The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries
Abstract
:Simple Summary
Abstract
1. Introduction
2. Why Are Ir Thyroid Disorders Important for Patients with Hematological Malignancies?
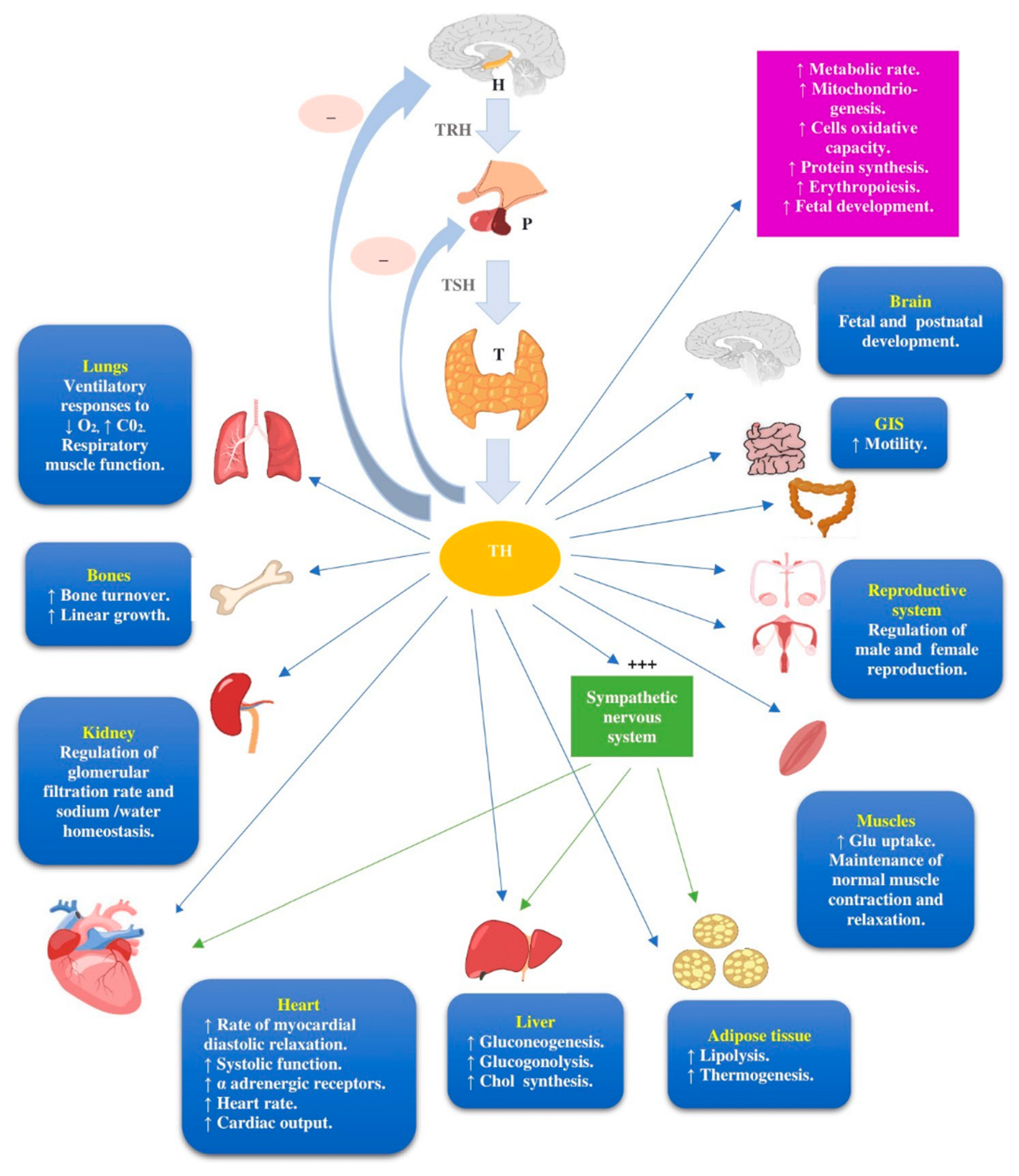
3. The Biological Background of Ir Thyroid Disorders
4. The Epidemiological Profile of Ir Thyroid Disorders
5. The Natural History of Ir Thyroid Disorders
6. The Clinical Presentation of Ir Thyroid Disorders
Remarkable Clinical Aspects of ir Thyroid Disorders
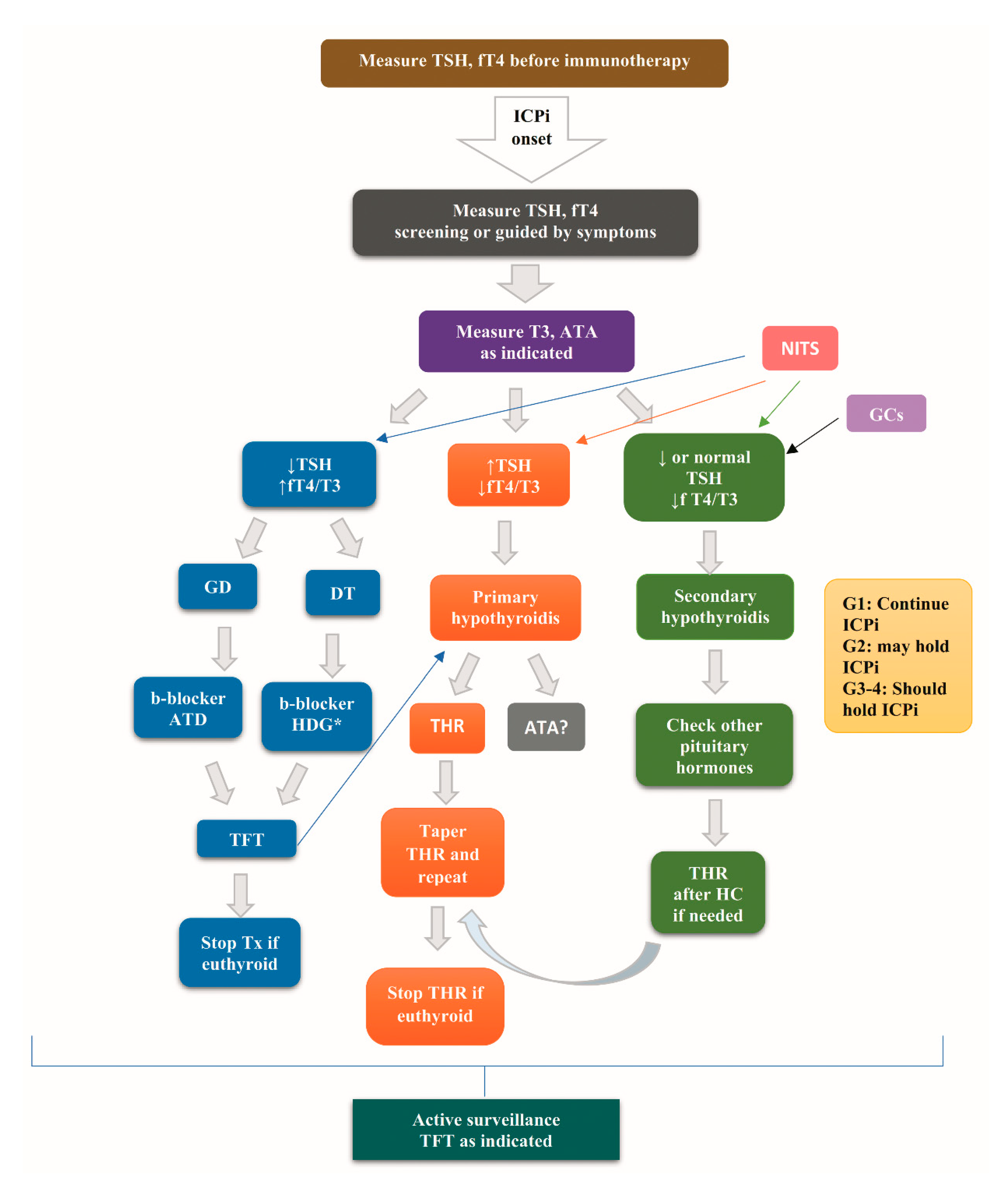
7. Diagnostic Evaluation of Ir Thyroid Disorders
8. Treatment of Ir Thyroid Disorders
9. The Contribution of Antithyroid Antibodies to Ir Thyroid Disorders
10. The Association of Ir Thyroid Disorders with ICPi Efficacy
11. An Integrative Framework of the Association of ICPi with Ir Thyroid Disorders
12. Is There a Distinguishing Feature of Ir Thyroid Disorders in the Setting of Hematological Cancers Compared to Solid Tumors That Hampers the Appliance of Data Derived from the Latter in the Former?
13. Current Challenges and Future Perspectives
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hattersley, R.; Nana, M.; Lansdown, A.J. Endocrine complications of immunotherapies: A review. Clin. Med. 2021, 21, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Pianko, M.J.; Liu, Y.; Bagchi, S.; Lesokhin, A.M. Immune checkpoint blockade for hematologic malignancies: A review. Stem Cell Investig. 2017, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Salik, B.; Smyth, M.J.; Nakamura, K. Targeting immune checkpoints in hematological malignancies. J. Hematol. Oncol. 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.K.; Watson, D.E. Pharmacovigilance Assessment of Immune-Mediated Reactions Reported for Checkpoint Inhibitor Cancer Immunotherapies. Pharmacotherapy 2017, 37, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Mazzarella, A.; Antonazzo, I.C.; Bendinelli, N.; Forcesi, E.; Tuccori, M.; Moretti, U.; Poluzzi, E.; De Ponti, F. Toxicities with Immune Checkpoint Inhibitors: Emerging Priorities From Disproportionality Analysis of the FDA Adverse Event Reporting System. Target Oncol. 2019, 14, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr. Relat. Cancer 2019, 26, G1–G18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.H.; Tang, X.W.; Dong, Z.; Song, L.; Jia, Y.T. Adverse Event Profiles of Anti-CTLA-4 and Anti-PD-1 Monoclonal Antibodies Alone or in Combination: Analysis of Spontaneous Reports Submitted to FAERS. Clin. Drug Investig. 2019, 9, 319–330. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid J. 2018, 7, 167–186. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Graves’ Disease: Can It Be Cured? Endocrinol. Metab. 2019, 34, 29–38. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 25 March 2021).
- Orio, F.; Muscogiuri, G.; Palomba, S.; Serio, B.; Sessa, M.; Giudice, V.; Ferrara, I.; Tauchmanovà, L.; Colao, A.; Selleri, C. Endocrinopathies after allogeneic and autologous transplantation of hematopoietic stem cells. Sci. World J. 2014, 2014, 282147. [Google Scholar] [CrossRef]
- Cooper, D.S.; Landenson, P.W. Chapter 7. The Thyroid Gland. In Greenspan’s Basic and Clinical Endocrinology, 9th ed.; Gardner, D., Shoback, D., Eds.; McGraw-Hill Education—Europe: New York, NY, USA, 2011; pp. 163–226. [Google Scholar]
- Hamnvik, O.P.; Larsen, P.R.; Marqusee, E. Thyroid dysfunction from antineoplastic agents. J. Natl. Cancer Inst. 2011, 103, 1572–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.K.; Bass, A.R. Autoimmune complications of immunotherapy: Pathophysiology and management. BMJ 2020, 369, m736. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, G.; Wang, Y.; Riese, M.J.; You, M. Uncoupling Therapeutic Efficacy from Immune-Related Adverse Events in Immune Checkpoint Blockade. IScience 2020, 23, 101580. [Google Scholar] [CrossRef]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowen, M.F.; Giles, K.M.; Simpson, D.; Tchack, J.; Zhou, H.; Moran, U.; Dawood, Z.; Pavlick, A.C.; Hu, S.; Wilson, M.A.; et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J. Transl. Med. 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torimoto, K.; Okada, Y.; Nakayamada, S.; Kubo, S.; Tanaka, Y. Anti-PD-1 Antibody Therapy Induces Hashimoto’s Disease with an Increase in Peripheral Blood Follicular Helper T Cells. Thyroid 2017, 27, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Gustafson, M.P.; Bornschlegl, S.; Kottschade, L.; Delivanis, D.A.; Dietz, A.B.; Gandhi, M.; Ryder, M. Immune Checkpoint Inhibitor-Induced Thyroiditis Is Associated with Increased Intrathyroidal T Lymphocyte Subpopulations. Thyroid 2020, 30, 1440–1450. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, M.P.; Bornschlegl, S.; Merten, M.M.; Kottschade, L.; Withers, S.; Dietz, A.B.; Ryder, M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Angell, T.E.; Min, L.; Wieczorek, T.J.; Hodi, F.S. Unique Cytologic Features of Thyroiditis Caused by Immune Checkpoint Inhibitor Therapy for Malignant Melanoma. Genes Dis. 2018, 5, 46–48. [Google Scholar] [CrossRef]
- Neppl, C.; Kaderli, R.M.; Trepp, R.; Schmitt, A.M.; Berger, M.D.; Wehrli, M.; Seiler, C.A.; Langer, R. Histology of Nivolumab-Induced Thyroiditis. Thyroid 2018, 28, 1727–1728. [Google Scholar] [CrossRef]
- Young, A.; Quandt, Z.; Bluestone, J.A. The Balancing Act between Cancer Immunity and Autoimmunity in Response to Immunotherapy. Cancer Immunol. Res. 2018, 6, 1445–1452. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential Immune-Related Adverse Events Associated With Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Park, H.; Malone, D.C.; Wang, C.Y.; Wilson, D.L.; Yeh, Y.M.; Van Boemmel-Wegmann, S.; Lo-Ciganic, W.H. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2020, 3, e201611. [Google Scholar] [CrossRef]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Tan, P.; Zheng, X.; Huang, Y.; Lin, T.; Wei, Q.; Ai, J.; Yang, L. Immune-related adverse events following administration of anti-cytotoxic T-lymphocyte-associated protein-4 drugs: A comprehensive systematic review and meta-analysis. Drug Des. Devel. Ther. 2019, 13, 2215–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Q.; Zhou, Y.L.; Guo, X.; Ge, J.; Fu, J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2018, 63, 292–298. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, O.; ElHalawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef]
- Da, L.; Teng, Y.; Wang, N.; Zaguirre, K.; Liu, Y.; Qi, Y.; Song, F. Organ-Specific Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitor Monotherapy Versus Combination Therapy in Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 10, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelmachowska-Banaś, M.; Czajka-Oraniec, I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef]
- Muir, C.A.; Menzies, A.M.; Clifton-Bligh, R.; Tsang, V.H.M. Thyroid Toxicity Following Immune Checkpoint Inhibitor Treatment in Advanced Cancer. Thyroid 2020, 30, 1458–1469. [Google Scholar] [CrossRef]
- Tan, M.H.; Iyengar, R.; Mizokami-Stout, K.; Yentz, S.; MacEachern, M.P.; Shen, L.Y.; Redman, B.; Gianchandani, R. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: A scoping review of case reports. Clin. Diabetes Endocrinol. 2019, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.; Callahan, M.; Postow, M.A.; Wolchok, J.; Fagin, J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 2014, 21, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Patrizio, A.; Galdiero, M.R.; Baldini, E.; Ulisse, S.; Marone, G.; et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int. J. Mol. Sci. 2019, 20, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzerling, L.; de Toni, E.N.; Schett, G.; Hundorfean, G.; Zimmer, L. Checkpoint Inhibitors. Dtsch Arztebl Int. 2019, 116, 119–126. [Google Scholar] [CrossRef]
- Sznol, M.; Postow, M.A.; Davies, M.J.; Pavlick, A.C.; Plimack, E.R.; Shaheen, M.; Veloski, C.; Robert, C. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat. Rev. 2017, 58, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cukier, P.; Santini, F.C.; Scaranti, M.; Hoff, A.O. Endocrine side effects of cancer immunotherapy. Endocr. Relat. Cancer 2017, 24, T331–T347. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Ye, X.; Hu, F.; Xu, J.; Guo, X.; Zhuang, Y.; He, J. Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US Food and Drug Administration adverse events reporting system. J. Immunother. Cancer 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Chen, X.; Wu, X.; Huang, Y.; Zhuang, Y.; Lin, X. Immune checkpoint inhibitor-associated thyroid dysfunction: A disproportionality analysis using the WHO Adverse Drug Reaction Database, VigiBase. Eur. J. Endocrinol. 2020, 182, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garon-Czmil, J.; Petitpain, N.; Rouby, F.; Sassier, M.; Babai, S.; Yéléhé-Okouma, M.; Weryha, G.; Klein, M.; Gille, P. Immune check point inhibitors-induced hypophysitis: A retrospective analysis of the French Pharmacovigilance database. Sci. Rep. 2019, 9, 19419. [Google Scholar] [CrossRef]
- Morganstein, D.L.; Lai, Z.; Spain, L.; Diem, S.; Levine, D.; Mace, C.; Gore, M.; Larkin, J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin. Endocrinol. 2017, 86, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Quandt, Z.; Trupin, L.; Evans, M.; Schmajuk, G.; Anderson, M.S.; Bluestone, J.A.; Yazdany Jinoos, Y. SAT-418 Finding the Needles in the Haystack: Harnessing the Electronic Health Record to Find Thyroid Immune Related Adverse Events. J. Endocr. Soc. 2020, 4, SAT-418. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Pond, G.R.; Abou Alaiwi, S.; Nassar, A.H.; Flippot, R.; Curran, C.; Kilbridge, K.L.; Wei, X.X.; McGregor, B.A.; Choueiri, T.; et al. Conditional immune toxicity rate in patients with metastatic renal and urothelial cancer treated with immune checkpoint inhibitors. J. Immunother. Cancer 2020, 8, e000371. [Google Scholar] [CrossRef]
- Kauppila, M.; Koskinen, P.; Irjala, K.; Remes, K.; Viikari, J. Long-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Transplant. 1998, 22, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Demirkaya, M.; Sevinir, B.; Sağlam, H.; Özkan, L.; Akacı, O. Thyroid functions in long-term survivors of pediatric Hodgkin’s lymphoma treated with chemotherapy and radiotherapy. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.B.; Chapman, R.; Wrigley, P.F. Cyclical combination chemotherapy and thyroid function in patients with advanced Hodgkin’s disease. Med. Pediatr. Oncol. 1981, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Buchbinder, E.I.; Haq, R.; Tolaney, S.; Barroso-Sousa, R.; Zhang, K.; Donahue, H.; et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol. Res. 2017, 5, 1133–1140. [Google Scholar] [CrossRef] [Green Version]
- Gan, E.H.; Mitchell, A.L.; Plummer, R.; Pearce, S.; Perros, P. Tremelimumab-Induced Graves Hyperthyroidism. Eur. Thyroid J. 2017, 6, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Orlov, S.; Salari, F.; Kashat, L.; Walfish, P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J. Clin. Endocrinol. Metab. 2015, 100, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, G.; Lee, H.J.; Parekh, S.; Galsky, M.D.; Smith, C.B.; Friedlander, P.; Yanagisawa, R.T.; Gallagher, E.J. Rapid evolution of thyroid dysfunction in patients treated with nivolumab. Endocr. Pract. 2017, 23, 1223–1231. [Google Scholar] [CrossRef]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-Related Thyroiditis with Immune Checkpoint Inhibitors. Thyroid 2018, 28, 1243–1251. [Google Scholar] [CrossRef]
- Fidilio, E.; Navarro-González, E.; Romero-Lluch, A.R.; Iglesias, P.; Diez Gómez, J.J.; Anda Apiñániz, E.; Santos Mazo, E.; Zafón, C. Thyroid disorders associated with immune control point inhibitors. Endocrinol. Diabetes Nutri. 2020. [Google Scholar]
- Weber, K.; Haugen, B.R. Hypothyroidism in Endocrine secrets, 6th ed.; Dermott, M.T., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 283–288. [Google Scholar]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef]
- Sagiv, O.; Kandl, T.J.; Thakar, S.D.; Thuro, B.A.; Busaidy, N.L.; Cabanillas, M.; Jimenez, C.; Dadu, R.; Graham, P.H.; Debnam, J.M.; et al. Extraocular Muscle Enlargement and Thyroid Eye Disease-like Orbital Inflammation Associated with Immune Checkpoint Inhibitor Therapy in Cancer Patients. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Orloff, M.; Sato, T.; Shields, J.A. CHECKPOINT INHIBITOR IMMUNE THERAPY: Systemic Indications and Ophthalmic Side Effects. Retina 2018, 38, 1063–1078. [Google Scholar] [CrossRef]
- McElnea, E.; Ní Mhéalóid, Á.; Moran, S.; Kelly, R.; Fulcher, T. Thyroid-like ophthalmopathy in a euthyroid patient receiving ipilimumab. Orbit 2014, 33, 424–427. [Google Scholar] [CrossRef]
- Min, L.; Vaidya, A.; Becker, C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 2011, 164, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Borodic, G.E.; Hinkle, D. Ipilimumab-induced orbital inflammation resembling graves disease with subsequent development of systemic hyperthyroidism from CTLA-4 receptor suppression. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 83. [Google Scholar] [CrossRef]
- Sohrab, M.A.; Desai, R.U.; Chambers, C.B.; Lissner, G.S. Re: "Drug-induced Graves disease from CTLA-4 receptor suppression". Ophthalmic Plast. Reconstr. Surg. 2013, 29, 239–240. [Google Scholar] [CrossRef]
- Campredon, P.; Imbert, P.; Mouly, C.; Grunenwald, S.; Mazières, J.; Caron, P. Severe Inflammatory Ophthalmopathy in a Euthyroid Patient during Nivolumab Treatment. Eur. Thyroid J. 2018, 7, 84–87. [Google Scholar] [CrossRef]
- Johnson, E.D.; Kerrigan, K.; Butler, K.; Patel, S.B. Nivolumab-induced hypothyoidism with consequent hypothyroid related myopathy. J. Oncol. Pharm. Pract. 2020, 26, 224–227. [Google Scholar] [CrossRef]
- Yu, C.; Chopra, I.J.; Ha, E. A novel melanoma therapy stirs up a storm: Ipilimumab-induced thyrotoxicosis. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 140092. [Google Scholar] [CrossRef] [PubMed]
- Yonezaki, K.; Kobayashi, T.; Imachi, H.; Yoshimoto, T.; Kikuchi, F.; Fukunaga, K.; Sato, S.; Ibata, T.; Yamaji, N.; Lyu, J.; et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: A case report. J. Med. Case Rep. 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- McMillen, B.; Dhillon, M.S.; Yong-Yow, S. A rare case of thyroid storm. BMJ Case Rep. 2016, 2016, 10–1136. [Google Scholar] [CrossRef] [Green Version]
- Gummalla, S.; Manjunath, M.; Phillips, B. “Myxedema Coma: A Life-Threatening Condition in Patients Using Pembrolizumab”. Case Rep. Endocrinol. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Rizvi, H.; Sano, D.; Chiu, J.; Hadid, T. Nivolumab induced myxedema crisis. J. Immunother. Cancer 2017, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Cooksley, T.; Gupta, A.; Al-Sayed, T.; Lorigan, P. Emergency presentations in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2020, 130, 93–197. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.J.; Qdaisat, A.; Chaftari, P.; Lipe, D.; Merlin, J.; Rajha, E.; Wechsler, A.; Sandoval, M.; Viets, J.; Al-Breiki, A.; et al. Diagnosis and management of immune-related adverse effects of immune checkpoint therapy in the emergency department. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1637–1659. [Google Scholar] [CrossRef]
- Bahn, R.S.; Burch, H.B.; Cooper, D.S.; Garber, J.R.; Greenlee, M.C.; Klein, I.; Laurberg, P.; McDougall, I.R.; Montori, V.M.; Rivkees, S.A.; et al. American Thyroid Association, & American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr. Pract. 2011, 17, 456–520. [Google Scholar]
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952–1956. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. National Comprehensive Cancer Network Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Fliers, E.; Bianco, A.C.; Langouche, L.; Boelen, A. Thyroid function in critically ill patients. lancet Diabetes Endocrinol. 2015, 3, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.B.; Aleksyniene, R.; Gormsen, L.C.; Jødal, L.; Petersen, L.J. Effect of recent contrast-enhanced CT and patient age on image quality of thyroid scintigraphy. Clin. Nucl. Med. 2015, 40, 297–302. [Google Scholar] [CrossRef]
- Czepczyński, R. Nuclear medicine in the diagnosis of benign thyroid diseases. Nucl. Med. Rev. Cent. East. Eur. 2012, 15, 113–119. [Google Scholar]
- van Kooten, M.J.; van den Berg, G.; Glaudemans, A.W.; Hiltermann, T.J.; Groen, H.J.; Links, T.P. Transient thyrotoxicosis during nivolumab treatment. Neth. J. Med. 2017, 75, 204–207. [Google Scholar]
- Chen, W.; Parsons, M.; Torigian, D.A.; Zhuang, H.; Alavi, A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl. Med. Commun. 2009, 30, 240–244. [Google Scholar] [CrossRef]
- Chen, Y.K.; Chen, Y.L.; Liao, A.C.; Shen, Y.Y.; Kao, C.H. Elevated 18F-FDG uptake in skeletal muscles and thymus: A clue for the diagnosis of Graves’ disease. Nucl. Med. Commun. 2004, 25, 115–121. [Google Scholar] [CrossRef]
- Yamauchi, I.; Yasoda, A.; Matsumoto, S.; Sakamori, Y.; Kim, Y.H.; Nomura, M.; Otsuka, A.; Yamasaki, T.; Saito, R.; Kitamura, M.; et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 2019, 14, e0216954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhusseini, M.; Samantray, J. Hypothyroidism in Cancer Patients on Immune Checkpoint Inhibitors with anti-PD1 Agents: Insights on Underlying Mechanisms. Exp. Clin. Endocrinol. Diabetes 2017, 125, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Management of immunotherpy-Related Toxicities. National Comphrehensive Cancer Network (NCCN) Guidelines. Version 2. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 24 July 2019).
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv119–iv142. [Google Scholar] [CrossRef]
- Ohara, N.; Kobayashi, M.; Ohashi, K.; Ito, R.; Ikeda, Y.; Kawaguchi, G.; Yoneoka, Y.; Hasegawa, G.; Takada, T. Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: A case report and review of the literature. J. Med. Case Rep. 2019, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hodi, F.S.; Giobbie-Hurder, A.; Wang, X.; Zhou, J.; Zhang, A.; Zhou, Y.; Mao, F.; Angell, T.E.; Andrews, C.P.; et al. The Impact of High-Dose Glucocorticoids on the Outcome of Immune-Checkpoint Inhibitor-Related Thyroid Disorders. Cancer Immunol. Res. 2019, 7, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.S.; Long, G.V.; Guminski, A.; Clifton-Bligh, R.J.; Menzies, A.M.; Tsang, V.H. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur. J. Endocrinol. 2018, 178, 173–180. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujisawa, Y.; Maruyama, H.; Nakamura, Y.; Yoshino, K.; Ohtsuka, M.; Fujimoto, M. Nivolumab-induced thyroid dysfunction. Jpn. J. Clin. Oncol. 2016, 46, 575–579. [Google Scholar]
- Maekura, T.; Naito, M.; Tahara, M.; Ikegami, N.; Kimura, Y.; Sonobe, S.; Kobayashi, T.; Tsuji, T.; Minomo, S.; Tamiya, A.; et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo 2017, 31, 1035–1039. [Google Scholar]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimbara, S.; Fujiwara, Y.; Iwama, S.; Ohashi, K.; Kuchiba, A.; Arima, H.; Yamazaki, N.; Kitano, S.; Yamamoto, N.; Ohe, Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018, 109, 3583–3590. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Hayashi, T.; Takigami, K.; Imaizumi, K.; Shiroki, R.; Ohmiya, N.; Sugiura, K.; Kawada, K.; Sawaki, A.; Maeda, K.; et al. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer 2020, 20, 656. [Google Scholar] [CrossRef]
- Yano, S.; Ashida, K.; Nagata, H.; Ohe, K.; Wada, N.; Takeichi, Y.; Hanada, Y.; Ibayashi, Y.; Wang, L.; Sakamoto, S.; et al. Nivolumab-induced thyroid dysfunction lacking antithyroid antibody is frequently evoked in Japanese patients with malignant melanoma. BMC Endocr. Disord. 2018, 18, 36. [Google Scholar] [CrossRef] [Green Version]
- Olsson-Brown, A.; Lord, R.; Sacco, J.; Wagg, J.; Coles, M.; Pirmohamed, M. Two distinct clinical patterns of checkpoint inhibitor-induced thyroid dysfunction. Endocr. Connect. 2020, 9, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Mazarico, I.; Capel, I.; Giménez-Palop, O.; Albert, L.; Berges, I.; Luchtenberg, F.; García, Y.; Fernández-Morales, L.A.; De Pedro, V.J.; Caixàs, A.; et al. Low frequency of positive antithyroid antibodies is observed in patients with thyroid dysfunction related to immune check point inhibitors. J. Endocrinol. Investig. 2019, 42, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Bagnasco, M.; Giavarina, D.; Bizzaro, N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: Improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun. Rev. 2012, 12, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hesarghatta Shyamasunder, A.; Abraham, P. Measuring TSH receptor antibody to influence treatment choices in Graves’ disease. Clin. Endocrinol. (Oxf.) 2017, 86, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Beckett, G.; Zammitt, N.N.; Strachan, M.W.; Seckl, J.R.; Gibb, F.W. Thyrotropin Receptor Antibody Levels at Diagnosis and After Thionamide Course Predict Graves’ Disease Relapse. Thyroid 2016, 26, 1004–1009. [Google Scholar] [CrossRef]
- Azmat, U.; Liebner, D.; Joehlin-price, A.; Agrawal, A.; Nabhan, F.; Report, C. Case Report Treatment of Ipilimumab Induced Graves ‘ Disease in a Patient with Metastatic Melanoma. Case Rep. Endocrinol. 2016, 2016, 2087525. [Google Scholar]
- Iadarola, C.; Croce, L.; Quaquarini, E.; Teragni, C.; Pinto, S.; Bernardo, A.; Fonte, R.; Marinò, M.; Rotondi, M.; Chiovato, L. Nivolumab Induced Thyroid Dysfunction: Unusual Clinical Presentation and Challenging Diagnosis. Front. Endocrinol. (Lausanne) 2019, 17, 813. [Google Scholar] [CrossRef] [Green Version]
- Brancatella, A.; Viola, N.; Brogioni, S.; Montanelli, L.; Sardella, C.; Vitti, P.; Marcocci, C.; Lupi, I.; Latrofa, F. Graves’ Disease Induced by Immune Checkpoint Inhibitors: A Case Report and Review of the Literature. Eur. Thyroid J. 2019, 8, 192–195. [Google Scholar] [CrossRef]
- Park, E.S.; Rabinowits, G.; Hamnvik, O.R.; Dagi, L.R. A case of Graves’ ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J. AAPOS 2018, 22, 310–312. [Google Scholar] [CrossRef]
- Kurihara, S.; Oikawa, Y.; Nakajima, R.; Satomura, A.; Tanaka, R.; Kagamu, H.; Shimada, A. Simultaneous development of Graves’ disease and type 1 diabetes during anti-programmed cell death-1 therapy: A case report. J. Diabetes Investig. 2020, 11, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Okajima, F.; Onda, T.; Fujimori, S.; Emoto, N.; Sugihara, H. New-onset graves’ disease after the initiation of nivolumab therapy for gastric cancer: A case report. BMC Endocr. Disord. 2020, 20, 132. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Ge, W.; Lei, J.; Cao, D. The association between immune-related adverse events and the prognosis of solid cancer patients treated with immunotherapy: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2020, 92, 102134. [Google Scholar] [CrossRef]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kijima, T.; Fukushima, H.; Kusuhara, S.; Tanaka, H.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Numao, N.; Sakai, Y.; et al. Association Between the Occurrence and Spectrum of Immune-Related Adverse Events and Efficacy of Pembrolizumab in Asian Patients With Advanced Urothelial Cancer: Multicenter Retrospective Analyses and Systematic Literature Review. Clin. Genitourin. Cancer 2021, 19, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Nakagawa, K. Association of immune-related adverse events with immune checkpoint inhibitor efficacy: Real or imaginary? BMC Med 2020, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Uchino, J.; Chihara, Y.; Komori, S.; Asai, J.; Narukawa, T.; Arai, A.; Kobayashi, T.; Tsunezuka, H.; et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol. Lett. 2019, 18, 2140–2147. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Kim, M.; Lee, S.H.; Park, S.Y.; Kim, Y.N.; Kim, H.; Jeon, M.J.; Kim, T.Y.; Kim, S.W.; Kim, W.B.; et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 2017, 7, e1375642. [Google Scholar] [CrossRef] [Green Version]
- Campredon, P.; Mouly, C.; Lusque, A.; Bigay-Game, L.; Bousquet, E.; Mazières, J.; Caron, P. Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: Retrospective study of 105 patients. Presse Med. 2019, 48, e199–e207. [Google Scholar] [CrossRef]
- Fujii, T.; Colen, R.R.; Bilen, M.A.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; Tsimberidou, A.; Janku, F. Incidence of immune-related adverse events and its association with treatment outcomes: The MD Anderson Cancer Center experience. Investig. New Drugs 2018, 36, 638–646. [Google Scholar] [CrossRef]
- Judd, J.; Zibelman, M.; Handorf, E.; O’Neill, J.; Ramamurthy, C.; Bentota, S.; Doyle, J.; Uzzo, R.G.; Bauman, J.; Borghaei, H. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 2017, 22, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
- Quach, H.T.; Dewan, A.K.; Davis, E.J.; Ancell, K.K.; Fan, R.; Ye, F.; Johnson, D.B. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 2019, 5, 906–908. [Google Scholar] [CrossRef]
- Zhan, L.; Feng, H.F.; Liu, H.Q.; Guo, L.T.; Chen, C.; Yao, X.L.; Sun, S.R. Immune Checkpoint Inhibitors-Related Thyroid Dysfunction: Epidemiology, Clinical Presentation, Possible Pathogenesis, and Management. Front. Endocrinol. (Lausanne) 2021, 12, 649863. [Google Scholar] [CrossRef]
- Jelinek, T.; Jelinek, T.; Mihalyova, J.; Kascak, M.; Duras, J.; Hajek, R. PD-1/PD-L1 inhibitors in haematological malignancies: Update 2017. Immunology 2017, 152, 357–371. [Google Scholar] [CrossRef]
- Mariniello, K.; Ruiz-Babot, G.; McGaugh, E.C.; Nicholson, J.G.; Gualtieri, A.; Gaston-Massuet, C.; Nostro, M.C.; Guasti, L. Stem Cells, Self-Renewal, and Lineage Commitment in the Endocrine System. Front. Endocrinol. (Lausanne) 2019, 10, 772. [Google Scholar] [CrossRef]
- Gundgurthi, A.; Garg, M.K.; Nair, V.; Pakhetra, R.; Das, S.; Sharma, S.; Dutta, M.K.; Kharb, S.; Kapoor, R. Endocrine complications after busulphan and cyclophosphamide based hematopoietic stem cell transplant: A single tertiary care centre experience. Indian J. Endocrinol. Metab. 2013, 17, 855–863. [Google Scholar] [PubMed]
- Ataca Atilla, P.; Akkus, E.; Atilla, E.; Gokmen, N.; Civriz Bozdag, S.; Kurt Yuksel, M.; Toprak, S.K.; Baskal, N.; Akan, H.; Demirer, T.; et al. Thyroid dysfunctions in adult patients after allogeneic hematopoietic stem cell transplantation. Clin. Transplant. 2020, 34, e14049. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.W.; Blum, I.D.; Storch, K.F. Clocks within the Master Gland: Hypophyseal Rhythms and Their Physiological Significance. J. Biol. Rhythms 2015, 30, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Reiners, C.; Drozd, V.; Yamashita, S. Hypothyroidism after radiation exposure: Brief narrative review. J. Neural Transm.(Vienna) 2020, 127, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, M.C.; Cornillon, J.; Decanter, C.; Defrance, F.; Karrouz, W.; Leroy, C.; Le Mapihan, K.; Couturier, M.A.; De Berranger, E.; Hermet, E.; et al. Management of endocrino-metabolic dysfunctions after allogeneic hematopoietic stem cell transplantation. Orphanet J. Rare Dis. 2014, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Syrjala, K.L.; Martin, P.J.; Lee, S.J. Delivering care to long-term adult survivors of hematopoietic cell transplantation. J. Clin. Oncol. 2012, 30, 3746–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cima, L.N.; Martin, S.C.; Lambrescu, I.M.; Stejereanu, L.; Zaharia, C.; Colita, A.; Fica, S. Long-term thyroid disorders in pediatric survivors of hematopoietic stem cell transplantation after chemotherapy-only conditioning. J. Pediatr. Endocrinol. Metab. 2018, 31, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuka, T.; Brixb, T.H.; Schimac, W.; Zettinigd, G.; Kahaly, G.J. European Thyroid Association Guidelines for the Management of Iodine-Based Contrast Media-Induced Thyroid Dysfunction. Eur. Thyroid J. 2021, 10, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Younes, A.; Armand, P.; Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma after Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-up of the Multicohort Single-Arm Phase Ii Checkmate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for Classical Hodgkin’s Lymphoma after Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multicentre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Armand, P.; Engert, A.; Younes, A.; Lee, H.J.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed or Refractory Classical Hodgkin Lymphoma (Chl) after Autologous Hematopoietic Cell Transplantation (Auto-HCT): Extended Follow-up of the Phase 2 Single-Arm Checkmate 205 Study. Blood 2018, 132, 2897. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase Ii Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Chen, R.W.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Two-Year Follow-up of Keynote-087 Study: Pembrolizumab Monotherapy in Relapsed/Refractory Classic Hodgkin Lymphoma. Blood 2018, 132, 2900. [Google Scholar] [CrossRef]
- Allen, P.; Savas, H.; Evens, A.M.; Pro, B.; Karmali, R.; Palmer, B.A.; Mou, E.; Bearden, J.; Scholtens, D.M.; Dillehay, G.; et al. Brief Pembrolizumab(PEM) Monotherapy Results in Complete and Near Complete Responses in the Majority of Untreated Patients with Classical Hodgkin Lymphoma (cHL): A Multicenter Phase 2 PET-Adapted Study of Sequential PEM and AVD. Blood 2019, 134, 235. [Google Scholar] [CrossRef]
- Ramchandren, R.; Domingo-Domènech, E.; Rueda, A.; Trněný, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J. Clin. Oncol. 2019, 37, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumor- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Banna, G.L.; Cantale, O.; Bersanelli, M.; Del Re, M.; Friedlaender, A.; Cortellini, A.; Addeo, A. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol. Rev. 2020, 14, 2. [Google Scholar] [CrossRef]
- Heinzerling, L.; Ascierto, P.A.; Dummer, R.; Gogas, H.; Grob, J.J.; Lebbe, C.; Long, G.V.; McArthur, G.; Moslehi, J.J.; Neilan, T.G.; et al. Adverse events 2.0-Let us get SERIOs: New reporting for adverse event outcomes needed in the era of immuno-oncology. Eur. J. Cancer 2019, 112, 29–31. [Google Scholar] [CrossRef]
- Nixon, A.B.; Schalper, K.A.; Jacobs, I.; Potluri, S.; Wang, I.M.; Fleener, C. Peripheral immune-based biomarkers in cancer immunotherapy: Can we realize their predictive potential? J. Immunother. Cancer 2019, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Oiso, N.; Taketomo, Y.; Okahashi, K.; Yamauchi, K.; Sato, M.; Uchida, S.; Matsuda, H.; Kawada, A. Serological aggravation of autoimmune thyroid disease in two cases receiving nivolumab. J. Dermatol. 2016, 43, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Madama, D.; Pego, A. Are patients with autoimmune disorders eligible for immunotherapy? Pulmonology 2020. Advance online publication. [Google Scholar] [CrossRef]
- Jia, X.H.; Geng, L.Y.; Jiang, P.P.; Xu, H.; Nan, K.J.; Yao, Y.; Jiang, L.L.; Sun, H.; Qin, T.J.; Guo, H. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2020, 39, 284. [Google Scholar] [CrossRef]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.S.; Wang, C.C.; Huang, Y.C.; Pavlidis, S.; Liu, C.Y.; Ko, H.W.; Chung, F.T.; Lin, T.Y.; Wang, C.L.; Guo, Y.K.; et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thorac. Cancer 2019, 10, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Brahmer, J.R.; Juergens, R.A.; Borghaei, H.; Gettinger, S.; Chow, L.Q.; Gerber, D.E.; Laurie, S.A.; Goldman, J.W.; et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.; Lawson, D.H.; Salama, A.K.; Koon, H.B.; Guthrie, T., Jr.; Thomas, S.S.; O’Day, S.J.; Shaheen, M.F.; Zhang, B.; Francis, S.; et al. Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAF-mutated metastatic melanoma. J. Immunother. Cancer 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.J.; Waypa, J.; Blaydorn, L.; Coats, J.; McGahey, K.; Sangal, A.; Niu, J.; Lynch, C.A.; Farley, J.H.; Khemka, V. A Phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br. J. Cancer 2017, 117, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Lawrence, D.; Cecilia Lezcano, C. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res. 2014, 2, 632–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A Phase I dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.B.; McSweeney, P.; et al. Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Westin, J.R.; Fowler, N.H.; Fanale, M.A.; Samaniego, F.; Oki, Y.; Obi, C.; Cao, J.J.; Cheng, X.; Ma, M.C.J.; et al. Response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: Interim results of an on open-label, phase II study. J. Clin. Oncol. 2017, 35, 7519. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031. [Google Scholar] [CrossRef] [PubMed]


| Ref. Enrolled Studies/ Type of Studies Ca Type (N * = Number of Studies; N ** = Number of Patients) | ICPi | Hypothyroidism | Hyperthyroidism |
|---|---|---|---|
| [26] 35 trials addressing irAEs in advanced melanoma, involving 6331 patients. Systematic Review and Meta-analysis Advanced Melanoma (N * = 35, N ** = 6331) |
|
|
|
| [27] 9 RCTs addressing irAEs in advanced melanoma, involving 5051 patients. Systematic review and network meta-analysis Advanced melanoma (N * = 9, N ** = 5051) |
|
| NS |
| [28] 13 studies addressing anti-PD-1/anti-PD-L1 toxicity, involving 6676 patients. Systematic Review and meta-analysis
|
|
| NS |
| [29] 11 studies addressing anti-CTLA-4 toxicity, involving 7088 patients. Systematic Review and meta-analysis
|
| IPI:
| IPI:
|
| [30] 101 studies addressing endocrine irAEs, involving 19,922 patients. Systematic Review and meta-analysis
|
|
|
|
| [31] 38 RCT addressing irAEs in advanced solid tumors, involving 7551 patients. Systematic Review and meta-analysis
|
| Overall incidence: 6.6 (5.5–7.8) Predicted incidence:
| Overall incidence: 2.9 (2.4–3.7) Predicted incidence:
|
| [32] 11 RCTs addressing irAEs of ICPi combination, involving 5307 patients. Meta-analysis
|
| Combination: RR for all-grade hypothyroidism: 1.71 (95% CI, 1.38–2.13; p < 0.00001) | Combination: RR for all-grade hyperthyroidism: 2.84 (95% CI, 1.71–4.72, p < 0.0001 |
| [33] 21 trials addressing irAEs, involving 11,454 patients. Meta-analysis
|
| All ICPi:
6.81 (4.20–11) p < 0.001 Pooled RR for high grade: 1.15 (0.44–3.05)
| NS |
| [34] 10 clinical trials addressing irAEs, involving 5, 291 patients. Meta-analysis
|
| 1.6–8.9 RR for all grades: 8.26 (95% CI: 4.67–14.62 p < 0.00001) | 0.4–3.5 RR for all grades: 5.48 (95% CI: 1.33–22.53; p = 0.02) |
| [35] 10 studies addressing irAEs, including 8 RCTs involving 2716 patients. Meta-analysis
|
|
|
|
| Ir Hypothyroidism | Ir Hyperthyroidism | |||||
|---|---|---|---|---|---|---|
| Ref. | Anti-CTL-A4 | Anti-PD-1/PDL-1 | Combo or Sequential Regimens | Anti-CTL-A4 | Anti-PD-1/PDL-1 | Combo or Sequential Regimens |
| [36] | 2.5–5.2 | 3.9–8.5 | 10.2–16.4 | 0.2–1.7 | 0.6–3.7 | 8.0–11.1 |
| [38] | 2/29 cases | 16/29 cases | 11/29 cases (9/29 cases for sequential 2/29 for combo) | IPI: 4/6 cases | NIVO: 1/6 cases | IPI + anti-PD-1/anti-PDL-1: 1/6 cases |
| [39] | IPI: 6 | NS | IPI + NIVO: 22 | NS | NS | NS |
| [40] | TREME: 2.3 | 5.9 | 13.9 | TREME: 2.6 | 3.3 | 8 |
| [41] | 4.3–11.0 a 5.2–5.9 b | 5.9 | 22 a 17 b | 2 | 1–4.7 | 10 |
| [42] | IPI: Any G: 5 G 3–4: 0 | NIVO: Any G: 11 G 3–4: 0 | NIVO + IPI: Any G: 17 G 3–4: <1 | IPI: Any G: 1 G 3–4: 0 | NIVO: Any G: 4 G 3–4: 0 | NIVO + IPI: Any G: 11 G 3–4: 1 |
| [43] | NS | PEM melanoma: 8.7 NIVO melanoma: 8.6 NIVO SC- NSCLC: 4 NIVO NS- NSCLC: 6.6 | NS | NS | PEM melanoma: 3.2 NIVO melanoma: 4.2 NIVO NS- NSCLC:1.4 | NIVO + IPI: 9.9 |
| [44] | IPI: 1.5–6.8 TREME: 2.3 | NIVO: 9–10.8 PEM: 7–9.1 AVE: 5 ATE: 2.5–4.2 DUVRA: 5.5–9.6 | NIVO + IPI: 4–27 PEM + IPI: 6–13.6 DUVRA + TREME: 5.9 | IPI: 4 TREME: 0–3 | NIVO: 2.7 PEM: 3.4–7.8 AVE: 0.4 ATE: 0.6–1.1 DUVRA: 4.9–5.7 | NIVO + IPI: 4.3–14 PEM + IPI: 4.5–6 DUVRA + TREME: NR |
| [45] | IPI: 1.5–13.3 TREME: 2–3 | Anti-PD-1: 2–3 Anti-PD-L1: 3 | NS | TREME: <1–2.5 | Anti-PD-1: <1 | NS |
| [37] | Any ir thyroid disorder | |||||
| Anti-CTLA-4 | Anti-PD-1/anti-PDL-1 | Combo or sequential | ||||
| IPI: 4.7 (range 2.0–10.4) | ATE: 22.2 NIVO: 8.8 (range 2.0–10.4)
| IPI + NIVO: 16.0
| ||||
| Ref. | Type of Study/Methods | Key Messages |
|---|---|---|
| [5] | Review and critical appraisal of 30 pharmacovigilance studies addressing irAEs as of 25 February 2020 using both a disproportionality and descriptive approach. The aim of the review was to provide a global perspective for the management of irAEs in clinical practice. |
|
| [7] | Disproportionality analysis of signals of irAEs. Data source: the FAERS database from the respective FDA approval dates for each specific druga through 2017 Q2. Evaluation of signals of disproportionality reporting using the pharmacovigilance index reporting odds ratio (ROR) with 95% CI. |
|
| [46] | Retrospective disproportionality analysis of FAERS database from 2014 Q1 through 2019 Q1. Disproportionality was calculated by the informataion component (IC) or ROR with full database as comparator, and only ROR when comparing different drug strategies. A signal was considered significant if lower limit of the 95% confidence interval (ROR025) > 1, with at least 3 cases. Threshold for statistical signal detection IC025 > 0 (IC025: lower end of a 95% confidence interval for the IC). |
|
| [47] | Observational, retrospective, and disproportionality analysis based on the VigiBase database, reporting suspected ir thyroid disorders from 1 January 2011 to 6 March 2019. |
|
| Ir Thyroid Disorder | Clinical Practice Guidelines Experts’ Committees [Ref] | |||
|---|---|---|---|---|
| ASCO [80] | SITC [89] | NCCN [90] | ESMO [91] | |
| Hypothyroidism | G 1: Continue ICPi. with frequent TFT. G 2: May hold ICPi until resolution of symptoms. Endocrine consultation. TSH > 10 mIU/L or TSH > 4 mIU/L plus symptoms: THR. TFT Q 6–8 wk for THR titration until TSH normalization and, accordingly, annually or guided by symptoms. G 3–4: THR Hold ICPi. Endocrine consultation. IV L-thyroxine for myxedema. | G ≤ 2: L-thyroxine: 1.6 μg/kg/d (young, healthy) 25–50 μg (elderly, patients with CVD). TFT Q 6–8 wk for titration. Increments of L-thyroxine dose by 12.5–25 μg if indicated. After TSH normalization, TFT Q 1 y, or earlier if needed. G ≥ 3: Hold ICPi THR as G ≤ 2. | SH: Continue ICPi TFT TSH > 10 and/or symptoms: Start L-thyroxine Continue ICPi if no symptoms Endocrine consultation TFT Q 4–6 wk | THR (L-thyroxine: 50–100 μg/day.) SH: THR if fatigue. Τitration of L-thyroxine until TSH normalization. Inflammatory thyroiditis: prednisone orally 1 mg/kg tapered gradually. Consider holding ICPi if patient is symptomatic. |
| Thyrotoxicosis | G 1: Continue ICPi with frequent TFT. G 2: May hold ICPi until resolution of symptoms. Administer b- blockers. Hydration and supportive care. Thyrotoxicosis >6 wk, or clinical suspicion of GD: Diagnostic work-up for GD. Treat GD as indicated, preferably starting with thionamide. G 3–4: As in G2. For severe symptoms:
| b-blockers (e.g., atenolol 25–50 mg daily, titrate for HR < 90 if BP allows). TFT (mainly f T4) Q 2 wk Treat GD per standard guidelines. Hold ICPi if G ≥ 3. | No symptoms: Continue ICPi Administer b-blockers (propranolol or atenolol or metoprolol). TFT in 4–6 weeks:
| Administer b-blockers (propranolol or atenolol) and rarely carbimazole or steroids. Hold ICPi until resolution of symptoms. |
| Ref. | Study Patients | Results |
|---|---|---|
| [87] | 200 patients treated with nivolumab at Kyoto University Hospital from 1 September 2014 to 31 August 2017. |
|
| [99] | 168 patients with advanced solid tumors treated with nivolumab from March 2009 to March 2016. |
|
| [101] | 51 patients with advanced NSCLC treated with pembrolizumab at Memorial Sloan Kettering Cancer Center as part of KEYNOTE-001 (NCT01295827). |
|
| [121] | 174 patients treated with nivolumab or pembrolizumab for metastatic or unresectable advanced cancers from September 2014 to July 2018. |
|
| [122] | 58 patients with metastatic NSCLC (stage IV) treated with nivolumab or pembrolizumab from January 2014 to December 2016. |
|
| [123] | 105 patients treated with nivolumab for NSCLC between May 2015 and December 2016. |
|
| Ref. | Type of Study N | Type of Malignacy | Treatment | Treatment Efficacy | Thyroid Immuno- Toxicity |
|---|---|---|---|---|---|
| [164] | Single-arm trial N = 70 | R/R AML | Azacitidine 75 mg/m2 days 1 to 7 IV or SC plus nivo 3 mg/kg IV on days 1 and 14 Q 4 to 6 weeks. |
| No |
| [165] | Phase 1/1 b multicenter, investigator-initiated study N = 28 | Relapsed hematological cancer a 3 mo or more after allogeneic HSCT | Ipilimumab at a dose of 3 or 10 mg/Kgr BW Q 3 weeks (4 doses), with additional doses Q 12 weeks for up to 60 weeks in case of clinical benefit. | Ipilimumab led to durable responses | No |
| [166] | Phase II, open-label study N = 121 | R/R DLBCL ineligible for auto-HSCT or after auto-HSCT failure | Nivo 3 mg/kg Q 2 weeks. | Median PFS:
| No |
| [167] | Open-label, phase II study N = 27 | Relapsed FL | R (375 mg/m2 IV) on days 1, 8, 15, and 22 of cycle 1 and Pembro (200 mg IV) Q 3 weeks for up to 16 cycles starting on day 2 of cycle 1. | Pre-planned interim analysis (N = 15):
| No |
| [142] | Single-arm phase II Study (KEYNOTE-087) N = 210 | R/R cHL | Pembro 200 mg once every 3 weeks. |
| Hypo-thyroidism was the most common irAE with an incidence of 12.4% |
| [145] | Multicenter, non- comparative, phase II trial (CheckMate 205) N = 51 | R/R cHL | Nivo 240 mg IV for 4 doses, followed by 12 doses of N-AVD; all doses Q 2 weeks. |
| Hypo-thyroidism was the most common irAE with an incidence of 16% (No severe case) |
| [168] | Phase 1 study N = 23 | R/R HL after auto-HSCT, or brentuximab vedotin | Nivo (at a dose of 3 mg/kgr/BW) Q 2 weeks. |
| Hypo- thyroidism: 9% (Incidence) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deligiorgi, M.V.; Sagredou, S.; Vakkas, L.; Trafalis, D.T. The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers 2021, 13, 5277. https://doi.org/10.3390/cancers13215277
Deligiorgi MV, Sagredou S, Vakkas L, Trafalis DT. The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers. 2021; 13(21):5277. https://doi.org/10.3390/cancers13215277
Chicago/Turabian StyleDeligiorgi, Maria V., Sofia Sagredou, Lampros Vakkas, and Dimitrios T. Trafalis. 2021. "The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries" Cancers 13, no. 21: 5277. https://doi.org/10.3390/cancers13215277
APA StyleDeligiorgi, M. V., Sagredou, S., Vakkas, L., & Trafalis, D. T. (2021). The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers, 13(21), 5277. https://doi.org/10.3390/cancers13215277






