Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. CT Image Analysis
2.3. RNA Isolation and Reverse-Transcription
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Patient Population and Clinical Evolution
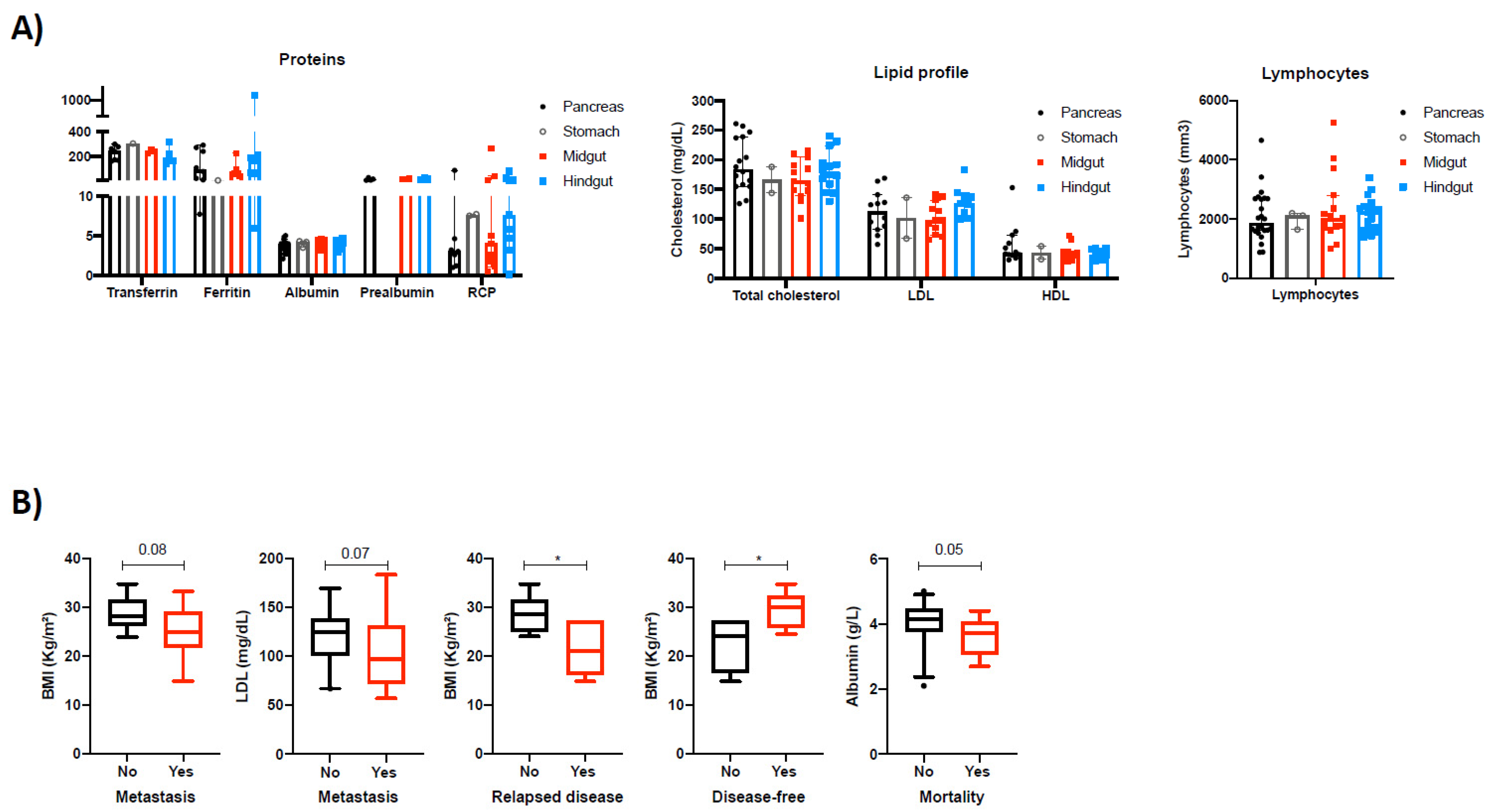
3.2. Cumulative Survival in NEN Patients Is Associated with Nutritional Parameters
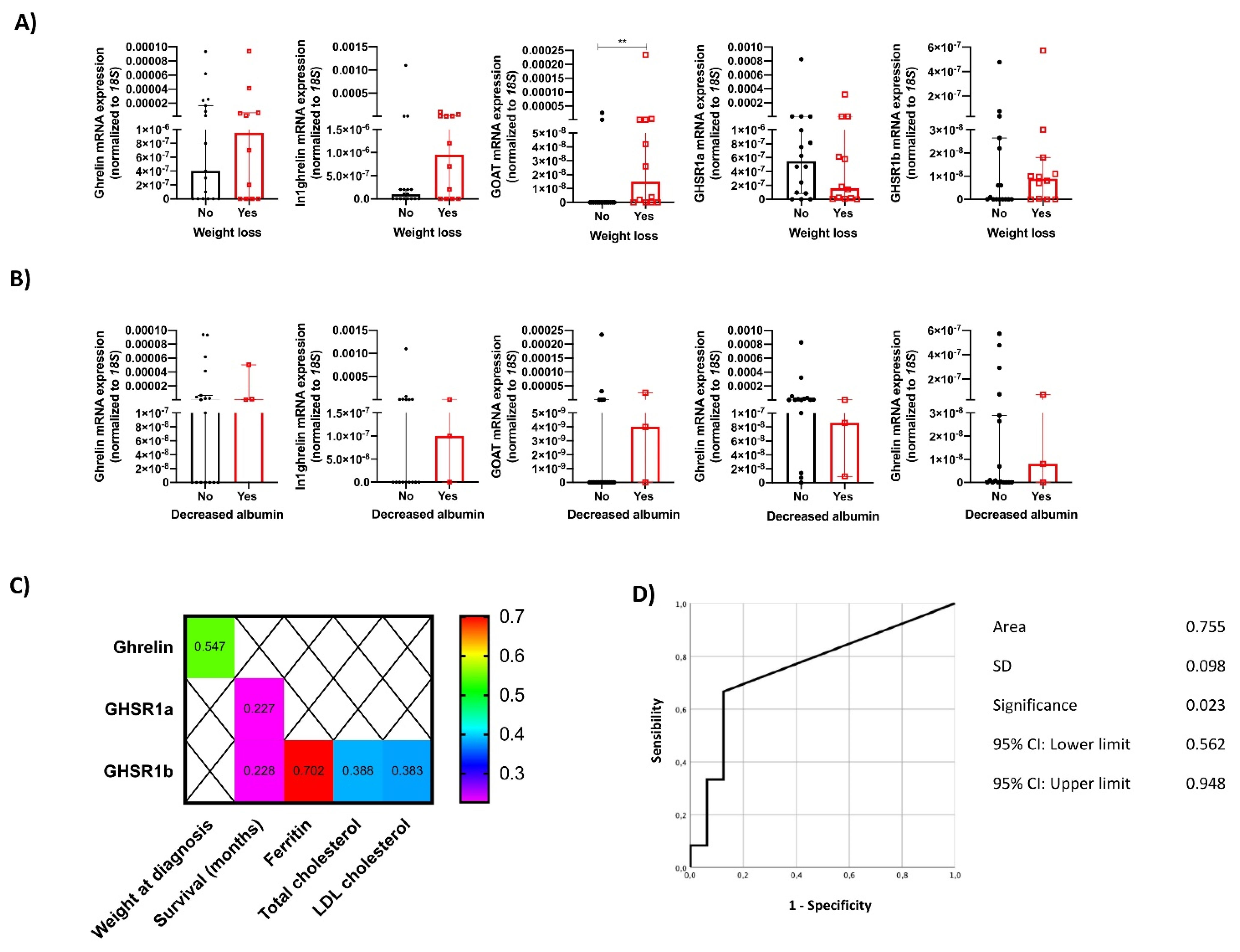
3.3. The Molecular Expression of the Ghrelin System and Its Relation to Nutritional Characteristics of NEN Patients
3.4. Sarcopenia at Diagnosis Found to Be Highly Prevalent in NEN Patients Using CT Scan Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Mendes, N.P.; De Barros, T.A.; Rosa, C.D.O.B.; Franceschini, S.D.C.C. Nutritional Screening Tools Used and Validated for Cancer Patients: A Systematic Review. Nutr. Cancer 2019, 71, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Muscogiuri, G.; Pizza, G.; Ruggeri, R.M.; Barrea, L.; Faggiano, A.; Colao, A.; NIKE Group. The management of neuroendocrine tumours: A nutritional viewpoint. Crit. Rev. Food Sci. Nutr. 2017, 59, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Byers, T.; Sedjo, R.L. Body fatness as a cause of cancer: Epidemiologic clues to biologic mechanisms. Endocrine-Related Cancer 2015, 22, R125–R134. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Schmitz, K.H.; Berger, N.A. Sarcopenia in aging, obesity, and cancer. Transl. Cancer Res. 2020, 9, 5760–5771. [Google Scholar] [CrossRef]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef] [Green Version]
- Altieri, B.; Barrea, L.; Modica, R.; Muscogiuri, G.; Savastano, S.; Colao, A.; Faggiano, A. Nutrition and neuroendocrine tumors: An update of the literature. Rev. Endocr. Metab. Disord. 2018, 19, 159–167. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rincón-Fernández, D.; Villa-Osaba, A.; Hormaechea-Agulla, D.; Ibañez-Costa, A.; Martínez-Fuentes, A.J.; Gracia-Navarro, F.; Castano, J.P.; Luque, R.M. Ghrelin gene products, receptors, and GOAT enzyme: Biological and pathophysiological insight. J. Endocrinol. 2013, 220, R1–R24. [Google Scholar] [CrossRef] [Green Version]
- Chopin, L.K. The ghrelin axis—Does it have an appetite for cancer progression? Endocr. Rev. 2012, 33, 849–891. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Kineman, R.D.; Luque, R.M. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in re-leasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 2007, 148, 4440–4449. [Google Scholar] [CrossRef] [Green Version]
- Nishi, Y.; Yoh, J.; Hiejima, H.; Kojima, M. Structures and molecular forms of the ghrelin-family peptides. Peptides 2011, 32, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Cordoba-Chacon, J.; Hergueta-Redondo, M.; Martínez-Fuentes, A.J.; Kineman, R.D.; Moreno-Bueno, G.; Luque, R.M.; Castaño, J.P. A Novel Human Ghrelin Variant (In1-Ghrelin) and Ghrelin-O-Acyltransferase Are Overexpressed in Breast Cancer: Potential Pathophysiological Relevance. PLoS ONE 2011, 6, e23302. [Google Scholar] [CrossRef] [Green Version]
- Leung, P.K. The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin recep-tor. Cell Signal 2007, 19, 1011–1022. [Google Scholar] [CrossRef]
- Lin, T.C.; Hsiao, M. Ghrelin, and cancer progression. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 51–57. [Google Scholar]
- Hormaechea-Agulla, D. Ghrelin O-acyltransferase (GOAT) enzyme is overexpressed in prostate cancer, and its levels are associated with patient’s metabolic status: Potential value as a non-invasive biomarker. Cancer Lett. 2016, 383, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Gahete, M.D.; Sánchez-Sánchez, R.; Salas, R.O.; Serrano-Blanch, R.; Salvatierra, Á.; Hofland, L.J.; Luque, R.M.; Gálvez-Moreno, M.A.; Castaño, J.P. The components of somatostatin and ghrelin systems are altered in neuroendocrine lung carcinoids and associated to clinical-histological features. Lung Cancer 2017, 109, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M. In1-ghrelin, a splice variant of ghrelin gene, is associated with the evolution and aggressiveness of human neu-roendocrine tumors: Evidence from clinical, cellular and molecular parameters. Oncotarget 2015, 6, 19619–19633. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Vacas, J.M.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Fuentes-Fayos, A.C.; Ruiz-Pino, F.; Guler, I.; Camargo, A.; Anglada, F.J.; Carrasco-Valiente, J.; Tena-Sempere, M.; et al. In1-Ghrelin Splicing Variant as a Key Element in the Pathophysiological Association Between Obesity and Prostate Cancer. J. Clin. Endocrinol. Metab. 2021, 106, e4956–e4968. [Google Scholar] [CrossRef]
- Sever, S.; White, D.L.; Garcia, J.M. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocr.-Relat. Cancer 2016, 23, R393–R409. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Martinez, A.D. Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Novel Potential Biomarker in Gastroenteropancre-atic Neuroendocrine Tumors. Clin. Transl. Gastroenterol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Niederle, B.; Pape, U.-F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconi, M. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendo-crine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Herrera-Martinez, A.D. Type 2 Diabetes in Neuroendocrine Tumors: Are Biguanides and Statins Part of the Solution? J. Clin. Endocrinol. Metab. 2019, 104, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martinez, A.D. Clinical and functional implication of the components of somatostatin system in gastroenteropancre-atic neuroendocrine tumors. Endocrine 2017, 59, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Hormaechea-Agulla, D.; Gahete, M.D.; Jiménez-Vacas, J.; Gómez-Gómez, E.; Ibáñez-Costa, A.; L-López, F.; Rivero-Cortés, E.; Sarmento-Cabral, A.; Rosa, O.-S.; Carrasco-Valiente, J.; et al. The oncogenic role of the In1-ghrelin splicing variant in prostate cancer aggressiveness. Mol. Cancer 2017, 16, 146. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Jiménez-Vacas, J.M.; Gómez-Gómez, E.; López, F.L.; Carrasco-Valiente, J.; Valero-Rosa, J.; Moreno, M.M.; Sánchez-Sánchez, R.; Ortega-Salas, R.; Gracia-Navarro, F.; et al. The oncogenic role of the spliced somatostatin receptor sst5TMD4 variant in prostate cancer. FASEB J. 2017, 31, 4682–4696. [Google Scholar] [CrossRef] [Green Version]
- Gahete, M.D.; Cordoba-Chacon, J.; Kineman, R.; Luque, R.M.; Castaño, J.P. Role of ghrelin system in neuroprotection and cognitive functions: Implications in Alzheimer’s disease. Peptides 2011, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez-Costa, A.; Gahete, M.D.; Rivero-Cortés, E.; Rincón-Fernández, D.; Nelson, R.; Beltrán, M.; De La Riva, A.; Japón, M.A.; Venegas-Moreno, E.; Gálvez, M.Á.; et al. In1-ghrelin splicing variant is overexpressed in pituitary adenomas and increases their aggressive features. Sci. Rep. 2015, 5, 8714. [Google Scholar] [CrossRef] [Green Version]
- Rincón-Fernández, D.; Culler, M.D.; Tsomaia, N.; Moreno-Bueno, G.; Luque, R.M.; Gahete, M.D.; Castaño, J.P. In1-ghrelin splicing variant is associated with reduced disease-free survival of breast cancer patients and increases malignancy of breast cancer cells lines. Carcinogenesis 2017, 39, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.L.; Clarke, S.J.; Engel, A.; Diakos, C.I.; Pavlakis, N.; Roach, P.J.; Bailey, D.L.; Bauer, J.; Findlay, M. Computed tomography (CT)-defined sarcopenia and myosteatosis are prevalent in patients with neuroendocrine neoplasms (NENs) treated with peptide receptor radionuclide therapy (PRRT). Eur. J. Clin. Nutr. 2021, 1–7. [Google Scholar] [CrossRef]
- Wang, J.-B.; Xue, Z.; Lu, J.; He, Q.-L.; Zheng, Z.-F.; Xu, B.-B.; Xie, J.-W.; Li, P.; Xu, Y.; Lin, J.-X.; et al. Effect of sarcopenia on short- and long-term outcomes in patients with gastric neuroendocrine neoplasms after radical gastrectomy: Results from a large, two-institution series. BMC Cancer 2020, 20, 1002. [Google Scholar] [CrossRef]
- Papotti, M. Ghrelin-producing endocrine tumors of the stomach and intestine. J. Clin. Endocrinol. Metab. 2001, 86, 5052–5059. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Savio, A.; Torsello, A.; Zoli, M.; Locatelli, V.; Cocchi, D.; Paolotti, D.; Solcia, E. Ghrelin expression in gut endocrine growths. Histochem. Cell Biol. 2002, 117, 521–525. [Google Scholar] [CrossRef]
- Volante, M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J. Clin. Endocrinol. Metab. 2002, 87, 1300–1308. [Google Scholar] [CrossRef]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; The ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2020, 36, 22–28. [Google Scholar] [CrossRef]
- Abbass, T.; Dolan, R.D.; Laird, B.J.; McMillan, D.C. The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review. Cancers 2019, 11, 1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederholm, T. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition commu-nity. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.; Hattori, M.; Fleming, G.; Jaskowiak, N.; Hedeker, D.; Olopade, O.I.; Huo, D. Impact of post-diagnosis weight change on survival outcomes in Black and White breast cancer patients. Breast Cancer Res. 2021, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Yueh, B. Weight loss predicts mortality after recurrent oral cavity and oropharyngeal carcinomas. Cancer 2002, 95, 553–562. [Google Scholar] [CrossRef]
- Gannavarapu, B.S.; Lau, S.K.; Carter, K.; Cannon, N.A.; Gao, A.; Ahn, C.; Meyer, J.J.; Sher, D.J.; Jatoi, A.; Infante, R.; et al. Prevalence and Survival Impact of Pretreatment Cancer-Associated Weight Loss: A Tool for Guiding Early Palliative Care. J. Oncol. Pract. 2018, 14, e238–e250. [Google Scholar] [CrossRef] [PubMed]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2015, 104, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, S.A.; Burch, N.; Druce, M.; Hattersley, A.; Khan, S.; Gopalakrishnan, K.; Darby, C.; Wong, J.L.H.; Davies, L.; Fletcher, S.; et al. Screening for malnutrition in patients with gastro-entero-pancreatic neuroendocrine tumours: A cross-sectional study. BMJ Open 2016, 6, e010765. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.; Gioulbasanis, I.; Senesse, P.; Baracos, V.E. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis Are Endemic in Overweight and Obese Patients. J. Parenter. Enter. Nutr. 2019, 44, 227–238. [Google Scholar] [CrossRef]
- Kikut, J.; Jasińska, A.; Pobłocki, J.; Brodowski, J.; Małgorzata, S. Assessment and State of Nutrition of Patients with Gastroenteropancreatic Neuroendocrine Neoplasms. Nutrients 2020, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Structure and Function of Ghrelin. Results Probl. Cell Differ. 2008, 46, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tsai, C.Y. Ghrelin, and motilin in the gastrointestinal system. Curr. Pharm. Des. 2012, 18, 4755–4765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A. Ghrelin prevents tumour-and cisplatin-induced muscle wasting: Characterization of multiple mechanisms in-volved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef]
- Korbonits, M. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2001, 86, 881–887. [Google Scholar] [CrossRef]
- Lin, T.-C.; Liu, Y.-P.; Chan, Y.-C.; Su, C.-Y.; Lin, Y.-F.; Hsu, S.-L.; Yang, C.-S.; Hsiao, M. Ghrelin promotes renal cell carcinoma metastasis via Snail activation and is associated with poor prognosis. J. Pathol. 2015, 237, 50–61. [Google Scholar] [CrossRef]
- Volante, M. Ghrelin in fetal thyroid and follicular tumors and cell lines: Expression and effects on tumor growth. Am. J. Pathol. 2003, 162, 645–654. [Google Scholar] [CrossRef]
- Duxbury, M.S.; Waseem, T.; Ito, H.; Robinson, M.K.; Zinner, M.J.; Ashley, S.W.; Whang, E.E. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem. Biophys. Res. Commun. 2003, 309, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.D.; Weeraratna, A.T.; Yang, H.; Bertak, D.; Cooper-Jenkins, A.; Riggins, G.J.; Eberhart, C.G.; Taub, D.D. Ghrelin and the Growth Hormone Secretagogue Receptor Constitute a Novel Autocrine Pathway in Astrocytoma Motility. J. Biol. Chem. 2006, 281, 16681–16690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Almeida, J.M. Morphofunctional assessment of patient s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [PubMed]


| Characteristic | Total (n = 104) |
|---|---|
| Sex (♂/♀) | 45.2/54.8 |
| Age at diagnosis (years) | 54.5 (52–58) |
| Functional tumors | 32.9 (27/82) |
| Incidental tumor | 38.7 (29/75) |
| Tobacco exposure | |
| No | 31.1 (14/45) |
| Active | 48.9 (22/45) |
| Previous exposure | 20 (9/45) |
| Other neoplasms | 20 (18/90) |
| Tumor localization | |
| Pancreas | 38.5 (40/104) |
| Stomach | 4.8 (5/104) |
| Small bowel | 21.2 (22/104) |
| Hindgut | 34 (35/104) |
| Other | 2 (2/104) |
| Nutritional characteristics | |
| Weight loss at diagnosis | 39.7 (25/63) |
| Weight at diagnosis (Kg) | 70 (65– 78) |
| BMI at diagnosis | 27.2 (24.5–28.7) |
| Metastasis at diagnosis | 49.1 (51/104) |
| Liver | 17.4 (8/46) |
| Spleen | 2.2 (1/46) |
| Lymph nodes | 50 (23/46) |
| Peritoneum | 2.2 (2/46) |
| Multiple invasion | 28.2 (13/46) |
| Relapsed disease | 25.4 (17/67) |
| Disease-free | 75.9 (44/58) |
| Mortality | 34.6 (36/104) |
| Histologic features | |
| Necrosis | 7.3 (9/33) |
| Multiple tumors | 7.5 (4/53) |
| Peritumoral invasion | 51.8 (44/85) |
| Vascular invasion | 28.2 (22/78) |
| Perineural invasion | 28 (21/75) |
| Tumor grade | |
| Grade 1 | 33.7 (35/104) |
| Grade 2 | 26.9 (28/104) |
| Grade 3 | 9.6 (10/104) |
| Unknown | 29.8% (31/104) |
| Biochemical analysis at diagnosis | |
| Lymphocytes | 1520 (390–2762) |
| Transferrin (mg/dL) | 240 (199–262) |
| Ferritin (mg/dL) | 81.7 (5–63) |
| Albumin (g/dL) | 3.9 (1.6–5.6) |
| Prealbumin (mg/dL) | 23 (9.7–41.6) |
| RCP (g/dL) | 2.6 (1.3–5.4) |
| Total cholesterol (mg/dL) | 165 (30–206) |
| LDL cholesterol (mg/dL) | 95 (53–124) |
| HDL cholesterol (mg/dL) | 72 (15–108) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Martínez, Y.; Alzas Teomiro, C.; León Idougourram, S.; Molina Puertas, M.J.; Calañas Continente, A.; Serrano Blanch, R.; Castaño, J.P.; Gálvez Moreno, M.Á.; Gahete, M.D.; Luque, R.M.; et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2022, 14, 111. https://doi.org/10.3390/cancers14010111
Herrera-Martínez Y, Alzas Teomiro C, León Idougourram S, Molina Puertas MJ, Calañas Continente A, Serrano Blanch R, Castaño JP, Gálvez Moreno MÁ, Gahete MD, Luque RM, et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2022; 14(1):111. https://doi.org/10.3390/cancers14010111
Chicago/Turabian StyleHerrera-Martínez, Yiraldine, Carlos Alzas Teomiro, Soraya León Idougourram, María José Molina Puertas, Alfonso Calañas Continente, Raquel Serrano Blanch, Justo P. Castaño, María Ángeles Gálvez Moreno, Manuel D. Gahete, Raúl M. Luque, and et al. 2022. "Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms" Cancers 14, no. 1: 111. https://doi.org/10.3390/cancers14010111
APA StyleHerrera-Martínez, Y., Alzas Teomiro, C., León Idougourram, S., Molina Puertas, M. J., Calañas Continente, A., Serrano Blanch, R., Castaño, J. P., Gálvez Moreno, M. Á., Gahete, M. D., Luque, R. M., & Herrera-Martínez, A. D. (2022). Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers, 14(1), 111. https://doi.org/10.3390/cancers14010111








