Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Histomorphological Characterization
2.1.2. Immunohistochemistry
2.2. Statistics
3. Results
3.1. Clinicopathological Features and Survival
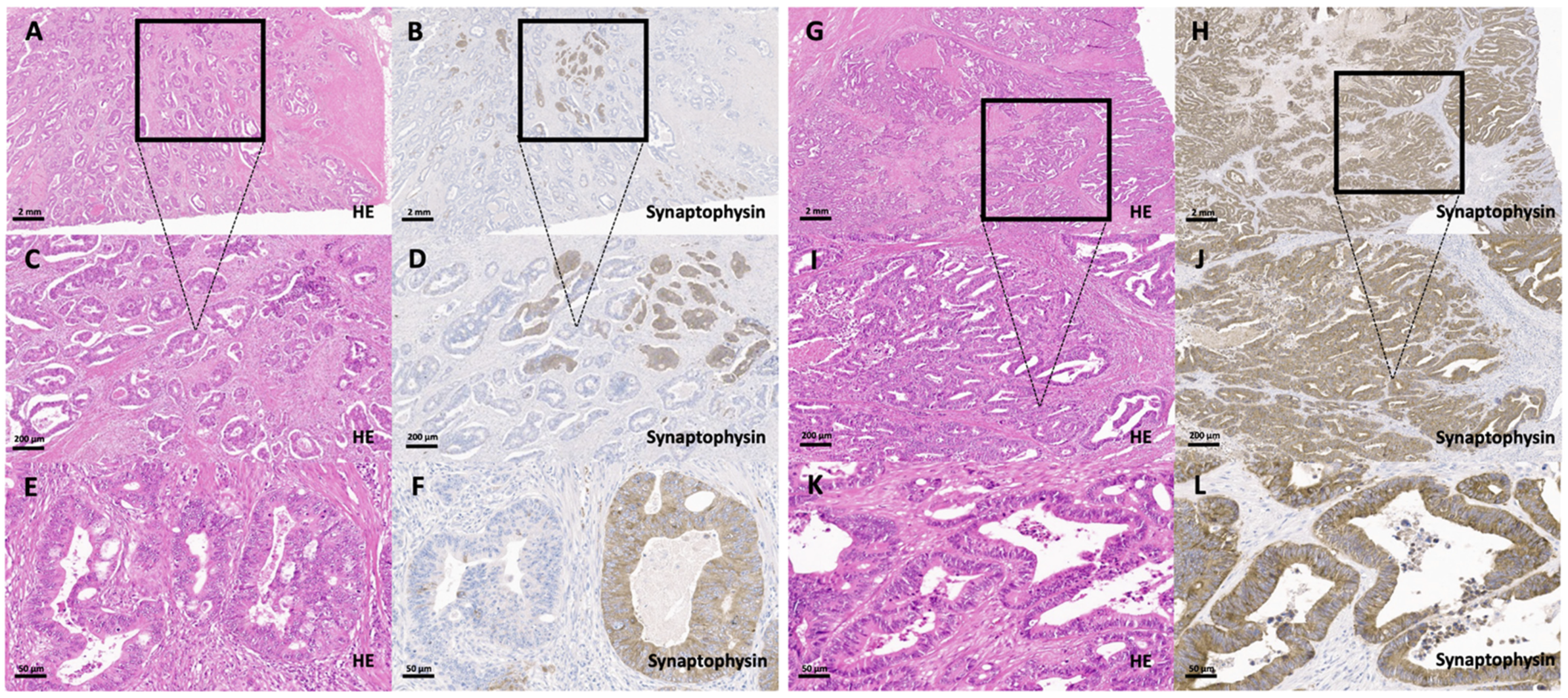
3.2. Synaptophysin Expression in Conventional Colorectal Adenocarcinomas without Histological Features Suggestive of a Neuroendocrine Differentiation
3.3. Correlation of Synaptophysin Expression in Conventional Colorectal Adenocarcinomas with Clinicopathological Data
3.4. Correlation of Synaptophysin Expression in Conventional Colorectal Adenocarcinomas with Survival Parameters
3.5. Survival of True Colorectal MANECs Compared to Colorectal Adenocarcinomas without Histological Features Suggestive of a Neuroendocrine Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- Woischke, C.; Schaaf, C.W.; Yang, H.M.; Vieth, M.; Veits, L.; Geddert, H.; Markl, B.; Stommer, P.; Schaeffer, D.F.; Frolich, M.; et al. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod. Pathol. 2017, 30, 95–103. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Konukiewitz, B.; Keller, G.; Kloor, M.; Steiger, K.; Reiche, M.; Penzel, R.; Endris, V.; Arsenic, R.; Hermann, G.; et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod. Pathol. 2017, 30, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Jesinghaus, M.; Schmitt, M.; Lang, C.; Reiser, M.; Scheiter, A.; Konukiewitz, B.; Steiger, K.; Silva, M.; Tschurtschenthaler, M.; Lange, S.; et al. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am. J. Surg. Pathol. 2021, 45, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Machida, N.; Morizane, C.; Kasuga, A.; Takahashi, H.; Sudo, K.; Nishina, T.; Tobimatsu, K.; Ishido, K.; Furuse, J.; et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014, 105, 1176–1181. [Google Scholar] [CrossRef]
- Tanaka, T.; Kaneko, M.; Nozawa, H.; Emoto, S.; Murono, K.; Otani, K.; Sasaki, K.; Nishikawa, T.; Kiyomatsu, T.; Hata, K.; et al. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology 2017, 105, 426–434. [Google Scholar] [CrossRef]
- La Rosa, S.; Marando, A.; Sessa, F.; Capella, C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers 2012, 4, 11–30. [Google Scholar] [CrossRef]
- Volante, M.; Rindi, G.; Papotti, M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: A comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006, 449, 499–506. [Google Scholar] [CrossRef]
- Wiedenmann, B.; Franke, W.W.; Kuhn, C.; Moll, R.; Gould, V.E. Synaptophysin: A marker protein for neuroendocrine cells and neoplasms. Proc. Natl. Acad. Sci. USA 1986, 83, 3500–3504. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Arends, M.; Odze, R.D.; Lam, A.K. Digestive System Tumours: Tumours of the Colon and Rectum, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2019. [Google Scholar]
- Grabowski, P.; Schindler, I.; Anagnostopoulos, I.; Foss, H.D.; Riecken, E.O.; Mansmann, U.; Stein, H.; Berger, G.; Buhr, H.J.; Scherubl, H. Neuroendocrine differentiation is a relevant prognostic factor in stage III-IV colorectal cancer. Eur. J. Gastroenterol. Hepatol. 2001, 13, 405–411. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Meng, Q.; Ma, S. Is neuroendocrine differentiation a prognostic factor in poorly differentiated colorectal cancer? World J. Surg. Oncol. 2017, 15, 71. [Google Scholar] [CrossRef]
- de Bruine, A.P.; Wiggers, T.; Beek, C.; Volovics, A.; von Meyenfeldt, M.; Arends, J.W.; Bosman, F.T. Endocrine cells in colorectal adenocarcinomas: Incidence, hormone profile and prognostic relevance. Int. J. Cancer 1993, 54, 765–771. [Google Scholar] [CrossRef]
- Shinji, S.; Naito, Z.; Ishiwata, T.; Tanaka, N.; Furukawa, K.; Suzuki, H.; Seya, T.; Kan, H.; Tsuruta, H.; Matsumoto, S.; et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int. J. Oncol. 2006, 29, 357–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamada, Y.; Oishi, A.; Shoji, T.; Takada, H.; Yamamura, M.; Hioki, K.; Yamamoto, M. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer 1992, 69, 2641–2646. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Lai, W.; Liu, L.; Wu, H.; Luo, X.X.; Wang, J.; Chu, Z.H. Prognostic significance of neuroendocrine differentiation in colorectal adenocarcinoma after radical operation: A meta-analysis. J. Gastrointest. Surg. 2014, 18, 968–976. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Schroeder, G.; Bauman, M.D.; Krook, J.E.; Jin, L.; Goldberg, R.M.; Farr, G.H., Jr. Prevalence and Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas. Endocr. Pathol. 1998, 9, 35–42. [Google Scholar] [CrossRef]
- Foley, E.F.; Gaffey, M.J.; Frierson, H.F., Jr. The frequency and clinical significance of neuroendocrine cells within stage III adenocarcinomas of the colon. Arch. Pathol. Lab. Med. 1998, 122, 912–914. [Google Scholar]
- Suresh, P.K.; Sahu, K.K.; Pai, R.R.; Sridevi, H.B.; Ballal, K.; Khandelia, B.; Minal, J.; Annappa, R. The Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas: Our Experience. J. Clin. Diagn. Res. 2015, 9, EC01–EC04. [Google Scholar] [CrossRef]
- Gospodarowicz, M.K.; Brierley, J.D.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yu, S.; Hornick, J.L.; Gonzalez, R.S. An algorithmic approach utilizing CK7, TTF1, beta-catenin, CDX2, and SSTR2A can help differentiate between gastrointestinal and pulmonary neuroendocrine carcinomas. Virchows Arch. 2021. [Google Scholar] [CrossRef]
- Chavez-Blanco, A.; Taja-Chayeb, L.; Cetina, L.; Chanona-Vilchis, G.; Trejo-Becerril, C.; Perez-Cardenas, E.; Segura-Pacheco, B.; Acuna-Gonzalez, C.; Duenas-Gonzalez, A. Neuroendocrine marker expression in cervical carcinomas of non-small cell type. Int. J. Gynecol. Pathol. 2002, 21, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.Y.; Zhou, J.L.; Lai, M.D.; Chen, X.Q.; Chen, P.H. Neuroendocrine markers in adenocarcinomas: An investigation of 356 cases. World J. Gastroenterol. 2003, 9, 858–861. [Google Scholar] [CrossRef]
- Ogimi, T.; Sadahiro, S.; Kamei, Y.; Chan, L.F.; Miyakita, H.; Saito, G.; Okada, K.; Suzuki, T.; Kajiwara, H. Distribution of Neuroendocrine Marker-Positive Cells in Colorectal Cancer Tissue and Normal Mucosal Tissue: Consideration of Histogenesis of Neuroendocrine Cancer. Oncology 2019, 97, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Baudin, E.; Ducreux, M.; Sabourin, J.C.; Rufie, P.; Aparicio, T.; Aparicio, T.; Lasser, P.; Elias, D.; Duvillard, P.; et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer 1999, 81, 1351–1355. [Google Scholar] [CrossRef] [PubMed]


| Overall | Adenocarcinoma Synaptophysin-Negative Cells | Adenocarcinoma 1–9% Synaptophysin-Positive Cells | Adenocarcinoma 10–29% Synaptophysin-Positive Cells | Adenocarcinoma 30–100% Synaptophysin-Positive Cells | p-Value | ||
|---|---|---|---|---|---|---|---|
| 1002 (100%) | 763 (76%) | 198 (20%) | 15 (1%) | 26 (3%) | |||
| Age | 0.46 | ||||||
| below median | 485 (48%) | 367 (48%) | 102 (51%) | 5 (33%) | 11 (42%) | ||
| above median | 517 (52%) | 396 (52%) | 96 (49%) | 10 (67%) | 15 (58%) | ||
| Sex | 0.52 | ||||||
| male | 575 (57%) | 431 (56%) | 118 (60%) | 8 (53%) | 18 (69%) | ||
| female | 427 (43%) | 332 (44%) | 80 (40%) | 7 (47%) | 8 (31%) | ||
| pT | 0.49 | ||||||
| 1 | 73 (7%) | 51(7%) | 21 (11%) | 0 | 1 (7%) | ||
| 2 | 180 (18%) | 136 (18%) | 36 (18%) | 4 (27%) | 4 (18%) | ||
| 3 | 558 (56%) | 429 (56%) | 104 (52%) | 7 (46%) | 18 (69%) | ||
| 4 | 191 (19%) | 147 (19%) | 37 (19%) | 4 (47%) | 3 (19%) | ||
| pN | <0.001 | ||||||
| 0 | 560 (56%) | 426 (56%) | 120 (61%) | 5 (33%) | 9 (35%) | ||
| 1 | 284(28%) | 225 (29%) | 46 (23%) | 7 (47%) | 6 (23%) | ||
| 2 | 158 (16%) | 112 (15%) | 32 (16%) | 3 (20%) | 11 (42%) | ||
| pM | 0.09 | ||||||
| 0 | 859 (86%) | 663 (87%) | 168 (85%) | 11 (73%) | 17 (64%) | ||
| 1 | 143 (14%) | 100 (13%) | 30 (15%) | 4 (27%) | 9 (36%) | ||
| UICC Stage | 0.01 | ||||||
| 1 | 201 (20%) | 144 (19%) | 51 (26%) | 2 (13%) | 4 (15%) | ||
| 2 | 342 (34%) | 269 (35%) | 66 (33%) | 2(13%) | 5 (19%) | ||
| 3 | 310 (31%) | 245 (32%) | 50 (25%) | 7 (47%) | 8 (31%) | ||
| 4 | 149 (15%) | 105 (14%) | 31 (16%) | 4 (27%) | 9 (35%) | ||
| Lymphatic | 0.02 | ||||||
| Invasion | not present | 498 (49%) | 381 (50%) | 106 (54%) | 4 (27%) | 7 (27%) | |
| present | 504 (51%) | 382 (50%) | 92 (46%) | 11 (73%) | 19 (73%) | ||
| Vascular | 0.19 | ||||||
| Invasion | not present | 867 (86.5%) | 663 (87%) | 169 (86%) | 15 (100%) | 20 (77%) | |
| present | 135 (13.5%) | 100 (13%) | 29 (14%) | 0 | 6 (23%) | ||
| Resection | 0.19 | ||||||
| Margin | R0 | 933 (93%) | 709 (93%) | 188 (85%) | 13 (87%) | 23 (88%) | |
| R1 | 41 (4%) | 34 (4%) | 3 (2%) | 2 (13%) | 2 (8%) | ||
| R2 | 28 (3%) | 20 (3%) | 7 (3%) | 0 | 1 (4%) | ||
| Localization | 0.51 | ||||||
| right colon | 488 (49%) | 368 (48%) | 96 (48%) | 10 (67%) | 14 (54%) | ||
| left colon | 514 (51%) | 395 (52%) | 102 (52%) | 5 (33%) | 12 (46%) | ||
| WHO Tumor Type | 0.01 | ||||||
| Adenocarcinoma NOS | 629 (63%) | 480 (61%) | 125 (62%) | 8 (53%) | 16 (61%) | ||
| Mucinous adenocarcinoma | 86 (8%) | 71 (9%) | 13 (6%) | 1 (7%) | 1 (4%) | ||
| Signet-ring cell carcinoma | 9 (1%) | 6 (1%) | 1 (1%) | 0 | 2 (8%) | ||
| Medullary carcinoma | 31 (3%) | 24 (4%) | 6 (3%) | 1 (7%) | 0 | ||
| Micropapillary adenocarcinoma | 128 (13%) | 102 (13%) | 17 (9%) | 3 (20%) | 6 (23%) | ||
| Serrated adenocarcinoma | 88 (9%) | 60 (8%) | 25 (13%) | 2 (13%) | 1 (4%) | ||
| Adenoma-like adenocarcinoma | 31 (3%) | 20 (4%) | 11 (6%) | 0 | 0 | ||
| Microsatellite | 0.19 | ||||||
| Status | microsatellite stable | 846 (84%) | 634 (83%) | 175 (88%) | 13 (87%) | 24 (92%) | |
| microsatellite instable | 156 (16%) | 129 (17%) | 23 (12%) | 2 (13%) | 2 (8%) | ||
| WHO Grade | 0.19 | ||||||
| low-grade | 687 (68%) | 520 (68%) | 144 (73%) | 8 (53%) | 15 (69%) | ||
| high-grade | 315 (32%) | 243 (32%) | 54 (27%) | 7 (47%) | 11 (31%) | ||
| HR (DFS) | Lower CI (95%) | Upper CI (95%) | p-Value | ||
|---|---|---|---|---|---|
| Conventional Adenocarcinoma Synaptophysin Subgroups | 0.49 | ||||
| Conventional adenocarcinoma synaptophysin negative | 1.0 | ||||
| Conventional adenocarcinoma 1–9% synaptophysin positive | 1.2 | 0.92 | 1.60 | ||
| Conventional adenocarcinoma 10–29% synaptophysin positive | 0.8 | 0.34 | 1.97 | ||
| Conventional adenocarcinoma 30–100% synaptophysin positive | 1.1 | 0.64 | 1.94 | ||
| WHO grade | 0.01 | ||||
| Low grade | 1.00 | ||||
| High grade | 1.34 | 1.07 | 1.69 | ||
| UICC Stage | I | 1.00 | <0.001 | ||
| II | 2.16 | 1.30 | 3.50 | ||
| III | 3.94 | 2.48 | 6.25 | ||
| IV | 11.87 | 7.40 | 19.05 | ||
| Age group | 0.72 | ||||
| Below median | 1.00 | ||||
| Median and above | 1.05 | 0.84 | 1.32 | ||
| Sex | male | 1.0 | 0.48 | ||
| female | 1.08 | 0.86 | 1.35 |
| HR (DFS) | Lower CI (95%) | Upper CI (95%) | p-Value | ||
|---|---|---|---|---|---|
| Conventional Adenocarcinoma Synaptophysin Subgroups | 0.001 | ||||
| versus MANEC/NEC | |||||
| Conventional Adenocarcinoma synaptophysin negative | 1.0 | ||||
| Conventional Adenocarcinoma 1–9% synaptophysin positive | 1.20 | 0.91 | 1.59 | ||
| Conventional Adenocarcinoma 10–29% synaptophysin positive | 0.83 | 0.34 | 2.01 | ||
| Conventional Adenocarcinoma 30–100% synaptophysin positive | 1.12 | 0.65 | 1.96 | ||
| MANEC/NEC | 3.87 | 1.79 | 8.37 | ||
| WHO grade | 0.011 | ||||
| Low grade | 1.00 | ||||
| High grade | 1.34 | 1.07 | 1.68 | ||
| UICC Stage | I | 1.00 | <0.001 | ||
| II | 2.10 | 1.31 | 3.44 | ||
| III | 4.17 | 2.52 | 6.33 | ||
| IV | 12.16 | 7.39 | 19.02 | ||
| Age group | 0.61 | ||||
| Below median | 1.00 | ||||
| Median and above | 1.05 | 0.86 | 1.36 | ||
| Sex | male | 1.0 | 0.37 | ||
| female | 1.14 | 0.83 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konukiewitz, B.; Kasajima, A.; Schmitt, M.; Schwamborn, K.; Groll, T.; Schicktanz, F.; Delbridge, C.; Schütze, L.M.; Wilhelm, D.; Lang, C.; et al. Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker? Cancers 2021, 13, 5111. https://doi.org/10.3390/cancers13205111
Konukiewitz B, Kasajima A, Schmitt M, Schwamborn K, Groll T, Schicktanz F, Delbridge C, Schütze LM, Wilhelm D, Lang C, et al. Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker? Cancers. 2021; 13(20):5111. https://doi.org/10.3390/cancers13205111
Chicago/Turabian StyleKonukiewitz, Björn, Atsuko Kasajima, Maxime Schmitt, Kristina Schwamborn, Tanja Groll, Felix Schicktanz, Claire Delbridge, Lisa Marie Schütze, Dirk Wilhelm, Corinna Lang, and et al. 2021. "Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker?" Cancers 13, no. 20: 5111. https://doi.org/10.3390/cancers13205111
APA StyleKonukiewitz, B., Kasajima, A., Schmitt, M., Schwamborn, K., Groll, T., Schicktanz, F., Delbridge, C., Schütze, L. M., Wilhelm, D., Lang, C., Lange, S., Foersch, S., Jank, P., Steiger, K., Werder, A. v., Denkert, C., Weichert, W., Klöppel, G., & Jesinghaus, M. (2021). Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker? Cancers, 13(20), 5111. https://doi.org/10.3390/cancers13205111








