/L | 45% | NR | 68% | 40% | 0% |
| Franzin et al. [35] | 2012 | 103 | 21.5 | 71 | 61% | 3% | IGF-1; GH < 2.5 µg/L | 58% | 80% | 43% | 8% | 0% |
| Jagannathan et al. [36] | 2008 | 95 | 22 | 57^ | 53% | NR | IGF-1 | NR | NR | 92% | 34% | 4% |
| Pollock et al. [37] | 2007 | 46 | 20 | 63 | 50% | NR | IGF-1; GH < 2 µg/L | 60% | NR | 70% | 33% | 0% |
| Vik-Mo et al. [38] | 2007 | 61 | 26 | 67^ | 57% | NR | IGF-1 | 58% | 86% | NR | 23% | NR |
| Jezkova et al. [39] | 2006 | 96 | NR | 54 | 57% | NR | IGF-1; OGT-GH < 1 µg/L | 44% | NR | 62% | 32% | 0% |
| Castinetti et al. [40] | 2005 | 82 | 26 | 49 | 17% | NR | IGF-1; GH < 2 µg/L | NR | NR | NR | 17% | NR |
| Attanasio et al. [41] | 2003 | 30 | 20 | 46 | 23% | NR | IGF-1; GH < 2.5 µg/L | NR | NR | 37% | 0% | 2% |
| Author | Year | No. | Median Dose (Gy) | Median FU (Months) | Remission Rate (%) | Recurrence (%) | Hormonal Criteria | RFS (5-y) | RFS (10-y) | Tumor Shrinkage (%) | New Hypopituitarism (%) | Optic Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bunevicius et al. [43] | 2020 | 134 | 22 ^ | 64 | 75% | 18% | UFC; cortisol | 72% | NR | 53% | 35% | 2% |
| Bunevicius et al. [42] * | 2020 | 255 | 23 ^ | 66 ^ | 69% | 18% | UFC; cortisol | 68% | NR | 41% | 26% | 2% |
| Mehta et al. [44] | 2017 | 278 | 24 ^ | 51 | 69% | 18% | UFC | 77% | 80% | NR | 25% | 1% |
| Shehaan et al. [47] | 2013 | 96 | 22 ^ | 48 | 70% | 16% | UFC; cortisol | 78% | NR | 70% | 36% | 2% |
| Losa et al. [45] | 2010 | 49 | 25 | 48 | 53% | NR | UFC | 66% | NR | NR | NR | NR |
| Castinetti et al. [46] | 2007 | 40 | 29.5 | 48 | 43% | NR | UFC; LDDST | NR | NR | NR | 15% | 0% |
| Author | Year | No. | Median Dose (Gy) | Median FU (Months) | Remission Rate (%) | Recurrence Rate (%) | Hormonal Criteria | RFS (5-y) | RFS (10-y) | Tumor Shrinkage (%) | New Hypopituitarism (%) | Optic Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kara et al. [48] | 2021 | 52 | 17 | 13 | 33% | NR | Normal PRL | NR | NR | 69% | 19% | 4% |
| Hung et al. [49] | 2019 | 289 | 22 | 43 | 43% | NR | Normal PRL | 41% | NR | NR | 25% | 3% |
| Cohen-Inbar et al. [50] | 2015 | 38 | 25 | 42 | 50% | NR | Normal PRL | NR | NR | NR | 26% | NR |
| Pan et al. [51] | 2000 | 128 | 31 ^ | 45 ^ | 15% | NR | Normal PRL | NR | NR | NR | NR | NR |
| Author | Year | No. | Median Dose (Gy) | Median FU (Months) | Overall Tumor Control (%) | PFS (5-y) | PFS (10-y) | Tumor Shrinkage (%) | New Hypopituitarism (%) | Optic Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|
| Ogino et al. [62] | 2021 | 53 | 12 | 118 | NR | 72% | 53% | NR | 2% | 2% |
| Tsugawa et al. [52] | 2020 | 242 | 11.4 ^ | 61 | NR | 62% | 43% | NR | 9% | 2% |
| Losa et al. [53] | 2018 | 50 | 14.3 ^ | 75 ^ | 86% | 90% | 78% | 64% | 20% | 2% |
| Lee et al. [54] | 2014 | 137 | 12 | 46 | 69% | 70% | 44% | 54% | 8% | 1% |
| Saleem et al. [55] | 2013 | 35 | 11.5 | 22 | 88% | NR | NR | NR | 0% | NR |
| Kobayashi et al. [56] | 2012 | 100 | 11.5 | 65 | 80% | 74% | 60% | NR | NR | NR |
| Xu et al. [57] | 2011 | 37 | 14.5 | 50 | 68% | 85% | 67% | 69% | 3% | 0% |
| Niranjan et al. [58] | 2010 | 46 | 13 | 62 ^ | 71% | 78% | NR | 78% | 0% | 0% |
| Hasegawa et al. [59] | 2010 | 97 | 11.4 ^ | 68 | 64% | 69% | 60% | NR | NR | 5% |
| Yu et al. [60] | 2000 | 38 | 8-18 * | 16 ^ | 90% | NR | NR | NR | NR | 0% |
| Chung et al. [61] | 2000 | 31 | 12.2 ^ | 36 ^ | 87% | NR | NR | NR | 0% | 3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, L.; Losa, M.; Barzaghi, L.R.; Niranjan, A.; Siddiqui, Z.; Flickinger, J.C.; Lunsford, L.D.; Mortini, P. Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4998. https://doi.org/10.3390/cancers13194998
Albano L, Losa M, Barzaghi LR, Niranjan A, Siddiqui Z, Flickinger JC, Lunsford LD, Mortini P. Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(19):4998. https://doi.org/10.3390/cancers13194998
Chicago/Turabian StyleAlbano, Luigi, Marco Losa, Lina Raffaella Barzaghi, Ajay Niranjan, Zaid Siddiqui, John C. Flickinger, Lawrence Dade Lunsford, and Pietro Mortini. 2021. "Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis" Cancers 13, no. 19: 4998. https://doi.org/10.3390/cancers13194998
APA StyleAlbano, L., Losa, M., Barzaghi, L. R., Niranjan, A., Siddiqui, Z., Flickinger, J. C., Lunsford, L. D., & Mortini, P. (2021). Gamma Knife Radiosurgery for Pituitary Tumors: A Systematic Review and Meta-Analysis. Cancers, 13(19), 4998. https://doi.org/10.3390/cancers13194998






