Quality of Life as a Mediator between Cancer Stage and Long-Term Mortality in Nasopharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Data
2.2. Treatment
2.3. Follow-Up
2.4. QoL Instruments
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Effects of AJCC Stage on Mortality
3.3. Effects of AJCC Stage on QoL Score
3.4. Effects of QoL Score on Mortality
3.5. Effect Changes Associated with QoL Score Mediation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Lee, A.W.; Ng, W.T.; Chan, L.L.; Hung, W.M.; Chan, C.C.; Sze, H.C.; Chan, O.S.H.; Chang, A.T.; Yeung, R.M. Evolution of treatment for nasopharyngeal cancer—Success and setback in the intensity-modulated radiotherapy era. Radiother. Oncol. 2014, 110, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Narita, N.; Fujimoto, Y.; Wakisaka, N.; Yoshizaki, T.; Kodaira, T.; Makita, C.; Sato, Y.; Yamazaki, K.; Wakaoka, T.; et al. Third Epidemiological Analysis of Nasopharyngeal Carcinoma in the Central Region of Japan from 2006 to 2015. Cancers 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, M.; Grenman, R.; Leivo, I.; Vahlberg, T.; Mäkitie, A.; Saarilahti, K.; Wigren, T.; Korpela, M.; Voutilainen, L.; Koivunen, P.; et al. Outcome of nasopharyngeal carcinoma in Finland: A nationwide study. Acta Oncol. 2018, 57, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sitlinger, A.; Zafar, S.Y. Health-Related Quality of Life: The impact on morbidity and mortality. Surg. Oncol. Clin. N. Am. 2018, 27, 675–684. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Ediebah, D.E.; Quinten, C.; Coens, C.; Ringash, J.; Dancey, J.; Zikos, E.; Gotay, C.; Brundage, M.; Tu, D.; Flechtner, H.; et al. Quality of life as a prognostic indicator of survival: A pooled analysis of individual patient data from Canadian cancer trials group clinical trials. Cancer 2018, 124, 3409–3416. [Google Scholar] [CrossRef]
- Yang, C.J.; Roh, J.-L.; Kim, M.-J.; Lee, S.-W.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Pretreatment quality of life as a prognostic factor for early survival and functional outcomes in patients with head and neck cancer. Qual. Life Res. 2016, 25, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.-M.; Tsai, W.-L.; Chien, C.-Y.; Chen, H.-C.; Hsu, H.-C.; Huang, T.-L.; Lee, T.-F.; Huang, H.-Y.; Lee, C.-H. Pretreatment Quality of Life as a Predictor of Distant Metastasis and Survival for Patients with Nasopharyngeal Carcinoma. J. Clin. Oncol. 2010, 28, 4384–4389. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-L.; Chien, C.-Y.; Huang, H.-Y.; Liao, K.-C.; Fang, F.-M. Prognostic value of quality of life measured after treatment on subsequent survival in patients with nasopharyngeal carcinoma. Qual. Life Res. 2013, 22, 715–723. [Google Scholar] [CrossRef]
- Guo, S.-S.; Hu, W.; Chen, Q.-Y.; Li, J.-M.; Zhu, S.-H.; He, Y.; Li, J.-W.; Xia, L.; Ji, L.; Lin, C.-Y.; et al. Pretreatment quality of life as a predictor of survival for patients with nasopharyngeal carcinoma treated with IMRT. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Huang, T.-L.; Chien, C.-Y.; Tsai, W.-L.; Liao, K.-C.; Chou, S.-Y.; Lin, H.-C.; Luo, S.D.; Lee, T.-F.; Lee, C.-H.; Fang, F.-M. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 2016, 38, E1026–E1032. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.J.; Rock, K.; Xu, W.; Chan, B.; Waldron, J.; Lu, L.; Ezzat, S.; Pothier, D.; Bernstein, L.; So, N.; et al. Long-Term Late Toxicity, Quality of Life, and Emotional Distress in Patients with Nasopharyngeal Carcinoma Treated with Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. 2018, 102, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Özyar, E.; Esassolak, M.; Altun, M.; Akmansu, M.; Şen, M.; Uzel, O.; Yavuz, A.; Dalmaz, G.; Uzal, C.; et al. Assessment of quality of life of nasopharyngeal carcinoma patients with EORTC QLQ-C30 and H&N-35 modules. Int. J. Radiat. Oncol. 2005, 63, 1347–1353. [Google Scholar] [CrossRef]
- Hong, J.; Tian, J.; Han, Q.; Ni, Q. Quality of Life of Nasopharyngeal Cancer Survivors in China. Curr. Oncol. 2015, 22, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yao, J.-J.; Zhou, G.-Q.; Zhang, W.; Liu, G.-L.; Liu, L.-Z.; Ma, J.; Sun, Y. The Impact of Clinical Stage on Radiation Doses to Organs at Risk Following Intensity-modulated Radiotherapy in Nasopharyngeal Carcinoma: A Prospective Analysis. J. Cancer 2016, 7, 2157–2164. [Google Scholar] [CrossRef]
- Haleshappa, R.A.; Thanky, A.H.; Kuntegowdanahalli, L.; Kanakasetty, G.B.; Dasappa, L.; Jacob, L. Epidemiology and outcomes of nasopharyngeal carcinoma: Experience from a regional cancer center in Southern India. South Asian J. Cancer 2017, 6, 122–124. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Foo, Y.C.; Yap, B.K.; Lee, C.M.L.; Hoo, L.P.; Lim, T.O. Retrospective Analysis of Cancer Care Performance and Survival Outcome for Nasopharyngeal Carcinoma at a leading Cancer Treatment Centre in Malaysia 2008–2012. Asian Pac. J. Cancer Prev. 2019, 20, 1701–1708. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Fang, F.; Huang, T.; Lin, Y.; Chien, C.; Chuang, H.; Luo, S.; Lin, H.; Lin, Y.; Li, S.; Liao, K.; et al. Concurrent chemoradiotherapy by simultaneously integrated boost volumetric-modulated arc therapy for nasopharyngeal carcinoma-toxicity/quality of life and survival. Head Neck 2019, 41, 1282–1289. [Google Scholar] [CrossRef]
- Chie, W.; Hong, R.; Lai, C.; Ting, L.; Hsu, M. Quality of life in patients of nasopharyngeal carcinoma: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and the EORTC QLQ-H&N35. Qual. Life Res. 2003, 12, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussel, Belgium, 2001. [Google Scholar]
- Bian, X.; Song, T.; Wu, S. Outcomes of xerostomia-related quality of life for nasopharyngeal carcinoma treated by IMRT: Based on the EORTC QLQ-C30 and H&N35 questionnaires. Expert Rev. Anticancer Ther. 2015, 15, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Ho, F.-M.; Cheng, N.-C.; Lee, M.-C.; Yeh, C.-J. BMI and all-cause mortality among middle-aged and older adults in Taiwan: A population-based cohort study. Public Health Nutr. 2015, 18, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Rosenzweig, M.; Linkov, F.; Brufsky, A.; Weissfeld, J.L.; Sereika, S.M. Comorbidity as a Mediator of Survival Disparity Between Younger and Older Women Diagnosed with Metastatic Breast Cancer. Hypertension 2012, 59, 205–211. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-Y.; Lin, W.-T.; Tsai, S.; Hung, Y.-C.; Wu, P.-W.; Yang, Y.-C.; Chan, T.-F.; Huang, H.-L.; Weng, Y.-L.; Chiu, Y.-W.; et al. Association of Parental Overweight and Cardiometabolic Diseases and Pediatric Adiposity and Lifestyle Factors with Cardiovascular Risk Factor Clustering in Adolescents. Nutrients 2016, 8, 567. [Google Scholar] [CrossRef]
- Lin, W.-T.; Chan, T.-F.; Huang, H.-L.; Lee, C.-Y.; Tsai, S.; Wu, P.-W.; Yang, Y.-C.; Wang, T.-N.; Lee, C.-H. Fructose-Rich Beverage Intake and Central Adiposity, Uric Acid, and Pediatric Insulin Resistance. J. Pediatr. 2016, 171, 90–96.e1. [Google Scholar] [CrossRef]
- Terrell, J.E.; Nanavati, K.; Esclamado, R.M.; Bradford, C.R.; Wolf, G.T. Health impact of head and neck cancer. Otolaryngol. Neck Surg. 1999, 120, 852–859. [Google Scholar] [CrossRef]
- Bottomley, A.; Therasse, P.; Piccart, M.; Efficace, F.; Coens, C.; Gotay, C.; Welnicka-Jaskiewicz, M.; Mauriac, L.; Dyczka, J.; Cufer, T.; et al. Health-related quality of life in survivors of locally advanced breast cancer: An international randomised controlled phase III trial. Lancet Oncol. 2005, 6, 287–294. [Google Scholar] [CrossRef]
- Barton, D.L.; Thanarajasingam, G.; Sloan, J.A.; Diekmann, B.; Fuloria, J.; Kottschade, L.A.; Lyss, A.P.; Jaslowski, A.J.; Mazurczak, M.A.; Blair, S.C.; et al. Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance). Cancer 2014, 120, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.P.; Gupta, D.; Grutsch, J.F.; Staren, E.D. Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health Qual. Life Outcomes 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Braun, D.P.; Staren, E.D. Prognostic value of changes in quality of life scores in prostate cancer. BMC Urol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.P.; Gupta, D.; Staren, E.D. Longitudinal Health-Related Quality of Life Assessment Implications for Prognosis in Stage IV Pancreatic Cancer. Pancreas 2013, 42, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-L.; Huang, T.-L.; Liao, K.-C.; Chuang, H.-C.; Lin, Y.-T.; Lee, T.-F.; Huang, H.-Y.; Fang, F.-M. Impact of late toxicities on quality of life for survivors of nasopharyngeal carcinoma. BMC Cancer 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.; Corry, J.; Ringash, J.; Rischin, D. Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Front. Oncol. 2020, 10, 930. [Google Scholar] [CrossRef]
- Hajdú, S.F.; Wessel, I.; Dalton, S.O.; Eskildsen, S.J.; Johansen, C. Swallowing Exercise During Head and Neck Cancer Treatment: Results of a Randomized Trial. Dysphagia 2021, 1–14. [Google Scholar] [CrossRef]
- Morais, M.O.; Martins, A.F.L.; De Jesus, A.P.G.; Neto, S.S.D.S.; Da Costa, A.W.F.; Pereira, C.H.; Oton-Leite, A.F.; De Freitas, N.M.A.; Leles, C.R.; Mendonça, E.F. A prospective study on oral adverse effects in head and neck cancer patients submitted to a preventive oral care protocol. Support. Care Cancer 2020, 28, 4263–4273. [Google Scholar] [CrossRef]
- Marimuthu, D.; Han, K.M.; Mohamad, M.S.F.; Azman, M. Saliva substitute mouthwash in nasopharyngeal cancer survivors with xerostomia: A randomized controlled trial. Clin. Oral Investig. 2020, 25, 3105–3115. [Google Scholar] [CrossRef]
- Shi, R.-C.; Meng, A.-F.; Zhou, W.-L.; Yu, X.-Y.; Huang, X.-E.; Ji, A.-J.; Chen, L. Effects of Home Nursing Intervention on the Quality of Life of Patients with Nasopharyngeal Carcinoma after Radiotherapy and Chemotherapy. Asian Pac. J. Cancer Prev. 2015, 16, 7117–7121. [Google Scholar] [CrossRef]
- Su, D.; He, Y.; Chen, L.; Li, J.; Zhang, L.; Chen, L.; Li, W.; Hu, W.; Li, C.; Fan, Y. Nutrition counseling combined with head and neck rehabilitation exercises can enhance outcomes among nasopharyngeal carcinoma patients in southern China: A prospective study in an epidemic area. Ann. Palliat. Med. 2020, 9, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fu, S.-N.; Chen, Y.-Z.; Yan, O.-Y.; Tong, F.; Peng, W.-L.; Zou, R.; Wen, M.-N.; Jiang, L.; Ma, H.-Z.; et al. Effects of Cognitive Behavioral Therapy for Depression and Anxiety, Response Rates and Adverse Events in Patients with Locoregional Advanced Nasopharyngeal Carcinoma. Integr. Cancer Ther. 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Temel, B.; Fuh, C.-X.; Kay, P.; Landay, S.; Lage, D.; Franco-Garcia, E.; Scott, E.; Stevens, E.; O’Malley, T.; et al. Pilot Randomized Trial of a Transdisciplinary Geriatric and Palliative Care Intervention for Older Adults with Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 591–598. [Google Scholar] [CrossRef] [PubMed]


| Factors | No. (%) | aHR a | (95% CI) |
|---|---|---|---|
| Age, year, mean (SD) | 49.4 (11.5) | - | - |
| ≤40 | 152 (22.3) | 1.0 | - |
| >40 | 530 (77.7) | 1.9 | (1.3 to 2.9) |
| Gender | |||
| Female | 163 (23.9) | 1.0 | - |
| Male | 519 (76.1) | 2.0 | (1.3 to 3.0) |
| Ethnicity | |||
| Minnan | 551 (80.8) | 1.0 | - |
| Other | 131 (19.2) | 1.2 | (0.9 to 1.7) |
| Educational level, year | |||
| >12 | 190 (27.9) | 1.0 | - |
| ≤12 | 491 (72.1) | 1.6 | (1.1 to 2.3) |
| Body mass index (kg/m2) b | |||
| Normal (18.5–23.9) | 268 (39.8) | 1.0 | - |
| Underweight (<18.5) | 16 (2.4) | 2.4 | (1.1 to 5.1) |
| Overweight (≥24.0) | 390 (57.9) | 1.0 | (0.7 to 1.3) |
| Charlson comorbidity index | |||
| 0 | 490 (72.5) | 1.0 | - |
| ≥1 | 186 (27.5) | 1.2 | (0.9 to 1.6) |
| Chemotherapy | |||
| No | 94 (13.8) | 1.0 | |
| Yes | 588 (86.2) | 0.7 | (0.4 to 1.2) |
| IMRT treatment period | |||
| Before 2011 | 436 (63.9) | 1.0 | |
| 2011 onward | 246 (36.1) | 0.5 | (0.3 to 0.7) |
| Follow-up time, months | |||
| Mean (SD) | 90.8 (50.8) | - | - |
| Median (range) | 90.5 (3.4–193.0) | - | - |
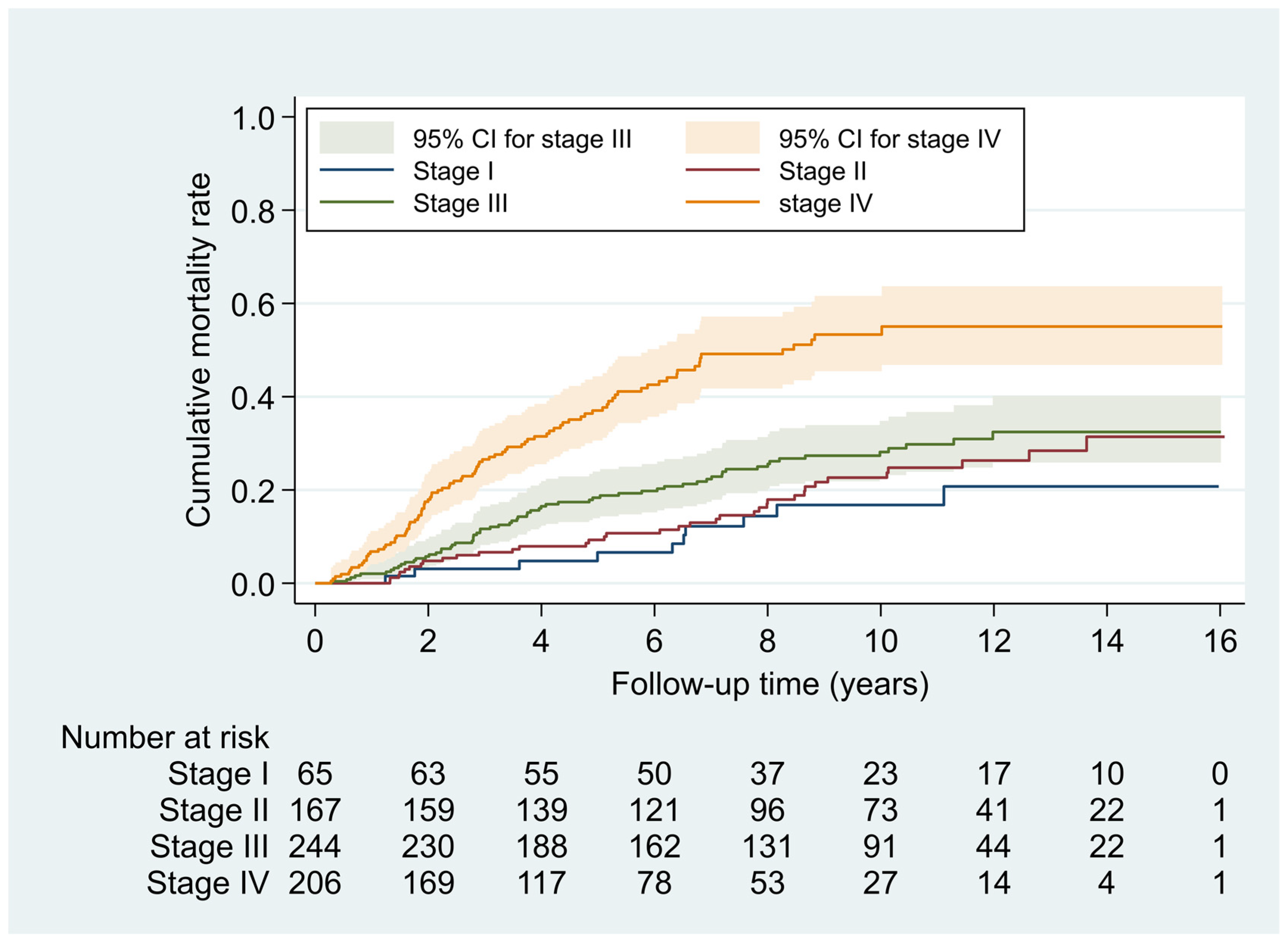
| Group | No. of Patients (%) | Years of Follow-Up | No. of Deaths | Mortality Density, per 100 PY | aHR a | (95% CI) | |
|---|---|---|---|---|---|---|---|
| Total | 682 | (100.0) | 5163.1 | 204 | 3.95 | - | - |
| AJCC stage | |||||||
| I | 65 | (9.5) | 583.0 | 10 | 1.7 | 1.0 | - |
| II | 167 | (24.5) | 1468.3 | 36 | 2.5 | 1.8 | (0.8 to 3.7) |
| III | 244 | (35.8) | 1983.9 | 65 | 3.3 | 2.5 | (1.2 to 5.3) |
| IV | 206 | (30.2) | 1127.9 | 93 | 8.2 | 5.7 | (2.7 to 12.3) |
| Factor | Global Health QoL a | Functioning QoL a | QoL-C30 Symptom a | QoL-HN35 Symptom a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adj. β b | (95% CI) | p | Adj. β b | (95% CI) | p | Adj. β b | (95% CI) | p | Adj. β b | (95% CI) | p | |
| Main effect | ||||||||||||
| AJCC stage c | ||||||||||||
| I–II | Ref. | - | - | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| III–IV | 1.3 | (−2.2 to 4.8) | 0.471 | −2.1 | (−4.5 to 0.4) | 0.103 | 0.08 | (−2.2 to 2.4) | 0.946 | 0.08 | (−2.3 to 2.4) | 0.946 |
| Time points c | ||||||||||||
| Pre-IMRT | 17.4 | (14.2 to 20.5) | <0.001 | 7.7 | (5.9 to 9.5) | <0.001 | −14.9 | (−16.6 to −13.1) | <0.001 | −25.3 | (−27.1 to −23.5) | <0.001 |
| During IMRT (40 Gy) | Ref. | - | - | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| 3 months post IMRT | 20.8 | (17.7 to 24.0) | <0.001 | 6.4 | (4.6 to 8.2) | <0.001 | −12.1 | (−13.9 to −10.3) | <0.001 | −13.7 | (−15.6 to −11.9) | <0.001 |
| 1 year post IMRT | 25.7 | (21.8 to 29.5) | <0.001 | 7.5 | (5.1 to 9.8) | <0.001 | −14.4 | (−16.7 to −12.1) | <0.001 | −16.3 | (−18.7 to −14.0) | <0.001 |
| 2 years post IMRT | 28.6 | (24.4 to 32.8) | <0.001 | 8.8 | (6.1 to 11.6) | <0.001 | −15.9 | (−18.4 to −13.3) | <0.001 | −20.2 | (−22.9 to −17.5) | <0.001 |
| Interaction effect (stage × time) | ||||||||||||
| III–IV × pre-IMRT | −6.3 | (−10.2 to −2.4) | 0.001 | −2.3 | (−4.5 to −0.1) | 0.043 | 4.0 | (1.8 to 6.2) | <0.001 | 4.3 | (2.0 to 6.6) | <0.001 |
| III–IV × 3 months post IMRT | −6.3 | (−10.3 to −2.3) | 0.002 | −1.7 | (−4.0 to 0.6) | 0.143 | 3.6 | (1.3 to 5.8) | 0.002 | 4.0 | (1.6 to 6.3) | 0.001 |
| III–IV × 1 year post IMRT | −2.3 | (−7.1 to 2.5) | 0.353 | 0.7 | (−2.3 to 3.7) | 0.633 | 1.2 | (−1.7 to 4.1) | 0.428 | 0.9 | (−2.1 to 3.9) | 0.540 |
| III–IV × 2 years post IMRT | −5.8 | (−11.2 to −0.3) | 0.038 | −0.6 | (−4.1 to 2.9) | 0.730 | 2.6 | (−0.8 to 5.9) | 0.132 | 3.6 | (0.1 to 7.0) | 0.043 |
| b QoL Scores | Pre-IMRT | During IMRT (40 Gy) | 3 Months Post IMRT | 1 Year Post IMRT | 2 Years Post IMRT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| aHR a | (95% CI) | aHR a | (95% CI) | aHR a | (95% CI) | aHR a | (95% CI) | aHR a | (95% CI) | |
| Global health QoL | 0.92 * | (0.86 to 0.99) | 0.94 | (0.87 to 1.02) | 0.97 | (0.89 to 1.05) | 0.88 * | (0.80 to 0.97) | 0.79 * | (0.69 to 0.91) |
| Functioning QoL | 0.90 | (0.82 to 1.00) | 0.97 | (0.88 to 1.06) | 0.93 | (0.83 to 1.03) | 0.85 * | (0.76 to 0.96) | 0.80 * | (0.67 to 0.96) |
| QoL-C30 symptom | 1.15 * | (1.03 to 1.28) | 1.00 | (0.90 to 1.11) | 1.08 | (0.97 to 1.21) | 1.19 * | (1.05 to 1.35) | 1.34 * | (1.11 to 1.63) |
| QoL-HN35 symptom | 1.17 * | (1.05 to 1.31) | 1.02 | (0.92 to 1.12) | 1.02 | (0.91 to 1.15) | 1.18 * | (1.04 to 1.34) | 1.34 * | (1.11 to 1.61) |
| Models/Variables | Stages III–IV vs. I–II | EC a | ||
|---|---|---|---|---|
| aHR | (95% CI) | p Value | ||
| Base modelb | 2.26 | (1.56 to 3.27) | <0.001 | Ref. |
| Base model + QoL scale c,d | ||||
| Pre-IMRT | ||||
| Global health QoL | 2.19 | (1.51 to 3.17) | <0.001 | 5.8% |
| QoL-C30 symptom | 2.14 | (1.47 to 3.10) | <0.001 | 9.8% |
| QoL-HN35 symptom | 2.14 | (1.48 to 3.10) | <0.001 | 9.3% |
| 2 years post IMRT | ||||
| Global health QoL | 1.64 | (0.92 to 2.90) | 0.091 | 49.4% |
| QoL-HN35 symptom | 1.76 | (0.99 to 3.12) | 0.052 | 39.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, K.-C.; Chuang, H.-C.; Chien, C.-Y.; Lin, Y.-T.; Tsai, M.-H.; Su, Y.-Y.; Yang, C.-H.; Lai, C.-C.; Huang, T.-L.; Li, S.-H.; et al. Quality of Life as a Mediator between Cancer Stage and Long-Term Mortality in Nasopharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy. Cancers 2021, 13, 5063. https://doi.org/10.3390/cancers13205063
Liao K-C, Chuang H-C, Chien C-Y, Lin Y-T, Tsai M-H, Su Y-Y, Yang C-H, Lai C-C, Huang T-L, Li S-H, et al. Quality of Life as a Mediator between Cancer Stage and Long-Term Mortality in Nasopharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy. Cancers. 2021; 13(20):5063. https://doi.org/10.3390/cancers13205063
Chicago/Turabian StyleLiao, Kuan-Cho, Hui-Ching Chuang, Chih-Yen Chien, Yu-Tsai Lin, Ming-Hsien Tsai, Yan-Ye Su, Chao-Hui Yang, Chi-Chih Lai, Tai-Lin Huang, Shau-Hsuan Li, and et al. 2021. "Quality of Life as a Mediator between Cancer Stage and Long-Term Mortality in Nasopharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy" Cancers 13, no. 20: 5063. https://doi.org/10.3390/cancers13205063
APA StyleLiao, K.-C., Chuang, H.-C., Chien, C.-Y., Lin, Y.-T., Tsai, M.-H., Su, Y.-Y., Yang, C.-H., Lai, C.-C., Huang, T.-L., Li, S.-H., Lee, T.-F., Lin, W.-T., Lee, C.-H., & Fang, F.-M. (2021). Quality of Life as a Mediator between Cancer Stage and Long-Term Mortality in Nasopharyngeal Cancer Patients Treated with Intensity-Modulated Radiotherapy. Cancers, 13(20), 5063. https://doi.org/10.3390/cancers13205063








