Basal Cell Carcinoma Treated with High Dose Rate (HDR) Brachytherapy—Early Evaluation of Clinical and Dermoscopic Patterns during Irradiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Clinical Evaluation
2.4. Histopathologic Assessment
2.5. Dermoscopic Procedure and Image Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Didkowska, J.; Michałek, J.; Olasek, P.; Ciuba, A. Cancer in Poland 2018, Krajowy Rejestr Nowotworów; Ministerstwo Zdrowia: Warszawa, Poland, 2020; pp. 16–21.
- Sng, J.; Koh, D.; Siong, W.C.; Choo, T.B. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J. Am. Acad. Dermatol. 2009, 61, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, R.; Rosso, S.; Martinez, C.; Nieto, A.; Miranda, A.; Mercier, M.; Loria, D.I.; Østerlind, A.; Greinert, R.; Navarro, C.; et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: A multi-centre case-case-control study. Br. J. Cancer 2006, 94, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errichetti, E.; Zalaudek, I.; Kittler, H.; Apalla, Z.; Argenziano, G.; Bakos, R.; Blum, A.; Braun, R.P.; Ioannides, D.; Lacarrubba, F.; et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): An expert consensus on behalf of the International Dermoscopy Society. Br. J. Dermatol. 2020, 182, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Kittler, H.; Marghoob, A.A.; Argenziano, G.; Carrera, C.; Curiel-Lewandrowski, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Menzies, S.; Puig, S.; Rabinovitz, H.; et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J. Am. Acad. Dermatol. 2016, 74, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lallas, A.; Apalla, Z.; Argenziano, G.; Longo, C.; Moscarella, E.; Specchio, F.; Zalaudek, I. The dermatoscopic universe of basal cell carcinoma. Dermatol. Pract. Concept. 2014, 4, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Pampena, R.; Parisi, G.; Benati, M.; Borsari, S.; Lai, M.; Paolino, G.; Cesinaro, A.M.; Ciardo, S.; Farnetani, F.; Bassoli, S.; et al. Clinical and Dermoscopic Factors for the Identification of Aggressive Histologic Subtypes of Basal Cell Carcinoma. Front. Oncol. 2021, 10, 630458. [Google Scholar] [CrossRef]
- Reiter, O.; Mimouni, I.; Dusza, S.; Halpern, A.C.; Leshem, Y.A.; Marghoob, A.A. Dermoscopic features of basal cell carcinoma and its subtypes: A systematic review. J. Am. Acad. Dermatol. 2021, 85, 653–664. [Google Scholar] [CrossRef]
- Mae, K.; Tsuboi, R.; Irisawa, R.; Sato, T.; Fukushima, N.; Harada, K. Recent reductions in the size of facial pigmented basal cell carcinoma at diagnosis and the surgical margin: A retrospective and comparative study. J. Dermatol. 2021, 5, 661–666. [Google Scholar] [CrossRef]
- Conforti, C.; Giuffrida, R.; Zalaudek, I.; Guarneri, F.; Cannavò, S.P.; Pizzichetta, M.A.; Bonin, S.; Corneli, P.; Bussani, R.; Bazzacco, G.; et al. Dermoscopic Findings in the Presurgical Evaluation of Basal Cell Carcinoma. A Prospective Study. Dermatol. Surg. 2021, 47, 37–41. [Google Scholar] [CrossRef]
- Yélamos, O.; Braun, R.P.; Liopyris, K.; Wolner, Z.J.; Kerl, K.; Gerami, P.; Marghoob, A.A. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J. Am. Acad. Dermatol. 2019, 80, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Van Limbergen, E.; Pötter, R.; Hoskin, P.; Baltas, D. The GEC ESTRO Handbook of Brachytherapy, 2nd ed.; European Society for Therapeutic Radiology and Oncology: Brussels, Belgium, 2019. [Google Scholar]
- NCCN Guidelines Non Melanoma Skin Cancer, 2021,V.1. Available online: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations (accessed on 30 May 2021).
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Rowe, D.E.; Carroll, R.J.; Day, C.L.J. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: Implications for patient follow-up. J. Dermatol. Surg. Oncol. 1989, 15, 315–328. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L.J. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J. Dermatol. Surg. Oncol. 1989, 15, 424–431. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. Cutaneous Squamous Cell Carcinoma of the Head and Neck. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 171–181. [Google Scholar]
- Messina, J.; Epstein, E.H.J.; Kossard, S.; McKenzie, C.; Patel, R.M.; Patterson, J.W.; Scolyer, R.A. Basal cell carcinoma. In WHO Classification of Skin Tumours; IARC Publications: Lyon, France, 2018; pp. 26–34. [Google Scholar]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Guinot, J.L.; Rembielak, A.; Perez-Calatayud, J.; Rodríguez-Villalba, S.; Skowronek, J.; Tagliaferri, L.; Guix, B.; Gonzalez-Perez, V.; Valentini, V.; Kovacs, G.; et al. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018, 126, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, L.; Cinotti, E.; Fiorani, D.; Couzan, C.; Cavarretta, C.; Chazelle, M.; Labeille, B.; Pianigiani, E.; Cevenini, G.; Perrot, J.L.; et al. Long-term therapy of multiple basal cell carcinomas: Clinicodermoscopic score for monitoring of intermittent vismodegib treatment. Dermatol. Ther. 2019, 32, e13097. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://cran.r-project.org (accessed on 30 May 2021).
- Dourmishev, L.A.; Rusinova, D.; Botev, I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol. Online J. 2013, 4, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; Cordova, M.; Liopyris, K.; Aleissa, S.; Rajadhyaksha, M.; Cohen, G.; Marghoob, A.A.; Rossi, A.M.; Barker, C.A. In vivo imaging characterization of basal cell carcinoma and cutaneous response to high-dose ionizing radiation therapy: A prospective study of reflectance confocal microscopy, dermoscopy, and ultrasonography. J. Am. Acad. Dermatol. 2021, 84, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Richtig, E.; Arzberger, E.; Hofmann-Wellenhof, R.; Fink-Puches, R. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy: A pilot study. Br. J. Dermatol. 2015, 172, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.B.; Llanas, O.P.; Calatayud, J.P.; Estrada, R.B. Dermoscopy margin delineation in radiotherapy planning for superficial or nodular basal cell carcinoma. Br. J. Dermatol. 2015, 172, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Diluvio, L.; Bavetta, M.; Di Prete, M.; Orlandi, A.; Bianchi, L.; Campione, E. Dermoscopic monitoring of efficacy of ingenolmebutate in the treatment of pigmented and non-pigmented basal cell carcinomas. Dermatol. Ther. 2017, 30, e12438. [Google Scholar] [CrossRef]
- Aguilar, J.A.; Garcés, M.H.; Bayona, J.I.Y.; Azcona Rodríguez, M.; Martínez de Espronceda Ezquerro, I.; Sarriugarte Aldecoa-Otalora, J. Criterios dermatoscópicos comopredictores de ausencia de respuesta a tratamiento con imiquimod en carcinomas basocelulares superficiales [Dermoscopic signs as predictors of non-response to imiquimod treatment in superficial basal cellcarcinoma]. An. Sist. Sanit. Navar. 2019, 42, 303–307. [Google Scholar]
- Husein-ElAhmed, H.; Fernandez-Pugnaire, M.A. Dermatoscopy-guided therapy of pigmented basal cell carcinoma with imiquimod. An. Bras. Dermatol. 2016, 91, 764–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörr, W. Radiobiology of tissue reactions. Ann. ICRP 2015, 44 (Suppl. S1), 58–68. [Google Scholar] [CrossRef] [Green Version]

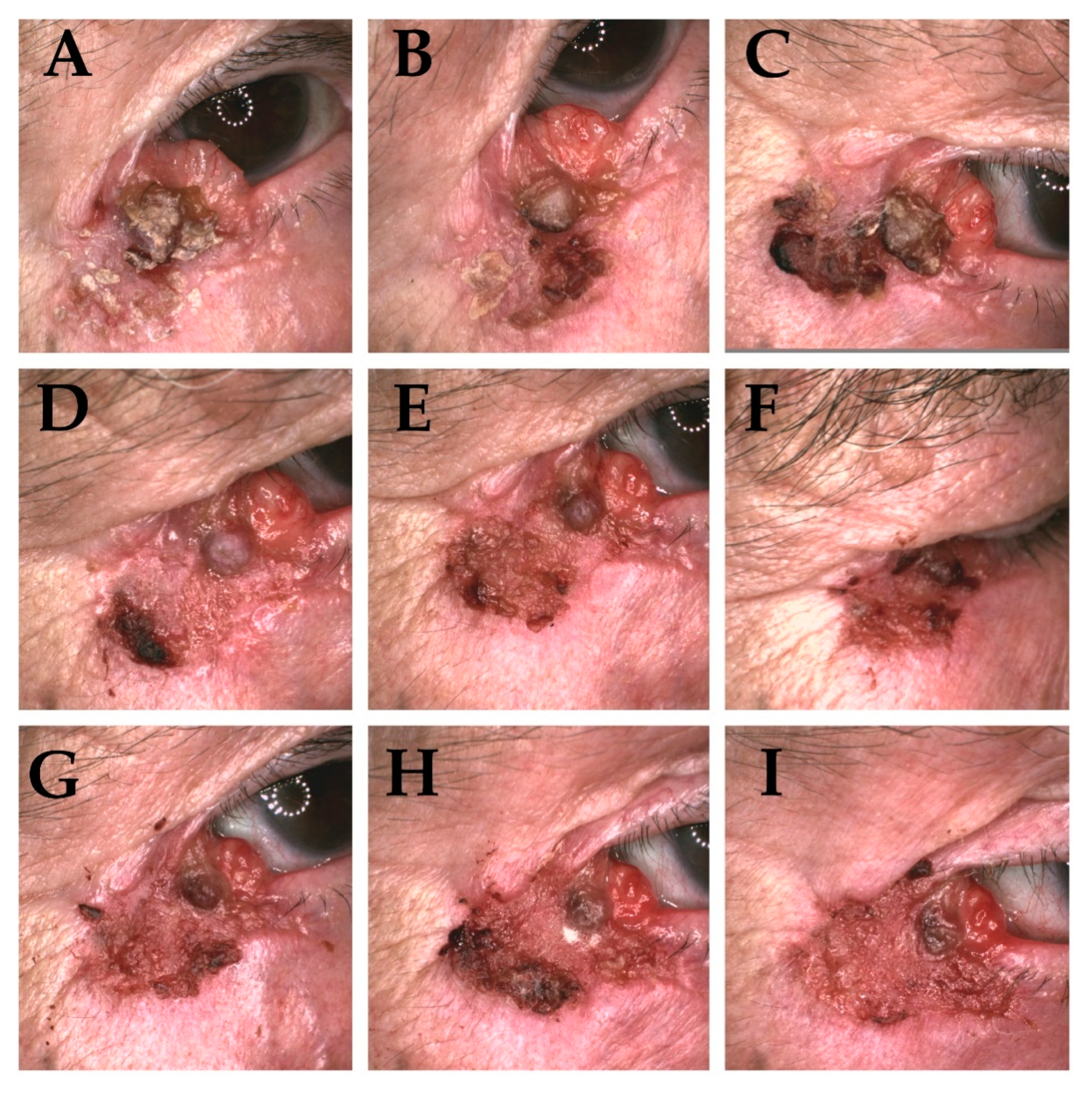





| Sex (M/F) | Median Age | Location of Tumor | Tumour Size According to AJCC [19] | Clinical Subtype [8] | Primary or Recurrent Type | High-Risk and Low-Risk BCC According to NCCN [15] | Histopathological Type (WHO Classification) [20] | Acute Morbidity According to RTOG [21] |
|---|---|---|---|---|---|---|---|---|
| 10/13 | 72 (58–95) | Forehead (2) Ear (1) Temporal region (1) Infraorbital region (2) Buccal region (3) Neck (2) Nose (9) Orbital region (2) | T1 (20) T2 (3) | Superficial (5) Nodular (12) Sclerodermiform (3) Infiltrating (3) | Primary (20) Recurrent (3) | High (23) | Nodular (12) Nodular (adenoid) (3) Superficial (4) Micronodular (1) Infiltrating (3) | G0–1 (11) G2 (2) G3 (3) G4 (7) |
| Clinical Structures | Description | Significance |
|---|---|---|
| Pink macules | Well-demarcated pink macules | Superficial BCC |
| Erythematous papules | Red papules | Nodular BCC |
| Erythematous nodules | Red nodules | Nodular BCC |
| Pearly-shiny papules | Translucent papules | Superficial BCC |
| Pearly-shiny nodules | Translucent nodules | Nodular BCC |
| Scar-like plaque | Ivory-white plaque resembling scar or morpheaform plaque | Scleroderma-like BCC |
| Erosions | Multiple small de-epithelized areas | Superficial/nodular BCC |
| Ulcerations | One or more large red to blackish red areas representing hematogenous crusts | Superficial/nodular BCC |
| Telangiectasia | Large dilated vessels | Superficial/nodular/scleroderma-like BCC |
| Short vessels | Short fine vessels | Superficial/nodular/scleroderma-like BCC |
| Pigmented structures | Hyperpigmented macule, papule, or nodule | Superficial/nodular/scleroderma-like BCC |
| Crust/scale | Small brown-red to brown-yellow crusts | Superficial/nodular/scleroderma-like BCC |
| Descriptive Terminology | Metaphoric Terminology | Description | Significance |
|---|---|---|---|
| Lines, white, perpendicular | Shiny white streaks (former: chrysalis, chrysalids, crystalline) | Short thick shiny orthogonal crossing lines | Melanoma, BCC, Spitz nevus, dermatofibroma |
| Lines, radial, connected to a common base | Leaf-like areas | Greyish/bluish brown peripheral globular extensions arising from pigmented network or adjacent confluent pigmented areas | BCC |
| Lines, radial, converging to a central dot or clod | Spoke wheel area | Brown or greyish well-circumscribed radial projections, usually around a dark brown/black, bluish central axis | BCC |
| Clods, brown or blue, concentric (clod within a clod) | Concentric globules | Gray/brown/black/blue globular structures with darker central areas | BCC |
| Clods, blue, large, clustered | Blue-grey ovoid nests | Pigmented ovoid or elongated structures well circumscribed and separate from pigmented tumor body | BCC |
| Clods, blue, small | Blue globules | Numerous loosely arranged round to oval well circumscribed structures, smaller than nests | BCC |
| Clods, white, shiny | Shiny white blotches and strands | White structures in the form of circles, oval structures, or large structureless areas, bright white. | BCC |
| Gray dots | In focus dots | Small well defined loosely arranged grey dots in focus at dermoepidermal junction | BCC |
| Structureless zone, polychromatic | Rainbow pattern | Many different colours of the rainbow ranging from red to violet | Various diagnoses |
| Structureless zone, blue | Blue-white veil-like structures | Irregularly margined confluent blue pigmentation with overlying white ground glass haze | Melanoma |
| Structureless zone, white | Scar-like depigmentation | White structureless areas | BCC presence, tumour fibrotic stroma |
| Vessel Morphology | |||
| Serpentine | Linear vessels with multiple bends | Linear vessels with multiple bends | Flat BCC, melanoma |
| Linear | Superficial fine vessels | Telangiectatic vessels in papillary dermis | BCC, inflammation |
| Vessel Arrangement | |||
| Branched | Arborizing vessels | Bright-red, sharply in focus, large of thick diameter vessels dividing into smaller vessels | BCC |
| Others | Erosions | Thin crusts overlaying superficial loss of epidermis | BCC |
| Ulcerations | Loss of epidermis with/without haematogenous crusts | Various diagnoses | |
| Clinical Outcome | OR (CI 95%), p-Value |
|---|---|
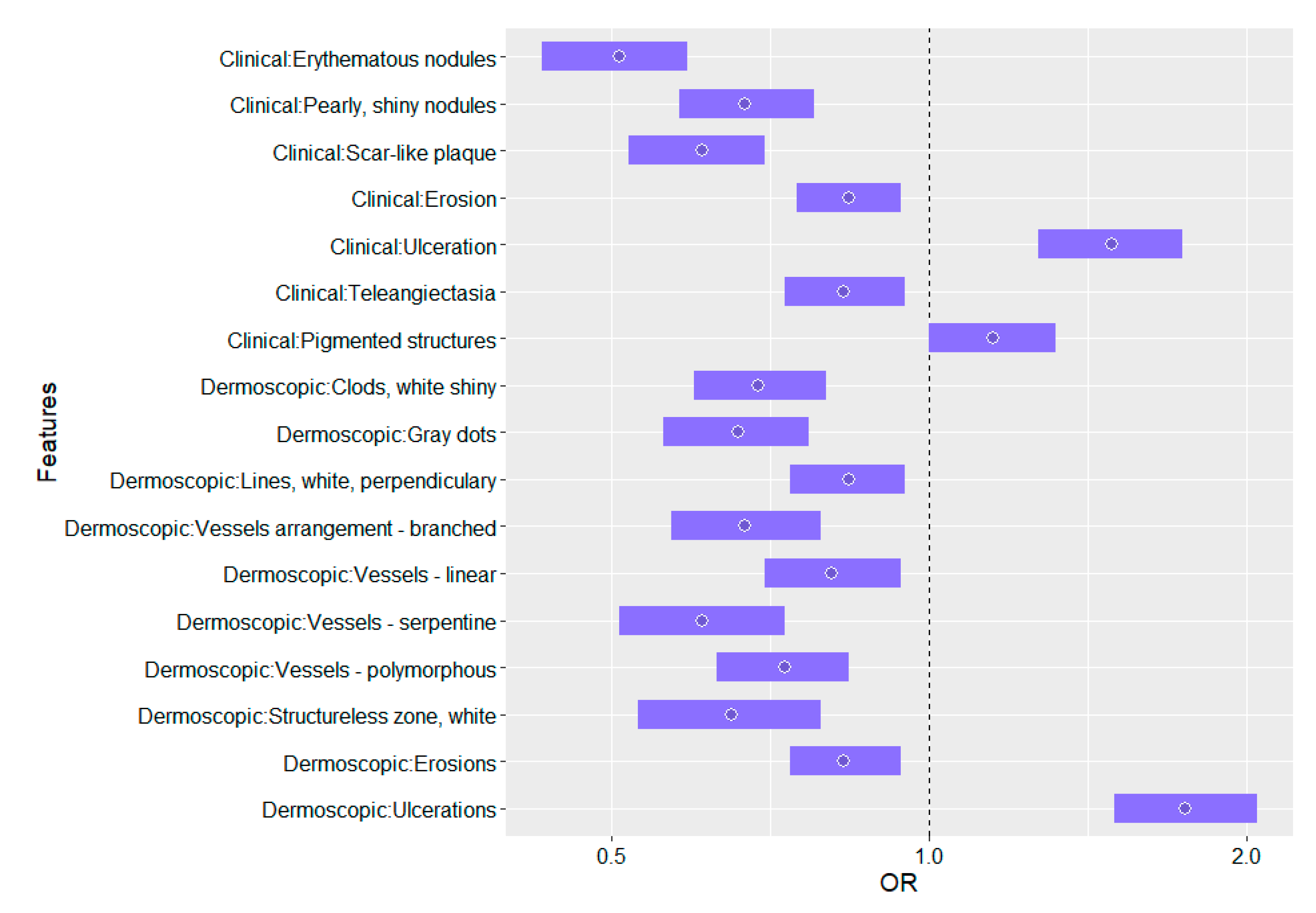
| Erythematous nodules | 0.51 (0.43,0.59), <0.0001 |
| Pearly, shiny nodules | 0.67 (0.58,0.78), <0.0001 |
| Scar-like plaque | 0.61 (0.52,0.70), <0.0001 |
| Erosion | 0.84 (0.75,0.94), 0.0029 |
| Ulceration | 1.49 (1.27,1.74), <0.0001 |
| Telangiectasia | 0.83 (0.73,0.95), 0.0055 |
| Pigmented structures | 1.15 (1.00,1.32), 0.0465 |
| Dermoscopic Outcome | OR (CI 95%), p-Value |
| Clods, white, shiny | 0.69 (0.60,0.80), <0.0001 |
| Gray dots | 0.66 (0.56,0.77), <0.0001 |
| Lines, white, perpendicular | 0.84 (0.74,0.95), 0.0046 |
| Vessels arrangement—branched | 0.67 (0.57,0.79), <0.0001 |
| Vessels—linear | 0.81 (0.70,0.94), 0.0049 |
| Vessels—serpentine | 0.61 (0.51,0.73), <0.0001 |
| Vessels—polymorphous | 0.73 (0.63,0.84), <0.0001 |
| Structureless zone, white | 0.65 (0.53,0.79), <0.0001 |
| Erosions | 0.83 (0.74,0.94), 0.0028 |
| Ulcerations | 1.75 (1.50,2.05), <0.0001 |
| Neoplastic: | Risk Factor | OR (CI 95%), p-Value |
|---|---|---|
| Yes | BT fraction | 0.77 (0.76,0.78), <0.0001 |
| Age | 0.98 (0.97,0.99), 0.0005 | |
| No | BT fraction | 0.94 (0.85,1.04), 0.2190 |
| Age | 1.01 (0.87,1.16), 0.9440 |
| TERMINOLOGY | G0 | G1 | G2 | G3 | G4 | |
|---|---|---|---|---|---|---|
| VESSELS | Dotted | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| MORPHOLOGY | Linear | 24.3% | 35.7% | 0.0% | 50.0% | 8.3% |
| Branched | 24.3% | 31.4% | 0.0% | 50.0% | 8.3% | |
| Curved | 4.9% | 4.3% | 0.0% | 0.0% | 8.3% | |
| VESSELS | Uniform | 2.9% | 0.0% | 0.0% | 0.0% | 4.2% |
| DISTRIBUTION | Clustered | 1.9% | 0.0% | 0.0% | 0.0% | 0.0% |
| Peripheral | 1.0% | 2.9% | 0.0% | 0.0% | 0.0% | |
| Reticular | 1.9% | 7.1% | 0.0% | 0.0% | 4.2% | |
| Unspecific | 19.6% | 30.0% | 0.0% | 50.0% | 8.3% | |
| SCALE | White | 46.6% | 45.7% | 25.0% | 0.0% | 12.5% |
| COLOUR | Yellow | 38.2% | 34.3% | 100.0% | 83.3% | 8.3% |
| Brown | 12.6% | 22.9% | 50.0% | 50.0% | 0.0% | |
| SCALES | Diffuse | 30.1% | 27.1% | 100.0% | 16.7% | 4.2% |
| DISTRIBUTION | Central | 1.9% | 11.4% | 0.0% | 0.0% | 0.0% |
| Peripheral | 20.4% | 22.9% | 0.0% | 0.0% | 8.3% | |
| Patchy | 13.6% | 11.4% | 0.0% | 66.7% | 0.0% | |
| FOLLICULAR | Plugs | 13.6% | 15.7% | 0.0% | 0.0% | 8.3% |
| FINDINGS | Red dots | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Peripheral White colour | 3.9% | 2.9% | 0.0% | 16.7% | 0.0% | |
| Peripheral Pigmentation | 5.8% | 4.3% | 0.0% | 0.0% | 0.0% | |
| OTHER | White | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| STRUCTURES | Brown | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| COLOR | Grey | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Blue | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Orange | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Yellow | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Purple | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| OTHER | Structureless | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| STRUCTURES | Dots | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| MORPHOLOGY | Lines | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Circles | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzysztofiak, T.; Kamińska-Winciorek, G.; Tukiendorf, A.; Suchorzepka, M.; Wojcieszek, P. Basal Cell Carcinoma Treated with High Dose Rate (HDR) Brachytherapy—Early Evaluation of Clinical and Dermoscopic Patterns during Irradiation. Cancers 2021, 13, 5188. https://doi.org/10.3390/cancers13205188
Krzysztofiak T, Kamińska-Winciorek G, Tukiendorf A, Suchorzepka M, Wojcieszek P. Basal Cell Carcinoma Treated with High Dose Rate (HDR) Brachytherapy—Early Evaluation of Clinical and Dermoscopic Patterns during Irradiation. Cancers. 2021; 13(20):5188. https://doi.org/10.3390/cancers13205188
Chicago/Turabian StyleKrzysztofiak, Tomasz, Grażyna Kamińska-Winciorek, Andrzej Tukiendorf, Magdalena Suchorzepka, and Piotr Wojcieszek. 2021. "Basal Cell Carcinoma Treated with High Dose Rate (HDR) Brachytherapy—Early Evaluation of Clinical and Dermoscopic Patterns during Irradiation" Cancers 13, no. 20: 5188. https://doi.org/10.3390/cancers13205188
APA StyleKrzysztofiak, T., Kamińska-Winciorek, G., Tukiendorf, A., Suchorzepka, M., & Wojcieszek, P. (2021). Basal Cell Carcinoma Treated with High Dose Rate (HDR) Brachytherapy—Early Evaluation of Clinical and Dermoscopic Patterns during Irradiation. Cancers, 13(20), 5188. https://doi.org/10.3390/cancers13205188







