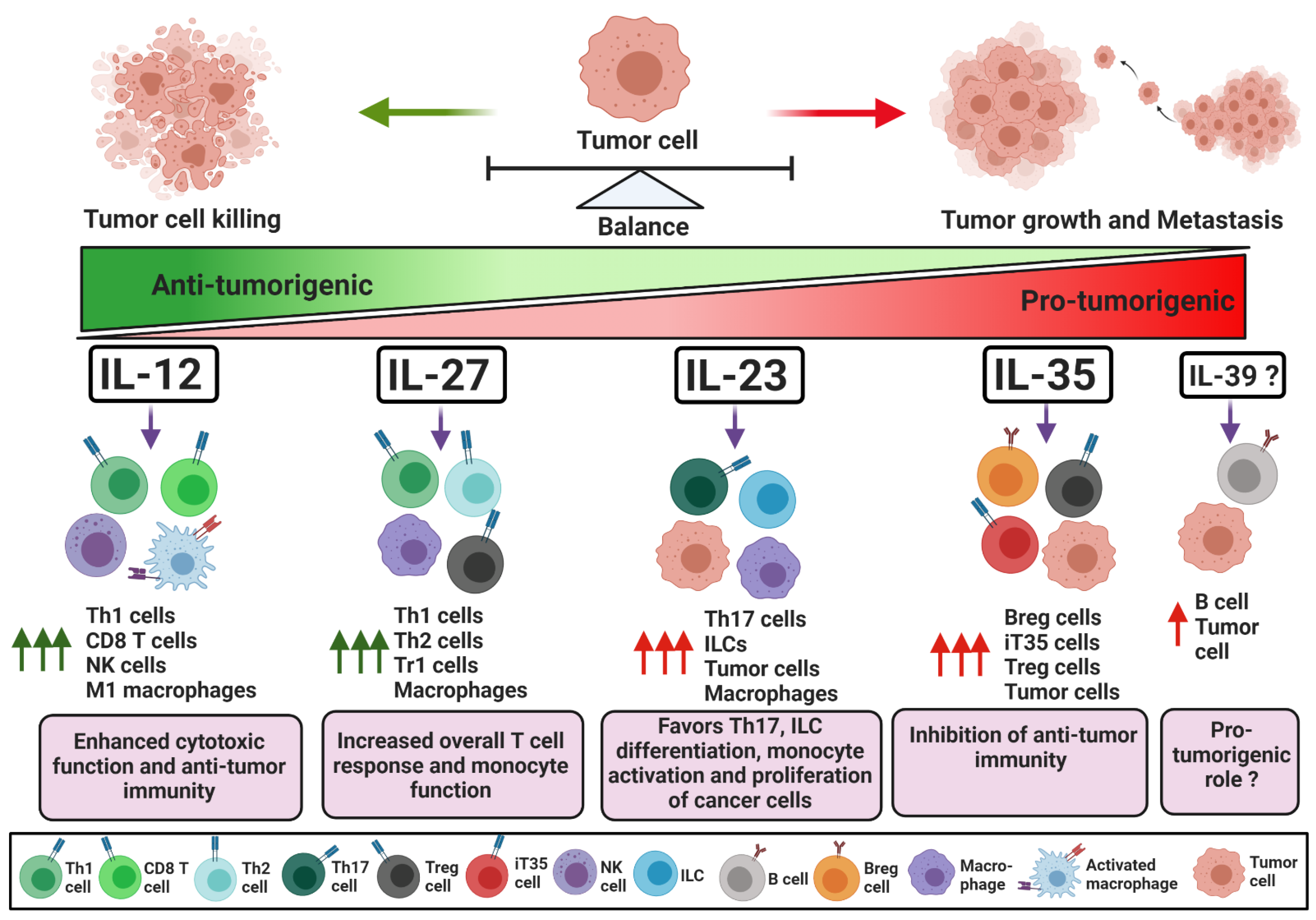
IL-12 Family Cytokines in Cancer and Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction. IL-12 Family Cytokines: Composition, Signaling and Mechanism of Action
1.1. IL-12
1.2. IL-12 in Cancer Immunotherapy
1.3. IL-23
1.3.1. IL-23 as a Suppressor of Anti-Tumor Immunity
1.3.2. IL-23 as an Activator of Anti-Tumor Immunity
1.4. IL-23 in Cancer Immunotherapy
1.5. IL-27
1.5.1. IL-27 as a Suppressor of Anti-Tumor Immunity
1.5.2. IL-27 as an Activator of Anti-Tumor Immunity
1.6. IL-27 in Cancer Immunotherapy
1.7. IL-35
1.8. IL-35 in Cancer Immunotherapy
1.8.1. IL-35 in Inhibiting Tumor Growth
1.8.2. IL-35 in Promoting Tumor Growth
1.9. IL-39
2. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Wojno, E.D.T.; Hunter, C.A.; Stumhofer, J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Mal, X.; Trinchieri, G. Regulation of interleukin-12 production in antigen-presenting cells. Dev. Funct. Myeloid Subsets 2001, 79, 55–92. [Google Scholar]
- Vignali, D.A.; Kuchroo, V.K. Il-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar]
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Berg, J.V.; Kulig, P.; Becher, B. New insights into il-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thieu, V.T.; Yu, Q.; Chang, H.-C.; Yeh, N.; Nguyen, E.T.; Sehra, S.; Kaplan, M.H. Stat4 is required for t-bet to promote il-12-dependent th1 fate determination. Immunity 2008, 29, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.L.; Schilling, M.M.; Cho, S.H.; Lee, K.; Wei, M.; Boothby, M. Stat4 and t-bet are required for the plasticity of ifn-γ expression across th2 ontogeny and influence changes in ifng promoter DNA methylation. J. Immunol. 2013, 191, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Jenner, R.G.; Townsend, M.J.; Jackson, I.; Sun, K.; Bouwman, R.D.; Young, R.A.; Glimcher, L.H.; Lord, G.M. The transcription factors t-bet and gata-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. USA 2009, 106, 17876–17881. [Google Scholar]
- Vilgelm, A.E.; Richmond, A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.; Bobanga, I.D.; Rauhe, P.; Barkauskas, D.; Teich, N.; Tong, C.; Myers, J.; Huang, A.Y. Ccl3 augments tumor rejection and enhances cd8+ T cell infiltration through nk and cd103+ dendritic cell recruitment via ifnγ. Oncoimmunology 2018, 7, e1393598. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.; Rauhe, P.; Askew, D.; Tong, A.A.; Nthale, J.; Eid, S.; Myers, J.T.; Tong, C.; Huang, A.Y. Ccl3 enhances antitumor immune priming in the lymph node via ifnγ with dependency on natural killer cells. Front. Immunol. 2017, 8, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwirner, N.W.; Ziblat, A. Regulation of nk cell activation and effector functions by the il-12 family of cytokines: The case of il-27. Front. Immunol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, N.; Markova, T.; Tsuzuki, K.; Li, W.; El-Darawish, Y.; Pencheva-Demireva, M.; Yamanishi, K.; Yamanishi, H.; Sakagami, M.; Tanaka, Y. Il-12 regulates the expansion, phenotype, and function of murine nk cells activated by il-15 and il-18. Cancer Immunol. Immunother. 2020, 69, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, B.; Ma, T.; Lin, Y.; Cheng, F.; Xiong, H.; Xie, C.; Liu, C.; Wang, Q.; Li, Z. Overexpression of il-12 reverses the phenotype and function of m2 macrophages to m1 macrophages. Int. J. Clin. Exp. Pathol. 2016, 9, 8963–8972. [Google Scholar]
- Suzuki, S.; Umezu, Y.; Saijo, Y.; Satoh, G.; Abe, Y.; Satoh, K.; Nukiwa, T. Exogenous recombinant human il-12 augments mhc class i antigen expression on human cancer cells in vitro. Tohoku J. Exp. Med. 1998, 185, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Kerkar, S.P.; Goldszmid, R.S.; Muranski, P.; Chinnasamy, D.; Yu, Z.; Reger, R.N.; Leonardi, A.J.; Morgan, R.A.; Wang, E.; Marincola, F.M. Il-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Investig. 2011, 121, 4746–4757. [Google Scholar] [PubMed] [Green Version]
- Lazarevic, V.; Chen, X.; Shim, J.; Hwang, E.; Jang, E.; Bolm, A.; Oukka, M.; Kuchroo, V.; Glimcher, L. Transcription factor t-bet represses th17 differentiation by preventing runx1-mediated activation of the ror7t gene. Nat. Immunol. 2011, 12, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.-W.; Wu, L.-X.; Xie, Y.; Ou, X.; Tian, P.-K.; Liu, X.-P.; Min, J.; Wang, J.; Chen, R.-F.; Chen, Y.-J. The expression levels of transcription factors t-bet, gata-3, rorγt and foxp3 in peripheral blood lymphocyte (pbl) of patients with liver cancer and their significance. Int. J. Med Sci. 2015, 12, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory t cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 1–23. [Google Scholar]
- O’Malley, J.T.; Sehra, S.; Thieu, V.T.; Yu, Q.; Chang, H.C.; Stritesky, G.L.; Nguyen, E.T.; Mathur, A.N.; Levy, D.E.; Kaplan, M.H. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology 2009, 127, 587–595. [Google Scholar]
- Lin, L.; Rayman, P.; Pavicic, P.G.; Tannenbaum, C.; Hamilton, T.; Montero, A.; Ko, J.; Gastman, B.; Finke, J.; Ernstoff, M. Ex vivo conditioning with il-12 protects tumor-infiltrating cd8+ T cells from negative regulation by local ifn-γ. Cancer Immunol. Immunother. 2019, 68, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J. Successful anti-pd-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines ifn-γ and il-12. Immunity 2018, 49, 1148–1161.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Leonard, K.; Collins, L.I.; Cai, S.F.; Mayer, J.C.; Payton, J.E.; Walter, M.J.; Piwnica-Worms, D.; Schreiber, R.D.; Ley, T.J. Interleukin 12 stimulates ifn-γ–mediated inhibition of tumor-induced regulatory T-cell proliferation and enhances tumor clearance. Cancer Res. 2009, 69, 8700–8709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhao, J.; Perlman, S. Differential effects of il-12 on tregs and non-treg T cells: Roles of ifn-γ, il-2 and il-2r. PLoS ONE 2012, 7, e46241. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood J. Am. Soc. Hematol. 1997, 90, 2541–2548. [Google Scholar]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G. Re-designing interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1–15. [Google Scholar]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef]
- Li, J.; Lin, W.; Chen, H.; Xu, Z.; Ye, Y.; Chen, M. Dual-target il-12-containing nanoparticles enhance t cell functions for cancer immunotherapy. Cell. Immunol. 2020, 349, 104042. [Google Scholar] [CrossRef]
- Díaz-Montero, C.M.; el Naggar, S.; al Khami, A.; el Naggar, R.; Montero, A.J.; Cole, D.J.; Salem, M.L. Priming of naive cd8+ T cells in the presence of il-12 selectively enhances the survival of cd8+ cd62l hi cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol. Immunother. 2008, 57, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Chinnasamy, D.; Yu, Z.; Kerkar, S.P.; Zhang, L.; Morgan, R.A.; Restifo, N.P.; Rosenberg, S.A. Local delivery of lnterleukin-12 using T cells targeting vegf receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012, 18, 1672–1683. [Google Scholar] [CrossRef] [Green Version]
- Kerkar, S.P.; Muranski, P.; Kaiser, A.; Boni, A.; Sanchez-Perez, L.; Yu, Z.; Palmer, D.C.; Reger, R.N.; Borman, Z.A.; Zhang, L. Tumor-specific cd8+ t cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010, 70, 6725–6734. [Google Scholar] [CrossRef] [Green Version]
- Chiocca, E.A.; John, S.Y.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11, eaaw5680. [Google Scholar] [CrossRef] [PubMed]
- Kueberuwa, G.; Kalaitsidou, M.; Cheadle, E.; Hawkins, R.E.; Gilham, D.E. Cd19 car T cells expressing il-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol. Ther.-Oncolytics 2018, 8, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. Il-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 2015, 4, e994446. [Google Scholar] [PubMed] [Green Version]
- Liu, Y.; Di, S.; Shi, B.; Zhang, H.; Wang, Y.; Wu, X.; Luo, H.; Wang, H.; Li, Z.; Jiang, H. Armored inducible expression of il-12 enhances antitumor activity of glypican-3–targeted chimeric antigen receptor–engineered T cells in hepatocellular carcinoma. J. Immunol. 2019, 203, 198–207. [Google Scholar] [CrossRef]
- Kunert, A.; Chmielewski, M.; Wijers, R.; Berrevoets, C.; Abken, H.; Debets, R. Intra-tumoral production of il18, but not il12, by tcr-engineered T cells is non-toxic and counteracts immune evasion of solid tumors. Oncoimmunology 2018, 7, e1378842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in car T-cell therapy. Mol. Ther.-Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.L.; Bailey, D.; Zielinski, J.; Apte, A.; Musenge, F.; Karp, R.; Burke, S.; Garcon, F.; Mishra, A.; Gurumurthy, S. Intratumoral interleukin-12 mrna therapy promotes th1 transformation of the tumor microenvironment. Clin. Cancer Res. 2020, 26, 6284–6298. [Google Scholar] [CrossRef]
- Fallon, J.K.; Vandeveer, A.J.; Schlom, J.; Greiner, J.W. Enhanced antitumor effects by combining an il-12/anti-DNA fusion protein with avelumab, an anti-pd-l1 antibody. Oncotarget 2017, 8, 20558–20571. [Google Scholar] [CrossRef] [Green Version]
- El-Shemi, A.G.; Ashshi, A.M.; Na, Y.; Li, Y.; Basalamah, M.; Al-Allaf, F.A.; Oh, E.; Jung, B.-K.; Chae-Ok, Y. Combined therapy with oncolytic adenoviruses encoding trail and il-12 genes markedly suppressed human hepatocellular carcinoma both in vitro and in an orthotopic transplanted mouse model. J. Exp. Clin. Cancer Res. 2016, 35, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupi, L.A.; Delella, F.K.; Cucielo, M.S.; Romagnoli, G.G.; Kaneno, R.; Nunes, I.d.S.; Domeniconi, R.F.; Martinez, M.; Martinez, F.E.; Fávaro, W.J. P-mapa and interleukin-12 reduce cell migration/invasion and attenuate the toll-like receptor-mediated inflammatory response in ovarian cancer skov-3 cells: A preliminary study. Molecules 2020, 25, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekaii-Saab, T.S.; Roda, J.M.; Guenterberg, K.D.; Ramaswamy, B.; Young, D.C.; Ferketich, A.K.; Lamb, T.A.; Grever, M.R.; Shapiro, C.L.; Carson, W.E. A phase i trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with her2/neu-expressing malignancies. Mol. Cancer Ther. 2009, 8, 2983–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplanque, G.; Shabafrouz, K.; Obeid, M. Can local radiotherapy and il-12 synergise to overcome the immunosuppressive tumor microenvironment and allow “in situ tumor vaccination”? Cancer Immunol. Immunother. 2017, 66, 833–840. [Google Scholar] [CrossRef]
- Eckert, F.; Jelas, I.; Oehme, M.; Huber, S.M.; Sonntag, K.; Welker, C.; Gillies, S.D.; Strittmatter, W.; Zips, D.; Handgretinger, R. Tumor-targeted il-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology 2017, 6, e1323161. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Wang, W.-Q.; Gong, X.-J.; Gao, L.; Yang, L.-R.; Yu, W.-N.; Shen, H.-Y.; Wan, L.-Q.; Jia, X.-F.; Wang, Y.-S. Study of recombinant human interleukin-12 for treatment of complications after radiotherapy for tumor patients. World J. Clin. Oncol. 2017, 8, 158–167. [Google Scholar] [CrossRef]
- Mills, B.N.; Connolly, K.A.; Ye, J.; Murphy, J.D.; Uccello, T.P.; Han, B.J.; Zhao, T.; Drage, M.G.; Murthy, A.; Qiu, H. Stereotactic body radiation and interleukin-12 combination therapy eradicates pancreatic tumors by repolarizing the immune microenvironment. Cell Rep. 2019, 29, 406–421.e5. [Google Scholar] [CrossRef] [Green Version]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. Il-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Hunter, C.A.; Cua, D.J. Discovery and biology of il-23 and il-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, A.; Breda, C.N.S.; Camara, N.O.S. Innate lymphoid cells in tissue homeostasis and diseases. World J. Hepatol. 2017, 9, 979–989. [Google Scholar] [CrossRef]
- Tian, Z.; van Velkinburgh, J.C.; Wu, Y.; Ni, B. Innate lymphoid cells involve in tumorigenesis. Int. J. Cancer 2016, 138, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F. Human neutrophils activated via tlr8 promote th17 polarization through il-23. J. Leukoc. Biol. 2019, 105, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Cauli, A.; Piga, M.; Floris, A.; Mathieu, A. Current Perspective on the Role of the Interleukin-23/Interleukin-17 Axis in Inflammation and Disease (Chronic Arthritis and Psoriasis). ImmunoTargets Ther. 2015, 4, 185–190. Available online: https://www.dovepress.com/front_end/cr_data/cache/pdf/download_1606621601_5fc319a1519b0/ITT-62870-current-perspectives-on-the-role-of-the-interleukin-23-inter_100115.pdf (accessed on 30 November 2020). [CrossRef] [PubMed] [Green Version]
- Wang, K.; Karin, M. The il-23 to il-17 cascade inflammation-related cancers. Clin. Exp. Rheumatol. 2015, 33, S87–S90. [Google Scholar]
- Qian, X.; Gu, L.; Ning, H.; Zhang, Y.; Hsueh, E.C.; Fu, M.; Hu, X.; Wei, L.; Hoft, D.F.; Liu, J. Increased th17 cells in the tumor microenvironment is mediated by il-23 via tumor-secreted prostaglandin e2. J. Immunol. 2013, 190, 5894–5902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, D.; Shi, Q.; Huang, X.; Ju, X. Umbilical cord blood-derived helios-positive regulatory t cells promote angiogenesis in acute lymphoblastic leukemia in mice via ccl22 and the vegfa-vegfr2 pathway. Mol. Med. Rep. 2019, 19, 4195–4204. [Google Scholar] [CrossRef] [Green Version]
- Martinenaite, E.; Ahmad, S.M.; Hansen, M.; Met, Ö.; Westergaard, M.W.; Larsen, S.K.; Klausen, T.W.; Donia, M.; Svane, I.M.; Andersen, M.H. Ccl22-specific T cells: Modulating the immunosuppressive tumor microenvironment. Oncoimmunology 2016, 5, e1238541. [Google Scholar] [CrossRef] [Green Version]
- Wiedemann, G.M.; Röhrle, N.; Makeschin, M.-C.; Fesseler, J.; Endres, S.; Mayr, D.; Anz, D. Peritumoural ccl1 and ccl22 expressing cells in hepatocellular carcinomas shape the tumour immune infiltrate. Pathology 2019, 51, 586–592. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, C.; Li, W.; Mao, Z.; Shi, Y.; Shi, H.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Tumor-educated neutrophils activate mesenchymal stem cells to promote gastric cancer growth and metastasis. Front. Cell Dev. Biol. 2020, 8, 788. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, J.; Shi, Y.; Li, W.; Shi, H.; Ji, R.; Mao, F.; Qian, H.; Xu, W.; Zhang, X. Cxcl5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis 2020, 9, 1–14. [Google Scholar]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.-Y.; Österreicher, C.H.; Hung, K.E. Adenoma-linked barrier defects and microbial products drive il-23/il-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S. Commensal microbiota promote lung cancer development via γδ T cells. Cell 2019, 176, 998–1013.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The il-23–il-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H. The il-23/il-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Cheng, Q.; Cai, Y.; Gong, H.; Wu, Y.; Yu, X.; Shi, L.; Wu, D.; Dong, C.; Liu, H. Il-17a produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014, 74, 1969–1982. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K. Interleukin-23-independent il-17 production regulates intestinal epithelial permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Nagai, N.; Tsunekawa, N.; Sato-Matsushita, M.; Yoshimoto, T.; Cua, D.J.; Iwakura, Y.; Yagita, H.; Okada, F.; Tahara, H. Il-17a-producing cd 30+ vδ1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016, 107, 1206–1214. [Google Scholar] [CrossRef]
- Pastor-Fernández, G.; Mariblanca, I.R.; Navarro, M.N. Decoding il-23 signaling cascade for new therapeutic opportunities. Cells 2020, 9, 2044. [Google Scholar] [CrossRef]
- Tang, L.; Wang, K. Chronic inflammation in skin malignancies. J. Mol. Signal. 2016, 11, 2. [Google Scholar]
- Wang, K.; Kim, M.K.; di Caro, G.; Wong, J.; Shalapour, S.; Wan, J.; Zhang, W.; Zhong, Z.; Sanchez-Lopez, E.; Wu, L.-W. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 2014, 41, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Kirshberg, S.; Izhar, U.; Amir, G.; Demma, J.; Vernea, F.; Beider, K.; Shlomai, Z.; Wald, H.; Zamir, G.; Shapira, O.M. Involvement of ccr6/ccl20/il-17 axis in nsclc disease progression. PLoS ONE 2011, 6, e24856. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, T.; Liu, X.; Guo, J.n.; Xie, T.; Ding, Y.; Lin, M.; Yang, H. Il-17 promotes tumor angiogenesis through stat3 pathway mediated upregulation of vegf in gastric cancer. Tumor Biol. 2016, 37, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating vegf production of cancer cells via the stat3/giv signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. Il-17 can promote tumor growth through an il-6–stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [PubMed] [Green Version]
- Liu, J.; Wang, L.; Wang, T.; Wang, J. Expression of il-23r and il-17 and the pathology and prognosis of urinary bladder carcinoma. Oncol. Lett. 2018, 16, 4325–4330. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Smyth, M.J.; Teng, M.W. Interleukin (il)-12 and il-23 and their conflicting roles in cancer. Cold Spring Harb. Perspect. Biol. 2018, 10, a028530. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Zhang, J.; Wei, Y.; Li, K.; Huang, L.; Zhang, S.; Gao, B.; Wang, X.; Lin, P. Interleukin 23 regulates proliferation of lung cancer cells in a concentration-dependent way in association with the interleukin-23 receptor. Carcinogenesis 2013, 34, 658–666. [Google Scholar]
- Tao, Y.; Tao, T.; Gross, N.; Peng, X.; Li, Y.; Huang, Z.; Liu, L.; Li, G.; Chen, X.; Yang, J. Combined effect of il-12rβ2 and il-23r expression on prognosis of patients with laryngeal cancer. Cell. Physiol. Biochem. 2018, 50, 1041–1054. [Google Scholar] [CrossRef]
- Cocco, C.; Canale, S.; Frasson, C.; di Carlo, E.; Ognio, E.; Ribatti, D.; Prigione, I.; Basso, G.; Airoldi, I. Interleukin-23 acts as antitumor agent on childhood b-acute lymphoblastic leukemia cells. Blood J. Am. Soc. Hematol. 2010, 116, 3887–3898. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Cao, A.; Yao, S.; Evans-Marin, H.L.; Liu, H.; Wu, W.; Carlsen, E.D.; Dann, S.M.; Soong, L.; Sun, J. Mtor mediates il-23 induction of neutrophil il-17 and il-22 production. J. Immunol. 2016, 196, 4390–4399. [Google Scholar]
- Lee, P.W.; Smith, A.J.; Yang, Y.; Selhorst, A.J.; Liu, Y.; Racke, M.K.; Lovett-Racke, A.E. Il-23r–activated stat3/stat4 is essential for th1/th17-mediated cns autoimmunity. JCI Insight 2017, 2, e91663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Hedl, M.; Abraham, C. Il23 group> il23r recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the ibd-protective il23r r381q variant modulates these outcomes. Gut 2020, 69, 264–273. [Google Scholar] [CrossRef] [PubMed]
- McCuaig, S.; Barras, D.; Mann, E.; Friedrich, M.; Bullers, S.J.; Janney, A.; Garner, L.C.; Domingo, E.; Koelzer, V.H.; Delorenzi, M. The interleukin 22 pathway interacts with mutant kras to promote poor prognosis in colon cancer. Clin. Cancer Res. 2020, 26, 4313–4325. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Rehman, A.; Falk-Paulsen, M.; Secher, T.; Kuiper, J.; Tran, F.; Pfeuffer, S.; Sheibani-Tezerji, R.; Breuer, A.; Luzius, A. Epithelial il-23r signaling licenses protective il-22 responses in intestinal inflammation. Cell Rep. 2016, 16, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Markota, A.; Endres, S.; Kobold, S. Targeting interleukin-22 for cancer therapy. Hum. Vaccines Immunother. 2018, 14, 2012–2015. [Google Scholar]
- Guéry, L.; Hugues, S. Th17 cell plasticity and functions in cancer immunity. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar]
- Bailey, S.R.; Nelson, M.H.; Himes, R.A.; Li, Z.; Mehrotra, S.; Paulos, C.M. Th17 cells in cancer: The ultimate identity crisis. Front. Immunol. 2014, 5, 276. [Google Scholar]
- Li, J.; Lau, G.; Chen, L.; Yuan, Y.-F.; Huang, J.; Luk, J.M.; Xie, D.; Guan, X.-Y. Interleukin 23 promotes hepatocellular carcinoma metastasis via nf-kappa b induced matrix metalloproteinase 9 expression. PLoS ONE 2012, 7, e46264. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-H.; Young, S.H.; Sinnett-Smith, J.; Chou, C.E.N.; Moro, A.; Hertzer, K.M.; Hines, O.J.; Rozengurt, E.; Eibl, G. Prostaglandin e2 activates the mtorc1 pathway through an ep4/camp/pka-and ep1/ca2+-mediated mechanism in the human pancreatic carcinoma cell line panc-1. Am. J. Physiol.-Cell Physiol. 2015, 309, C639–C649. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Zhang, J.; Ai, J.; Hao, X.; Luan, R. Aberrant expression of il-23/il-23r in patients with breast cancer and its clinical significance. Mol. Med. Rep. 2018, 17, 4639–4644. [Google Scholar]
- Elessawi, D.F.; Alkady, M.M.; Ibrahim, I.M. Diagnostic and prognostic value of serum il-23 in colorectal cancer. Arab J. Gastroenterol. 2019, 20, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Stanilov, N.; Miteva, L.; Jovchev, J.; Cirovski, G.; Stanilova, S. The prognostic value of preoperative serum levels of il-12p40 and il-23 for survival of patients with colorectal cancer. Apmis 2014, 122, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Il-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hmid, A.B.; Selmi, O.; Rekik, R.; Lamari, H.; Zamali, I.; Ladeb, S.; Safra, I.; Othman, T.B.; Romdhane, N.B.; Ahmed, M.B. Rorc overexpression as a sign of th17 lymphocytes accumulation in multiple myeloma bone marrow. Cytokine 2020, 134, 155210. [Google Scholar] [CrossRef]
- Koh, J.; Kim, H.Y.; Lee, Y.; Park, I.K.; Kang, C.H.; Kim, Y.T.; Kim, J.-E.; Choi, M.; Lee, W.-W.; Jeon, Y.K. Il23-producing human lung cancer cells promote tumor growth via conversion of innate lymphoid cell 1 (ilc1) into ilc3. Clin. Cancer Res. 2019, 25, 4026–4037. [Google Scholar] [CrossRef] [Green Version]
- Khodadadi, A.; Razmkhah, M.; Eskandari, A.-R.; Hosseini, A.; Habibagahi, M.; Ghaderi, A.; Jaberipour, M. Il-23/il-27 ratio in peripheral blood of patients with breast cancer. Iran. J. Med. Sci. 2014, 39, 350–356. [Google Scholar]
- Liu, Y.; Song, Y.; Lin, D.; Lei, L.; Mei, Y.; Jin, Z.; Gong, H.; Zhu, Y.; Hu, B.; Zhang, Y. Ncr−group 3 innate lymphoid cells orchestrate il-23/il-17 axis to promote hepatocellular carcinoma development. EBioMedicine 2019, 41, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Calcinotto, A.; Spataro, C.; Zagato, E.; di Mitri, D.; Gil, V.; Crespo, M.; de Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E. Il-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar]
- Błogowski, W.; Deskur, A.; Budkowska, M.; Sałata, D.; Madej-Michniewicz, A.; Dąbkowski, K.; Dołęgowska, B.; Starzyńska, T. Selected cytokines in patients with pancreatic cancer: A preliminary report. PLoS ONE 2014, 9, e97613. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.M.; Reed, L.F.; Krasnick, B.A.; Miranda-Carboni, G.; Fields, R.C.; Bi, Y.; Elahi, A.; Ajidahun, A.; Dickson, P.V.; Deneve, J.L. Il23 and tgf-ss diminish macrophage associated metastasis in pancreatic carcinoma. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caughron, B.; Yang, Y.; Young, M.R.I. Role of il-23 signaling in the progression of premalignant oral lesions to cancer. PLoS ONE 2018, 13, e0196034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, A.-M.; Leonard, J.; Naicker, K.M.; Kilmartin, L.; O’Byrne, K.J.; Gray, S.G. Il-23 is pro-proliferative, epigenetically regulated and modulated by chemotherapy in non-small cell lung cancer. Lung Cancer 2013, 79, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Ehara, M.; Suzuki, S.; Ohmori, Y.; Sakashita, H. Il-23 promotes growth and proliferation in human squamous cell carcinoma of the oral cavity. Int. J. Oncol. 2010, 36, 1355–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, W.; Yu, T.; Sang, Y.; Gao, X. Tumor-promoting effect of il-23 in mammary cancer mediated by infiltration of m2 macrophages and neutrophils in tumor microenvironment. Biochem. Biophys. Res. Commun. 2017, 482, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.M.; Sodir, N.M.; Wilson, C.H.; Burkhart, D.L.; Pellegrinet, L.; Swigart, L.B.; Littlewood, T.D.; Evan, G.I. Myc cooperates with ras by programming inflammation and immune suppression. Cell 2017, 171, 1301–1315.e14. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Zhang, Q.; Xiong, Z.; Wang, A.R.; Myers, L.; Melamed, J.; Tang, W.W.; You, Z. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate 2014, 74, 869–879. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Shao, Y.; Zhang, X.; Lu, G.; Liu, B. Il-23 and psma-targeted duo-car t cells in prostate cancer eradication in a preclinical model. J. Transl. Med. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and molecular mechanisms underlying prostate cancer development: Therapeutic implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Shou, P.; Smith, C.; Chen, Y.; Du, H.; Sun, C.; Kren, N.P.; Michaud, D.; Ahn, S.; Vincent, B. Interleukin-23 engineering improves car t cell function in solid tumors. Nat. Biotechnol. 2020, 38, 448–459. [Google Scholar] [CrossRef]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Dual roles of il-27 in cancer biology and immunotherapy. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pot, C.; Apetoh, L.; Awasthi, A.; Kuchroo, V.K. Induction of regulatory tr1 cells and inhibition of th17 cells by il-27. Semin. Immunol. 2011, 23, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Pot, C.; Apetoh, L.; Awasthi, A.; Kuchroo, V.K. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J. Interf. Cytokine Res. 2010, 30, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Saha, B. Il-27 in tumor immunity and immunotherapy. Trends Mol. Med. 2013, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Mittal, A.; Lopez-Diego, R.; Maier, L.M.; Anderson, D.E.; Weiner, H.L. Il-27 is a key regulator of il-10 and il-17 production by human cd4+ T cells. J. Immunol. 2009, 183, 2435–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Zhang, Z.; Tang, X.; Li, W.; Chen, W.; Yao, G. Il-27 regulated cd4+ il-10+ T cells in experimental sjögren syndrome. Front. Immunol. 2020, 11, 1699. [Google Scholar] [CrossRef]
- Pot, C.; Jin, H.; Awasthi, A.; Liu, S.M.; Lai, C.-Y.; Madan, R.; Sharpe, A.H.; Karp, C.L.; Miaw, S.-C.; Ho, I.-C. Cutting edge: Il-27 induces the transcription factor c-maf, cytokine il-21, and the costimulatory receptor icos that coordinately act together to promote differentiation of il-10-producing tr1 cells. J. Immunol. 2009, 183, 797–801. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-K.; Liu, L.-B.; Jin, L.-P.; Zhang, B.; Mei, J.; Li, H.; Wei, C.-Y.; Zhou, W.-J.; Zhu, X.-Y.; Shao, J. Il-27 triggers il-10 production in th17 cells via a c-maf/ror γ t/blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017, 8, e2666. [Google Scholar] [CrossRef] [Green Version]
- Guzzo, C.; Mat, N.F.C.; Gee, K. Interleukin-27 induces a stat1/3-and nf-κb-dependent proinflammatory cytokine profile in human monocytes. J. Biol. Chem. 2010, 285, 24404–24411. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, W.; Gu, Y.; Chang, X.; Wei, G.; Rong, Z.; Qin, L.; Chen, X.; Zhou, F. Non-small cell lung cancer cells modulate the development of human cd1c+ conventional dendritic cell subsets mediated by cd103 and cd205. Front. Immunol. 2019, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Fujio, K.; Okamura, T.; Yamamoto, K. Interleukin-27 in t cell immunity. Int. J. Mol. Sci. 2015, 16, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Owaki, T.; Asakawa, M.; Morishima, N.; Hata, K.; Fukai, F.; Matsui, M.; Mizuguchi, J.; Yoshimoto, T. A role for il-27 in early regulation of th1 differentiation. J. Immunol. 2005, 175, 2191–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, S.; Owaki, T.; Morishima, N.; Fukai, F.; Mizuguchi, J.; Yoshimoto, T. An indispensable role for stat1 in il-27-induced t-bet expression but not proliferation of naive cd4+ T cells. J. Immunol. 2004, 173, 3871–3877. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.M.; Liu, N.; Yang, S.G.; Hu, L.Y.; Chen, X.L.; Fang, Z.H.; Ren, Q.; Lu, S.H.; Liu, B.; Han, Z.C. Regulation of the class ii and class i mhc pathways in human thp-1 monocytic cells by interleukin-27. Biochem. Biophys. Res. Commun. 2008, 367, 553–559. [Google Scholar] [CrossRef]
- Torrado, E.; Fountain, J.; Pearl, J.; Cooper, A. Il-27 sustains t-bet expression and promotes the development of terminally differentiated cd4 T cells during tuberculosis (59.5). Am. Assoc. Immnol. 2012, 188 (Suppl. 1), 59.5. [Google Scholar]
- Hirahara, K.; Onodera, A.; Villarino, A.V.; Bonelli, M.; Sciumè, G.; Laurence, A.; Sun, H.-W.; Brooks, S.R.; Vahedi, G.; Shih, H.-Y. Asymmetric action of stat transcription factors drives transcriptional outputs and cytokine specificity. Immunity 2015, 42, 877–889. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.; Yaneva, T.; Beauseigle, D.; El-Khoury, L.; Arbour, N. Il-27 increases the proliferation and effector functions of human naive cd8+ t lymphocytes and promotes their development into tc1 cells. Eur. J. Immunol. 2011, 41, 47–59. [Google Scholar] [CrossRef]
- Morishima, N.; Mizoguchi, I.; Okumura, M.; Chiba, Y.; Xu, M.; Shimizu, M.; Matsui, M.; Mizuguchi, J.; Yoshimoto, T. A pivotal role for interleukin-27 in cd8+ T cell functions and generation of cytotoxic t lymphocytes. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Gotthardt, D.; Trifinopoulos, J.; Sexl, V.; Putz, E.M. Jak/stat cytokine signaling at the crossroad of nk cell development and maturation. Front. Immunol. 2019, 10, 2590. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Lim, E.J.; Kim, S.W.; Moon, Y.W.; Park, K.S.; An, H.-J. Il-27 enhances il-15/il-18-mediated activation of human natural killer cells. J. Immunother. Cancer 2019, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Hisada, M.; Kamiya, S.; Fujita, K.; Belladonna, M.L.; Aoki, T.; Koyanagi, Y.; Mizuguchi, J.; Yoshimoto, T. Potent antitumor activity of interleukin-27. Cancer Res. 2004, 64, 1152–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarino, A.V.; Stumhofer, J.S.; Saris, C.J.; Kastelein, R.A.; de Sauvage, F.J.; Hunter, C.A. Il-27 limits il-2 production during th1 differentiation. J. Immunol. 2006, 176, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owaki, T.; Asakawa, M.; Kamiya, S.; Takeda, K.; Fukai, F.; Mizuguchi, J.; Yoshimoto, T. Il-27 suppresses cd28-medicated il-2 production through suppressor of cytokine signaling 3. J. Immunol. 2006, 176, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.-J.; Chang, K.-K.; Wu, K.; Yang, H.-L.; Mei, J.; Xie, F.; Li, D.-J.; Li, M.-Q. Rapamycin synergizes with cisplatin in antiendometrial cancer activation by improving il-27–stimulated cytotoxicity of nk cells. Neoplasia 2018, 20, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, B.; Zhang, K.; Song, Y.; Zhang, L.; Xi, M. Il-27 suppresses skov3 cells proliferation by enhancing stat3 and inhibiting the akt signal pathway. Mol. Immunol. 2016, 78, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sato, A.; Shimozato, O.; Shingyoji, M.; Tada, Y.; Tatsumi, K.; Shimada, H.; Hiroshima, K.; Tagawa, M. Administration of DNA encoding the interleukin-27 gene augments antitumour responses through non-adaptive immunity. Scand. J. Immunol. 2015, 82, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, Y.; Mizoguchi, I.; Furusawa, J.; Hasegawa, H.; Ohashi, M.; Xu, M.; Owaki, T.; Yoshimoto, T. Interleukin-27 exerts its antitumor effects by promoting differentiation of hematopoietic stem cells to m1 macrophages. Cancer Res. 2018, 78, 182–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Wang, M.; Niu, Z.; Liu, Q.; Gao, X.; Zhou, L.; Liao, Q.; Zhao, Y. Interleukin-27 inhibits malignant behaviors of pancreatic cancer cells by targeting m2 polarized tumor associated macrophages. Cytokine 2017, 89, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Carbotti, G.; Nikpoor, A.R.; Vacca, P.; Gangemi, R.; Giordano, C.; Campelli, F.; Ferrini, S.; Fabbi, M. Il-27 mediates hla class i up-regulation, which can be inhibited by the il-6 pathway, in hla-deficient small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 140. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, I.; Tupone, M.G.; Esposito, S.; Russo, M.V.; Barbarito, G.; Cipollone, G.; di Carlo, E. Interleukin-27 re-educates intratumoral myeloid cells and down-regulates stemness genes in non-small cell lung cancer. Oncotarget 2015, 6, 3694–3708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourko, O.; Smyth, R.; Cino, D.; Seaver, K.; Petes, C.; Eo, S.Y.; Basta, S.; Gee, K. Poly (i: C)-mediated death of human prostate cancer cell lines is induced by interleukin-27 treatment. J. Interf. Cytokine Res. 2019, 39, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Q.; Zhao, W.-H.; Zhao, X.-X.; Zhang, J.; Nan, K.-J. Association between il-27 2905t/g genotypes and the risk and survival of cervical cancer: A case-control study. Biomarkers 2016, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Majumder, D.; Debnath, R.; Maiti, D. Il-27 along with il-28b ameliorates the pulmonary redox impairment, inflammation and immunosuppression in benzo (a) pyrene induced lung cancer bearing mice. Life Sci. 2020, 260, 118384. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, J.-Q.; Shi, M.; Cheng, X.; Ding, M.; Zhang, J.C.; Davis, J.P.; Varikuti, S.; Satoskar, A.R.; Lu, L. Il-27 gene therapy induces depletion of tregs and enhances the efficacy of cancer immunotherapy. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Ding, M.; Zhu, J.; Liu, J.-Q.; Pan, X.; Ghoshal, K.; Bai, X.-F. Intra-tumoral delivery of il-27 using adeno-associated virus stimulates anti-tumor immunity and enhances the efficacy of immunotherapy. Front. Cell Dev. Biol. 2020, 8, 210. [Google Scholar] [CrossRef]
- Yan, H.; Viswanadhapalli, S.; Chupp, D.; Fernandez, M.; Wu, S.; Wang, J.; Moroney, J.; Taylor, J.; Im, J.; Rivera, C. B cell-produced il-27 up-regulates pd-l1 expression in the tumor microenvironment to promote breast cancer development. AACR 2019. [Google Scholar] [CrossRef]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating pd-l1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Carbotti, G.; Barisione, G.; Airoldi, I.; Mezzanzanica, D.; Bagnoli, M.; Ferrero, S.; Petretto, A.; Fabbi, M.; Ferrini, S. Il-27 induces the expression of ido and pd-l1 in human cancer cells. Oncotarget 2015, 6, 43267–43280. [Google Scholar] [CrossRef] [Green Version]
- Rolvering, C.; Zimmer, A.D.; Ginolhac, A.; Margue, C.; Kirchmeyer, M.; Servais, F.; Hermanns, H.M.; Hergovits, S.; Nazarov, P.V.; Nicot, N. The pd-l1-and il6-mediated dampening of the il27/stat1 anticancer responses are prevented by α-pd-l1 or α-il6 antibodies. J. Leukoc. Biol. 2018, 104, 969–985. [Google Scholar] [CrossRef]
- Horlad, H.; Ma, C.; Yano, H.; Pan, C.; Ohnishi, K.; Fujiwara, Y.; Endo, S.; Kikukawa, Y.; Okuno, Y.; Matsuoka, M. An il-27/stat3 axis induces expression of programmed cell death 1 ligands (pd-l1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016, 107, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Meissl, K.; Macho-Maschler, S.; Müller, M.; Strobl, B. The good and the bad faces of stat1 in solid tumours. Cytokine 2017, 89, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Sakuishi, K.; Xiao, S.; Sun, Z.; Zaghouani, S.; Gu, G.; Wang, C.; Tan, D.J.; Wu, C.; Rangachari, M. An il-27/nfil3 signalling axis drives tim-3 and il-10 expression and t-cell dysfunction. Nat. Commun. 2015, 6, 1–12. [Google Scholar]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, tim-3, and tigit: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-J.; Ryu, H.; Choi, G.; Kim, B.-S.; Hwang, E.S.; Kim, H.S.; Chung, Y. Il-27 confers a protumorigenic activity of regulatory t cells via cd39. Proc. Natl. Acad. Sci. USA 2019, 116, 3106–3111. [Google Scholar] [CrossRef] [Green Version]
- d’Almeida, S.M.; Kauffenstein, G.; Roy, C.; Basset, L.; Papargyris, L.; Henrion, D.; Catros, V.; Ifrah, N.; Descamps, P.; Croue, A. The ecto-atpdase cd39 is involved in the acquisition of the immunoregulatory phenotype by m-csf-macrophages and ovarian cancer tumor-associated macrophages: Regulatory role of il-27. Oncoimmunology 2016, 5, e1178025. [Google Scholar] [CrossRef] [Green Version]
- Pylayeva-Gupta, Y. Molecular pathways: Interleukin-35 in autoimmunity and cancer. Clin. Cancer Res. 2016, 22, 4973–4978. [Google Scholar] [CrossRef] [Green Version]
- Hao, S.; Chen, X.; Wang, F.; Shao, Q.; Liu, J.; Zhao, H.; Yuan, C.; Ren, H.; Mao, H. Breast cancer cell–derived il-35 promotes tumor progression via induction of il-35-producing induced regulatory T cells. Carcinogenesis 2018, 39, 1488–1496. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Lin, J.-C.; Hwang, W.-L.; Kuo, Y.-J.; Chen, H.-K.; Tai, S.-K.; Lin, C.-C.; Yang, M.-H. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat. Commun. 2018, 9, 1–18. [Google Scholar]
- Wang, Z.; Liu, J.-Q.; Liu, Z.; Shen, R.; Zhang, G.; Xu, J.; Basu, S.; Feng, Y.; Bai, X.-F. Tumor-derived il-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J. Immunol. 2013, 190, 2415–2423. [Google Scholar] [CrossRef] [Green Version]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A. The inhibitory cytokine il-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Wei, X.q.; Cai, B.; Hueber, A.J.; Leung, B.P.; McInnes, I.B.; Liew, F.Y. Il-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory t cells and suppression of th17 cells. Eur. J. Immunol. 2007, 37, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Billmeier, U.; Mchedlidze, T.; Blumberg, R.S.; Neurath, M.F. Interleukin-35 mediates mucosal immune responses that protect against t-cell–dependent colitis. Gastroenterology 2011, 141, 1875–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-Q.; Liu, Z.; Zhang, X.; Shi, Y.; Talebian, F.; Carl, J.W.; Yu, C.; Shi, F.-D.; Whitacre, C.C.; Trgovcich, J. Increased th17 and regulatory T cell responses in ebv-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J. Immunol. 2012, 188, 3099–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirlekar, B.; Michaud, D.; Searcy, R.; Greene, K.; Pylayeva-Gupta, Y. Il35 hinders endogenous antitumor t-cell immunity and responsiveness to immunotherapy in pancreatic cancer. Cancer Immunol. Res. 2018, 6, 1014–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnis, M.E.; Sawant, D.V.; Szymczak-Workman, A.L.; Andrews, L.P.; Delgoffe, G.M.; Yano, H.; Beres, A.J.; Vogel, P.; Workman, C.J.; Vignali, D.A. Interleukin-35 limits anti-tumor immunity. Immunity 2016, 44, 316–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhang, T.; Yan, M.-X.; Wu, W. Il-35 inhibits cd8 (+) T cells activity by suppressing expression of costimulatory molecule cd28 and th1 cytokine production. Transl. Cancer Res. 2019, 8, 1319–1325. [Google Scholar] [CrossRef]
- Yang, L.; Shao, X.; Jia, S.; Zhang, Q.; Jin, Z. Interleukin-35 dampens cd8+ T cells activity in patients with non-viral hepatitis-related hepatocellular carcinoma. Front. Immunol. 2019, 10, 1032. [Google Scholar] [CrossRef] [Green Version]
- Mirlekar, B.; Michaud, D.; Lee, S.J.; Kren, N.P.; Harris, C.; Greene, K.; Goldman, E.C.; Gupta, G.P.; Fields, R.C.; Hawkins, W.G. B cell–derived il35 drives stat3-dependent cd8+ T-cell exclusion in pancreatic cancer. Cancer Immunol. Res. 2020, 8, 292–308. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, J.; Li, J. Il-35-producing b cells in gastric cancer patients. Medicine 2018, 97, e0710. [Google Scholar] [CrossRef]
- Collison, L.W.; Delgoffe, G.M.; Guy, C.S.; Vignali, K.M.; Chaturvedi, V.; Fairweather, D.; Satoskar, A.R.; Garcia, K.C.; Hunter, C.A.; Drake, C.G. The composition and signaling of the il-35 receptor are unconventional. Nat. Immunol. 2012, 13, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A. Il-35-mediated induction of a potent regulatory t cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambuza, I.M.; He, C.; Choi, J.K.; Yu, C.-R.; Wang, R.; Mattapallil, M.J.; Wingfield, P.T.; Caspi, R.R.; Egwuagu, C.E. Il-12p35 induces expansion of il-10 and il-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat. Commun. 2017, 8, 1–12. [Google Scholar]
- Wang, R.-X.; Yu, C.-R.; Dambuza, I.M.; Mahdi, R.M.; Dolinska, M.B.; Sergeev, Y.V.; Wingfield, P.T.; Kim, S.-H.; Egwuagu, C.E. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 2014, 20, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Pan, Y.; Wang, Y.; Wang, H.; Xiong, S.; Li, Q.; Wang, J.; Tao, L.; Wang, Z.; Wu, F. Regulatory T cells-derived il-35 promotes the growth of adult acute myeloid leukemia blasts. Int. J. Cancer 2015, 137, 2384–2393. [Google Scholar] [CrossRef]
- Wang, H.-M.; Zhang, X.-H.; Feng, M.-M.; Qiao, Y.-J.; Ye, L.-Q.; Chen, J.; Fan, F.-F.; Guo, L.-L. Interleukin-35 suppresses the antitumor activity of T cells in patients with non-small cell lung cancer. Cell. Physiol. Biochem. 2018, 47, 2407–2419. [Google Scholar] [CrossRef]
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F. Il-35 induces n2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lin, Y.; Li, C.; Zhang, X.; Cheng, L.; Dai, L.; Wang, Y.; Wang, F.; Shi, G.; Li, Y. Il-35 decelerates the inflammatory process by regulating inflammatory cytokine secretion and m1/m2 macrophage ratio in psoriasis. J. Immunol. 2016, 197, 2131–2144. [Google Scholar] [CrossRef]
- Ye, J.; Huang, Y.; Que, B.; Chang, C.; Liu, W.; Hu, H.; Liu, L.; Shi, Y.; Wang, Y.; Wang, M. Interleukin-12p35 knock out aggravates doxorubicin-induced cardiac injury and dysfunction by aggravating the inflammatory response, oxidative stress, apoptosis and autophagy in mice. EBioMedicine 2018, 35, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Haller, S.; Duval, A.; Migliorini, R.; Stevanin, M.; Mack, V.; Acha-Orbea, H. Interleukin-35-producing cd8α+ dendritic cells acquire a tolerogenic state and regulate t cell function. Front. Immunol. 2017, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Dixon, K.O.; van der Kooij, S.W.; Vignali, D.A.; van Kooten, C. Human tolerogenic dendritic cells produce il-35 in the absence of other il-12 family members. Eur. J. Immunol. 2015, 45, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Zheng, Y.; Zhang, M.; Jin, X.; Kang, K.; Wang, Y.; Li, S.; Zhang, H.; Zhao, Q. Administration of interleukin-35-conditioned autologous tolerogenic dendritic cells prolong allograft survival after heart transplantation. Cell. Physiol. Biochem. 2018, 49, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yan, D.; Kan, Q. Interleukin-35 as an emerging player in tumor microenvironment. J. Cancer 2019, 10, 2074–2082. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yang, X.; Wang, Y.; Zhang, H.; Guo, Z. Interleukin-35 is associated with the tumorigenesis and progression of prostate cancer. Oncol. Lett. 2019, 17, 5094–5102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, L.; Kachler, K.; Siegmund, R.; Trufa, D.I.; Mittler, S.; Geppert, C.-I.; Friedrich, J.; Rieker, R.J.; Sirbu, H.; Finotto, S. Increased expression of the immunosuppressive interleukin-35 in patients with non-small cell lung cancer. Br. J. Cancer 2019, 120, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chen, L.; Xie, G.; Zhou, Y.; Yue, C.; Yuan, X.; Zheng, Y.; Wang, W.; Deng, L.; Shen, L. Elevated level of interleukin-35 in colorectal cancer induces conversion of t cells into itr35 by activating stat1/stat3. Oncotarget 2016, 7, 73003–73015. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, T.; Wang, S.; Wang, D.; Niu, Z.; Sun, Z.; Zhang, J. Interleukin-35 expression is associated with colon cancer progression. Oncotarget 2017, 8, 71563–71573. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Zheng, X.; Meng, Q.; Dong, Y.; Zhang, G.; Rao, D.; An, X.; Yang, Z.; Pan, L.; Zhang, S. Interleukin-35 expression in non-small cell lung cancer is associated with tumor progression. Cell. Physiol. Biochem. 2018, 51, 1839–1851. [Google Scholar] [CrossRef]
- Jiang, Q.; Ma, L.; Li, R.; Sun, J. Colon cancer-induced interleukin-35 inhibits beta-catenin-mediated pro-oncogenic activity. Oncotarget 2018, 9, 11989–11998. [Google Scholar] [CrossRef]
- Long, J.; Guo, H.; Cui, S.; Zhang, H.; Liu, X.; Li, D.; Han, Z.; Xi, L.; Kou, W.; Xu, J. Il-35 expression in hepatocellular carcinoma cells is associated with tumor progression. Oncotarget 2016, 7, 45678–45686. [Google Scholar] [CrossRef] [Green Version]
- Chatrabnous, N.; Ghaderi, A.; Ariafar, A.; Razeghinia, M.S.; Nemati, M.; Jafarzadeh, A. Serum concentration of interleukin-35 and its association with tumor stages and foxp3 gene polymorphism in patients with prostate cancer. Cytokine 2019, 113, 221–227. [Google Scholar] [CrossRef]
- Chen, G.; Liang, Y.; Guan, X.; Chen, H.; Liu, Q.; Lin, B.; Chen, C.; Huang, M.; Chen, J.; Wu, W. Circulating low il-23: Il-35 cytokine ratio promotes progression associated with poor prognosisin breast cancer. Am. J. Transl. Res. 2016, 8, 2255–2264. [Google Scholar]
- Larousserie, F.; Kebe, D.; Huynh, T.; Audebourg, A.; Tamburini, J.; Terris, B.; Devergne, O. Evidence for il-35 expression in diffuse large b-cell lymphoma and impact on the patient’s prognosis. Front. Oncol. 2019, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tao, Q.; Wang, H.; Wang, Z.; Wu, F.; Pan, Y.; Tao, L.; Xiong, S.; Wang, Y.; Zhai, Z. Elevated il-35 in bone marrow of the patients with acute myeloid leukemia. Hum. Immunol. 2015, 76, 681–686. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.; Li, Z.; Chang, A.; Chen, Y.; Zhao, T.; Li, Y.; Wang, X.; Zhang, W.; Wang, Z. Tumour-derived interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing icam1 expression. Nat. Commun. 2017, 8, 1–15. [Google Scholar]
- Nicholl, M.B.; Ledgewood, C.L.; Chen, X.; Bai, Q.; Qin, C.; Cook, K.M.; Herrick, E.J.; Diaz-Arias, A.; Moore, B.J.; Fang, Y. Il-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: Evidence for a role as an autocrine growth factor. Cytokine 2014, 70, 126–133. [Google Scholar] [CrossRef]
- Jin, P.; Ren, H.; Sun, W.; Xin, W.; Zhang, H.; Hao, J. Circulating il-35 in pancreatic ductal adenocarcinoma patients. Hum. Immunol. 2014, 75, 29–33. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Xiao, H.; Liu, X.; Zhang, Y.; Han, G.; Chen, G.; Hou, C.; Ma, N.; Shen, B. A novel il-23p19/ebi3 (il-39) cytokine mediates inflammation in lupus-like mice. Eur. J. Immunol. 2016, 46, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, X.; Zhang, Y.; Wang, Z.; Zhu, G.; Han, G.; Chen, G.; Hou, C.; Wang, T.; Ma, N. Interleukin (il)-39 [il-23p19/epstein–barr virus-induced 3 (ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin. Exp. Immunol. 2016, 186, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, A.A.; Zhao, L.; Zhu, Z.; Xiao, H.; Redington, C.G.; Ding, V.A.; Stewart-Hester, T.; Bai, Q.; Dunlap, J.; Wakefield, M.R. Il-39 acts as a friend to pancreatic cancer. Med. Oncol. 2019, 36, 22. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the il-23/il-17 signaling pathway and the treatment of psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef]
- Hasegawa, H.; Mizoguchi, I.; Chiba, Y.; Ohashi, M.; Xu, M.; Yoshimoto, T. Expanding diversity in molecular structures and functions of the il-6/il-12 heterodimeric cytokine family. Front. Immunol. 2016, 7, 479. [Google Scholar] [CrossRef] [Green Version]
- Floss, D.; Schönberg, M.; Franke, M.; Horstmeier, F.; Engelowski, E.; Schneider, A.; Rosenfeldt, E.; Scheller, J. Il-6/il-12 cytokine receptor shuffling of extra-and intracellular domains reveals canonical stat activation via synthetic il-35 and il-39 signaling. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Hu, J.; Sun, C.; Bernatchez, C.; Xia, X.; Hwu, P.; Dotti, G.; Li, S. T-cell homing therapy for reducing regulatory T cells and preserving effector t-cell function in large solid tumors. Clin. Cancer Res. 2018, 24, 2920–2934. [Google Scholar] [CrossRef] [Green Version]
- Berraondo, P.; Etxeberria, I.; Ponz-Sarvise, M.; Melero, I. Revisiting interleukin-12 as a cancer immunotherapy agent. Clin. Cancer Res. 2018, 24, 2716–2718. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. https://doi.org/10.3390/cancers13020167
Mirlekar B, Pylayeva-Gupta Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers. 2021; 13(2):167. https://doi.org/10.3390/cancers13020167
Chicago/Turabian StyleMirlekar, Bhalchandra, and Yuliya Pylayeva-Gupta. 2021. "IL-12 Family Cytokines in Cancer and Immunotherapy" Cancers 13, no. 2: 167. https://doi.org/10.3390/cancers13020167
APA StyleMirlekar, B., & Pylayeva-Gupta, Y. (2021). IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers, 13(2), 167. https://doi.org/10.3390/cancers13020167





