Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
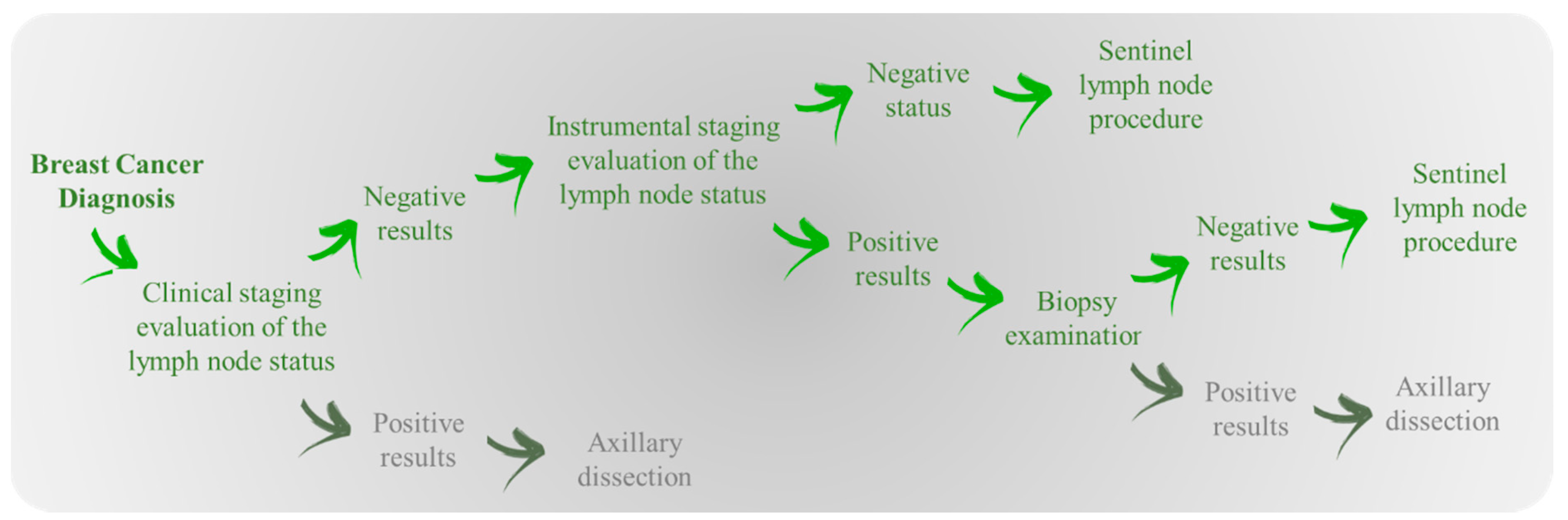
2.1. Experimental Data
2.2. Histological Evaluation Procedure
2.3. Algorithm Cancer Math
2.4. Performance Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consensus per L’irradiazione delle Stazioni Linfonodali Mammary; Associazione Italiana Radioterapia Oncologica: Milan, Italy, 2016.
- Diotaiuti, S.; De Summa, S.; Altieri, R.; Dantona, C.; Tommasi, S.; Di Gennaro, M.; Giuseppe, R.; Pastena, M.I.; Argentiero, A.; Zito, F.A.; et al. Biomarker phenotyping drives clinical management in axillary sentinel node: A retrospective study on women with primary breast cancer in 2002. Oncol. Lett. 2020, 20, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Nolan, M.E.; Silverstein, M.J.; Mihm, M.C.; Sober, A.J.; Tanabe, K.K.; Smith, B.L.; Younger, J.; Michaelson, J.S. The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer 2009, 115, 5071–5083. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Chen, L.L.; Silverstein, M.J.; Mihm, M.C.; Sober, A.J.; Tanabe, K.K.; Smith, B.L.; Younger, J. How cancer at the primary site and in the lymph nodes contributes to the risk of cancer death. Cancer 2009, 115, 5095–5107. [Google Scholar] [CrossRef]
- Qiu, S.-Q.; Zeng, H.-C.; Zhang, F.; Chen, C.; Huang, W.-H.; Pleijhuis, R.G.; Wu, J.-D.; van Dam, G.M.; Zhang, G.-J. A nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci. Rep. 2016, 6, 21196. [Google Scholar] [CrossRef] [Green Version]
- Early and Locally Advanced Breast Cancer: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2018.
- Tsujimoto, M.; Nakabayashi, K.; Yoshidome, K.; Kaneko, T.; Iwase, T.; Akiyama, F.; Kato, Y.; Tsuda, H.; Ueda, S.; Sato, K.; et al. One-step Nucleic Acid Amplification for Intraoperative Detection of Lymph Node Metastasis in Breast Cancer Patients. Clin. Cancer Res. 2007, 13, 4807–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.M.; Michalopoulos, N.V.; Williams, N.R.; Davidson, T.; El Sheikh, S.; McDermott, N.; Tran-Dang, M.-A.; Davison, S.; Keshtgar, M.R. Detailed evaluation of one step nucleic acid (OSNA) molecular assay for intra-operative diagnosis of sentinel lymph node metastasis and prediction of non-sentinel nodal involvement: Experience from a London Teaching Hospital. Breast 2014, 23, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Szychta, P.; Westfal, B.; Maciejczyk, R.; Smolarz, B.; Romanowicz, H.; Krawczyk, T.; Zadrozny, M. Intraoperative diagnosis of sentinel lymph node metastases in breast cancer treatment with onestep nucleic acid amplification assay (OSNA). Arch. Med. Sci. 2016, 12, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanfani, F.; Monterossi, G.; Ghizzoni, V.; Rossi, E.D.; Dinoi, G.; Inzani, F.; Fagotti, A.; Alletti, S.G.; Scarpellini, F.; Nero, C.; et al. One-Step Nucleic Acid Amplification (OSNA): A fast molecular test based on CK19 mRNA concentration for assessment of lymph-nodes metastases in early stage endometrial cancer. PLoS ONE 2018, 13, e0195877. [Google Scholar] [CrossRef] [Green Version]
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef] [Green Version]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral lymphedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Land, S.R.; Kopec, J.A.; Julian, T.B.; Brown, A.M.; Anderson, S.J.; Krag, D.N.; Christian, N.J.; Costantino, J.P.; Wolmark, N.; Ganz, P.A. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J. Clin. Oncol. 2010, 28, 3929–3936. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CancerMath. Available online: http://www.lifemath.net/cancer/ (accessed on 1 April 2019).
- Egner, J.R. AJCC Cancer Staging Manual. JAMA 2010, 304, 1726–1727. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Trihia, H.; Murray, S.; Price, K.; Gelber, R.D.; Golouh, R.; Goldhirsch, A.; Coates, A.S.; Castiglione-Gertsch, M.; Gusterson, B.A. Ki-67 expression in breast carcinoma: Its association with grading systems, clinical parameters, and other prognostic factors—A surrogate marker? Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Moore, D.H., II; Vartanian, R. Correlation of Ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum. Pathol. 1994, 25, 337–342. [Google Scholar] [CrossRef]
- Hoff, E.R.; Tubbs, R.R.; Myles, J.L.; Procop, G.W. HER2/neu amplification in breast cancer: Stratification by tumor type and grade. Am. J. Clin. Pathol. 2002, 117, 916–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazo, C.; Kearns, C.; Mooney, C.; Gallagher, W.M. Clinical Decision Support Systems in Breast Cancer: A Systematic Review. Cancers 2020, 12, 369. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.Y.; Oh, S.J.; Chun, Y.S.; Lee, W.K.; Park, H.K. Gene expression assay and Watson for Oncology for optimization of treatment in ER-positive, HER2-negative breast cancer. PLoS ONE 2018, 13, e0200100. [Google Scholar] [CrossRef]
- Park, C.; Ahn, J.; Kim, H.; Park, S. Integrative Gene Network Construction to Analyze Cancer Recurrence Using Semi-Supervised Learning. PLoS ONE 2014, 9, e86309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madekivi, V.; Boström, P.; Karlsson, A.; Aaltonen, R.; Salminen, E. Can a machine-learning model improve the prediction of nodal stage after a positive sentinel lymph node biopsy in breast cancer? Acta Oncol. 2020, 59, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Federated Learning Powered by NVIDIA Clara. Available online: https://developer.nvidia.com/blog/federated-learning-clara/ (accessed on 1 April 2019).
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as Novel Biomarkers Predicting Axillary Lymph Node Metastasis in T1 Breast Cancer. Clin. Cancer Res. 2005, 11, 5686–5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlilmann, B.; Senn, H.-J.; André, F.; Baselga, J.; et al. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.K.Y.; Thike, A.A.; Pathmanathan, N.; Jara-Lazaro, A.R.; Iqbal, J.; Sng, A.S.H.; Ye, H.S.; Lim, J.C.T.; Koh, V.C.Y.; Tan, J.S.Y.; et al. Using computer assisted image analysis to determine the optimal Ki67 threshold for predicting outcome of invasive breast cancer. Oncotarget 2018, 9, 11619–11630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoveling, L.A.; Van Maaren, M.C.; Hueting, T.; Strobbe, L.J.A.; Hendriks, M.P.; Sonke, G.S.; Siesling, S. Validation of the online prediction model CancerMath in the Dutch breast cancer population. Breast Cancer Res. Treat. 2019, 178, 665–681. [Google Scholar] [CrossRef]
- Miao, H.; Hartman, M.; Verkooijen, H.M.; Taib, N.A.; Wong, H.-S.; Subramaniam, S.; Yip, C.-H.; Tan, E.Y.; Chan, P.; Lee, S.-C.; et al. Validation of the CancerMath prognostic tool for breast cancer in Southeast Asia. BMC Cancer 2016, 16, 820. [Google Scholar] [CrossRef] [Green Version]
- Polchai, N.; Sa-Nguanraksa, D.; Numprasit, W.; Thumrongtaradol, T.; O’Charoenrat, E.; O’Charoenrat, P. A Comparison Between the Online Prediction Models CancerMath and PREDICT as Prognostic Tools in Thai Breast Cancer Patients. Cancer Manag. Res. 2020, 12, 5549–5559. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Lambaudie, E.; Classe, J.M.; Mazouni, C.; Giard, S.; Cohen, M.; Faure, C.; Charitansky, H.; Rouzier, R.; Darai, E.; et al. Lymph node positivity in different early breast carcinoma phenotypes: A predictive model. BMC Cancer 2019, 19, 45. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Li, S.; Jacobs, L. Development of nomograms to predict axillary lymph node status in breast cancer patients. BMC Cancer 2017, 17, 561. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.K.; Kim, M.K.; Kim, J.; Lee, E.; Yoo, T.-K.; Lee, H.-B.; Kang, Y.J.; Kim, J.; Moon, H.-G.; Chang, J.M.; et al. Can We Skip Intraoperative Evaluation of Sentinel Lymph Nodes? Nomogram Predicting Involvement of Three or More Axillary Lymph Nodes before Breast Cancer Surgery. Cancer Res. Treat. 2017, 49, 1088–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsang-Kitzis, H.; Mouttet-Boizat, D.; Guillot, E.; Feron, J.-G.; Fourchotte, V.; Alran, S.; Pierga, J.-Y.; Cottu, P.; Lerebours, F.; Stevens, D.; et al. Medico-economic impact of MSKCC non-sentinel node prediction nomogram for ER-positive HER2-negative breast cancers. PLoS ONE 2017, 12, e0169962. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [Green Version]
- Okuno, J.; Miyake, T.; Sota, Y.; Tanei, T.; Kagara, N.; Naoi, Y.; Shimoda, M.; Shimazu, K.; Kim, S.J.; Noguchi, S. Development of Prediction Model Including MicroRNA Expression for Sentinel Lymph Node Metastasis in ER-Positive and HER2-Negative Breast Cancer. Ann. Surg. Oncol. 2021, 28, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, J.L.B.; Kattan, M.W.; Fey, J.V.; Cody, H.S., III; Borgen, P.I.; Van Zee, K.J. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J. Clin. Oncol. 2007, 25, 3670–3679. [Google Scholar] [CrossRef]
- Yoo, T.K.; Kim, S.J.; Lee, J.; Lee, S.B.; Lee, S.J.; Park, H.Y.; Park, H.K.; Chae, B.J.; Eom, Y.H.; Kim, H.S.; et al. A N0 Predicting Model for Sentinel Lymph Node Biopsy Omission in Early Breast Cancer Upstaged from Ductal Carcinoma in Situ. Clin. Breast Cancer 2020, 20, e281–e289. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Q.; Zhouyang, L.; Lu, Z.; Deng, C.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. Eur. Radiol. 2018, 28, 582–591. [Google Scholar] [CrossRef]
- Luo, J.; Ning, Z.; Zhang, S.; Feng, Q.; Zhang, Y. Bag of deep features for preoperative prediction of sentinel lymph node metastasis in breast cancer. Phys. Med. Biol. 2018, 63, 245014. [Google Scholar] [CrossRef]
- Liu, M.; Mao, N.; Ma, H.; Dong, J.; Zhang, K.; Che, K.; Duan, S.; Zhang, X.; Shi, Y.; Xie, H. Pharmacokinetic parameters and radiomics model based on dynamic contrast enhanced MRI for the preoperative prediction of sentinel lymph node metastasis in breast cancer. Cancer Imaging 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Sun, D.; Chen, L.; Fang, Z.; Song, W.; Guo, D.; Ni, T.; Liu, C.; Feng, L.; Xia, Y.; et al. Radiomics analysis of dynamic contrast-enhanced magnetic resonance imaging for the prediction of sentinel lymph node metastasis in breast cancer. Front. Oncol. 2019, 9, 980. [Google Scholar] [CrossRef]
- Fanizzi, A.; Basile, T.M.A.; Losurdo, L.; Belloti, R.; Bottigli, U.; Dentamaro, R.; Didona, R.; Fausto, A.; Massafra, R.; Moschetta, M.; et al. A Machine Learning Approach on Multiscale Texture Analysis for Breast Microcalcification Diagnosis. BMC Bioinform. 2020, 21, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanizzi, A.; Basile, T.M.A.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Campobasso, F.; Didonna, V.; Fausto, A.; Massafra, R.; Tagliafico, A.; et al. Ensemble Discrete Wavelet Transform and Gray-Level Co-Occurrence Matrix for Microcalcification Cluster Classification in Digital Mammography. Appl. Sci. 2019, 9, 5388. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Lorusso, V.; Massafra, R.; Tamborra, P.; et al. Radiomics Analysis on Contrast-Enhanced Spectral Mammography Images for Breast Cancer Diagnosis:A Pilot Study. Entropy 2019, 21, 1110. [Google Scholar] [CrossRef] [Green Version]
- Fanizzi, A.; Losurdo, L.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Delogu, P.; Diacono, D.; Didonna, V.; Fausto, A.; Lombardi, A.; et al. Fully Automated Support System for Diagnosis of Breast Cancer in Contrast-Enhanced Spectral Mammography Images. J. Clin. Med. 2019, 8, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, T.M.A.; Fanizzi, A.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Fausto, A.; Massafra, R.; Moschetta, M.; et al. Microcalcification Detection in Full-Field Digital Mammograms: A Fully Automated Computer-Aided System. Phys. Med. 2019, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.M.A.; Fanizzi, A.; Losurdo, L.; Bellotti, R.; Tangaro, S.; La Forgia, D.; Didonna, V.; Massafra, R.; Tamborra, P.; Moschetta, M.; et al. Hough transform for clustered microcalcifications detection in full-field digital mammograms. Appl. Digit. Image Process. 2017, 10396, 41. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Fausto, A.; Massafra, R.; Monaco, A.; et al. A Combined Approach of Multiscale Texture Analysis and Interest Point/Corner Detectors for Microcalcifications Diagnosis. Lect. Notes Comput. Sci. 2018, 1, 302–313. [Google Scholar] [CrossRef]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Yang, L.; Wang, Y.; Li, H.; Zhou, X.; Zhao, W.; Ren, J.; Li, X.; Tian, J.; et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Mammography-Based Radiomics Method. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.A.; Hoskin, T.L.; Day, C.N.; Habermann, E.B.; Boughey, J.C. Effect of Primary Breast Tumor Location on Axillary Nodal Positivity. Ann. Surg. Oncol. 2018, 25, 3011–3018. [Google Scholar] [CrossRef]
| Prognostic Factor | N. Patients (%) | N. Positive (% of Total) | Prognostic Factor | N. Patients (%) | N. Positive (% of Total) |
|---|---|---|---|---|---|
| Overall | 993 (100%) | 208 (20.95%) | ER (≥1%) | ||
| Age | negative | 121 (12.19%) | 16 (13.22%) | ||
| 21–30 | 2 (0.20%) | 0 (0%) | positive | 872 (87.81%) | 192 (22.02%) |
| 31–40 | 61 (6.14%) | 14 (22.95%) | PR (≥1%) | ||
| 41–50 | 258 (25.98%) | 73 (28.29%) | negative | 234 (23.54%) | 39 (16.67%) |
| 51–60 | 292 (29.41%) | 62 (21.23%) | positive | 759 (80.06%) | 169 (22.27%) |
| 61–70 | 239 (24.07%) | 39 (16.32%) | Ki67 (≥20%) | ||
| 71–80 | 126 (12.69%) | 18 (14.26%) | negative | 664 (66.87%) | 132 (19.87%) |
| 81–90 | 14 (1.41%) | 2 (14.28%) | positive | 329 (33.13%) | 76 (23.10%) |
| >90 | 1 (0.10%) | 0 (0%) | HER2 | ||
| Diameter (mm) | negative | 870 (87.61%) | 180 (20.69%) | ||
| T1 (≤20) | 748 (75.33%) | 130 (17.38%) | positive | 117 (11.78%) | 26 (22.22%) |
| T2 (>20, ≤50) | 231 (23.26%) | 69 (29.87%) | unknown | 6 (0.61%) | 2 (33.33%) |
| T3 (>50) | 14 (1.41%) | 9 (64.29%) | Grading | ||
| Histologic type | G1 | 106 (10.68%) | 32 (30.19%) | ||
| ductal | 718 (72.31%) | 129 (17.97%) | G2 | 176 (17.72%) | 47 (26.70%) |
| lobular | 64 (6.44%) | 17 (26.56%) | G3 | 115 (11.58%) | 30 (26.09%) |
| unknown | 211 (21.25%) | 62 (29.38%) | unknown | 596 (60.02%) | 99 (16.61%) |
| Model | Performance Measure | Hold-Out Training Set | Hold-Out Test Set |
|---|---|---|---|
| CM on line | AUC (%) | 64.7 | 68.6 |
| Acc (%) | 68.3 | 66.2 | |
| Sens (%) | 46.4 | 41.5 | |
| Spec (%) | 73.6 | 75.2 | |
| CM features (A) | AUC (%) | 68.0 (67.6–68.3) | 68.6 |
| Acc (%) | 57.6 (55.4–66.2) | 51.5 | |
| Sens (%) | 72.3 (58.4–76.7) | 73.6 | |
| Spec (%) | 54.2 (50.5–67.9) | 43.4 | |
| CM features + Her2 (B) | AUC (%) | 67.6 (67.2–68.0) | 67.8 |
| Acc (%) | 56.4 (55.1–62.6) | 52.0 | |
| Sens (%) | 74.2 (62.9–77.7) | 73.6 | |
| Spec (%) | 52.1 (50.1–63.7) | 42.1 | |
| CM features + Ki67 (C) | AUC (%) | 67.4 (67.0–67.7) | 68.0 |
| Acc (%) | 56.4 (55.2–63.5) | 50.5 | |
| Sens (%) | 74.2 (61.6–76.5) | 69.8 | |
| Spec (%) | 52.0 (50.2–64.3) | 45.5 | |
| CM features + Ki67 + HER2 (D) | AUC (%) | 64.1 (63.8–64.6) | 65.4 |
| Acc (%) | 58.5 (56.1–61.3) | 53.8 | |
| Sens (%) | 68.1 (52.5–60.6) | 70.4 | |
| Spec (%) | 55.9 (63.2–71.9) | 48.3 |
| Characteristic | Sample Size (Pos) | CM on Line | A (CM) | ||
|---|---|---|---|---|---|
| Sens | Spec | Sens | Spec | ||
| Overall | 795 (155) | 42% | 79% | 72% | 54% |
| T1 | 595 (98) | 61% | 57% | 70% | 60% |
| T2 | 188 (49) | 78% | 35% | 61% | 51% |
| Age ≤ 45 | 134 (34) | 21% | 94% | 59% | 59% |
| 45 < Age ≤ 60 | 369 (82) | 77% | 42% | 70% | 58% |
| Age > 60 | 292 (39) | 46% | 86% | 64% | 63% |
| G1 | 43 (15) | 33% | 82% | 33% | 82% |
| G2 | 96 (24) | 58% | 75% | 88% | 39% |
| G3 | 61 (17) | 82% | 50% | 88% | 36% |
| Luminal A | 482 (90) | 64% | 57% | 77% | 52% |
| Luminal B | 202 (48) | 69% | 56% | 83% | 45% |
| Her2 pos | 37 (6) | 50% | 74% | 50% | 77% |
| Triple negative | 68 (9) | 89% | 31% | 100% | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanizzi, A.; Pomarico, D.; Paradiso, A.; Bove, S.; Diotaiuti, S.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers 2021, 13, 352. https://doi.org/10.3390/cancers13020352
Fanizzi A, Pomarico D, Paradiso A, Bove S, Diotaiuti S, Didonna V, Giotta F, La Forgia D, Latorre A, Pastena MI, et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers. 2021; 13(2):352. https://doi.org/10.3390/cancers13020352
Chicago/Turabian StyleFanizzi, Annarita, Domenico Pomarico, Angelo Paradiso, Samantha Bove, Sergio Diotaiuti, Vittorio Didonna, Francesco Giotta, Daniele La Forgia, Agnese Latorre, Maria Irene Pastena, and et al. 2021. "Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study" Cancers 13, no. 2: 352. https://doi.org/10.3390/cancers13020352
APA StyleFanizzi, A., Pomarico, D., Paradiso, A., Bove, S., Diotaiuti, S., Didonna, V., Giotta, F., La Forgia, D., Latorre, A., Pastena, M. I., Tamborra, P., Zito, A., Lorusso, V., & Massafra, R. (2021). Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers, 13(2), 352. https://doi.org/10.3390/cancers13020352









