A DOCK1 Gene-Derived Circular RNA Is Highly Expressed in Luminal Mammary Tumours and Is Involved in the Epithelial Differentiation, Growth, and Motility of Breast Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Biological, and Computational Resources
2.2. Generation of circDOCK1-1-Overexpressing and Knock-Out Cells
2.3. Measurement of the Selected circRNAs and Corresponding Linear mRNAs
2.4. Western Blot Analyses
2.5. Cell Proliferation, Single-Cell Motility, and Wound-Healing Assays
2.6. RNA-Sequencing Studies
2.7. Statistical Analyses
3. Results
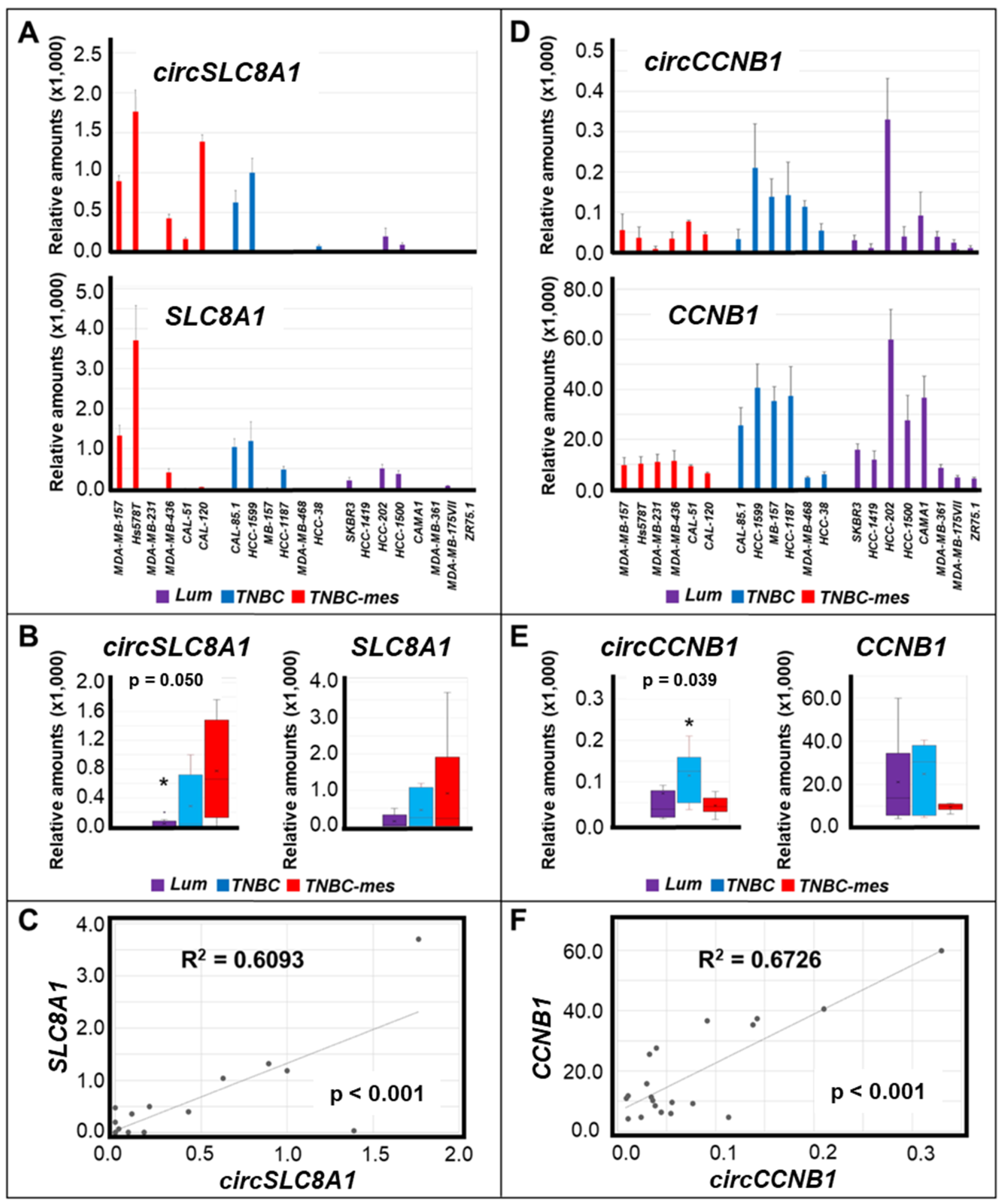
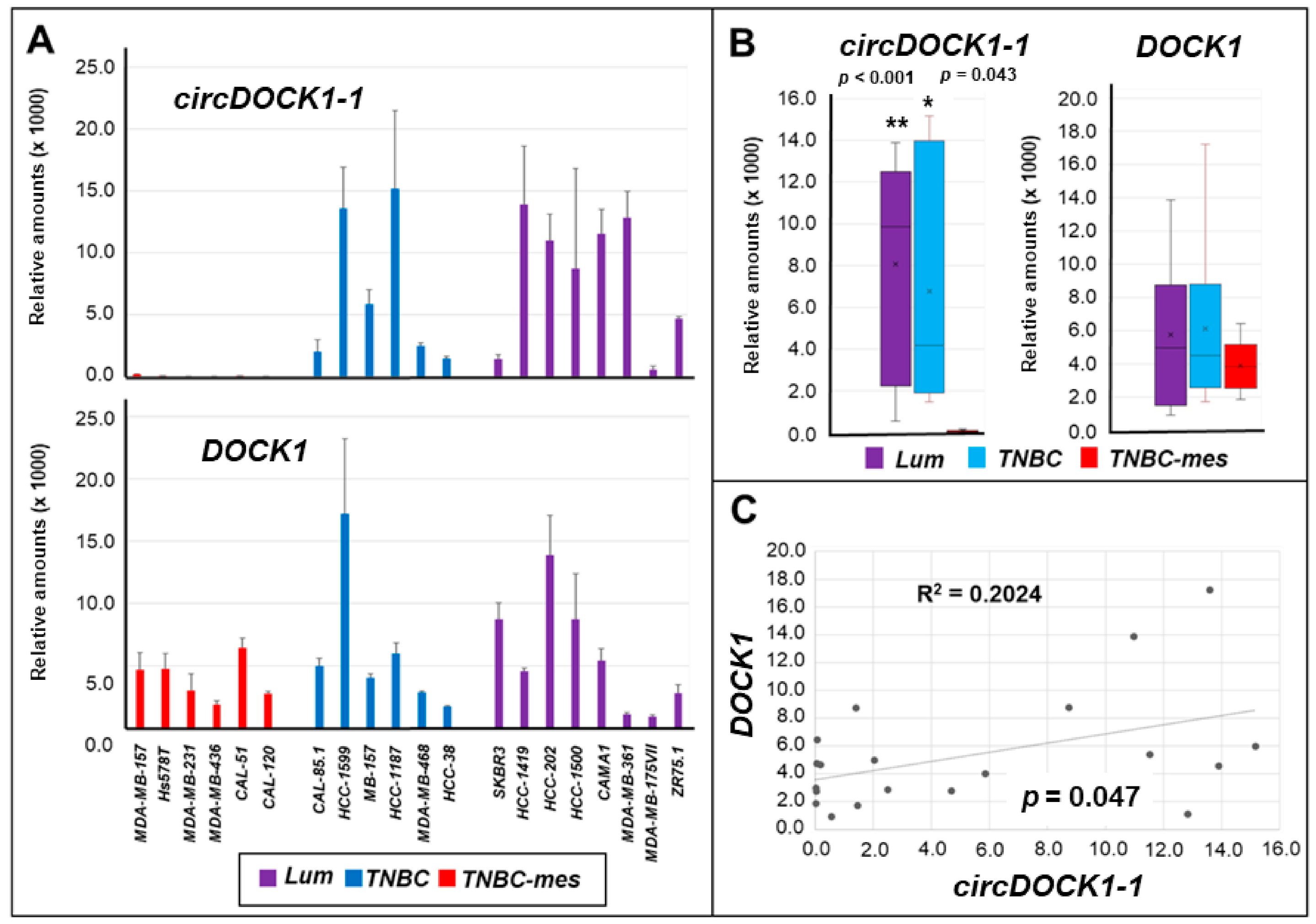
3.1. Expression of Selected circRNAs in Breast Cancer Cell Lines
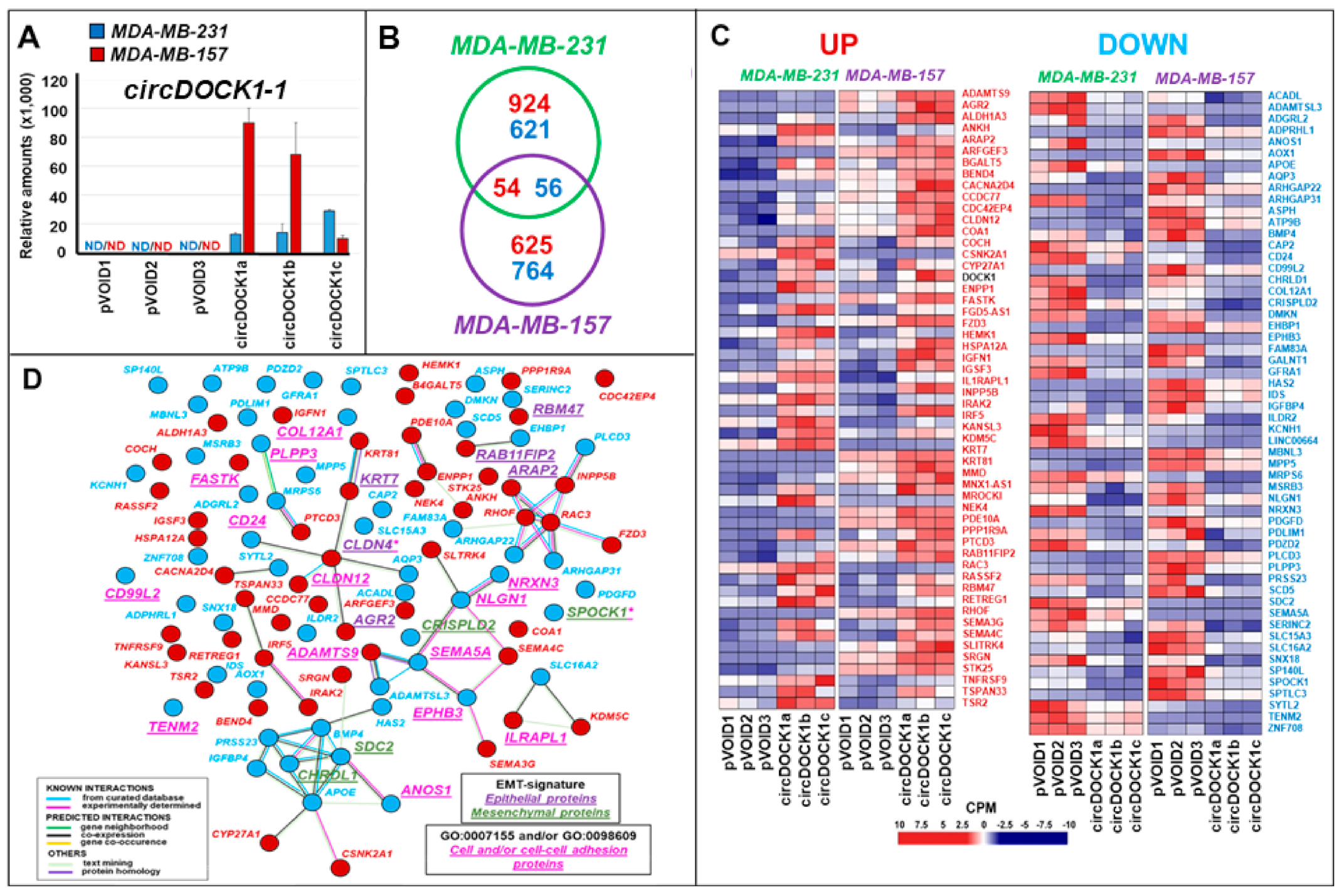
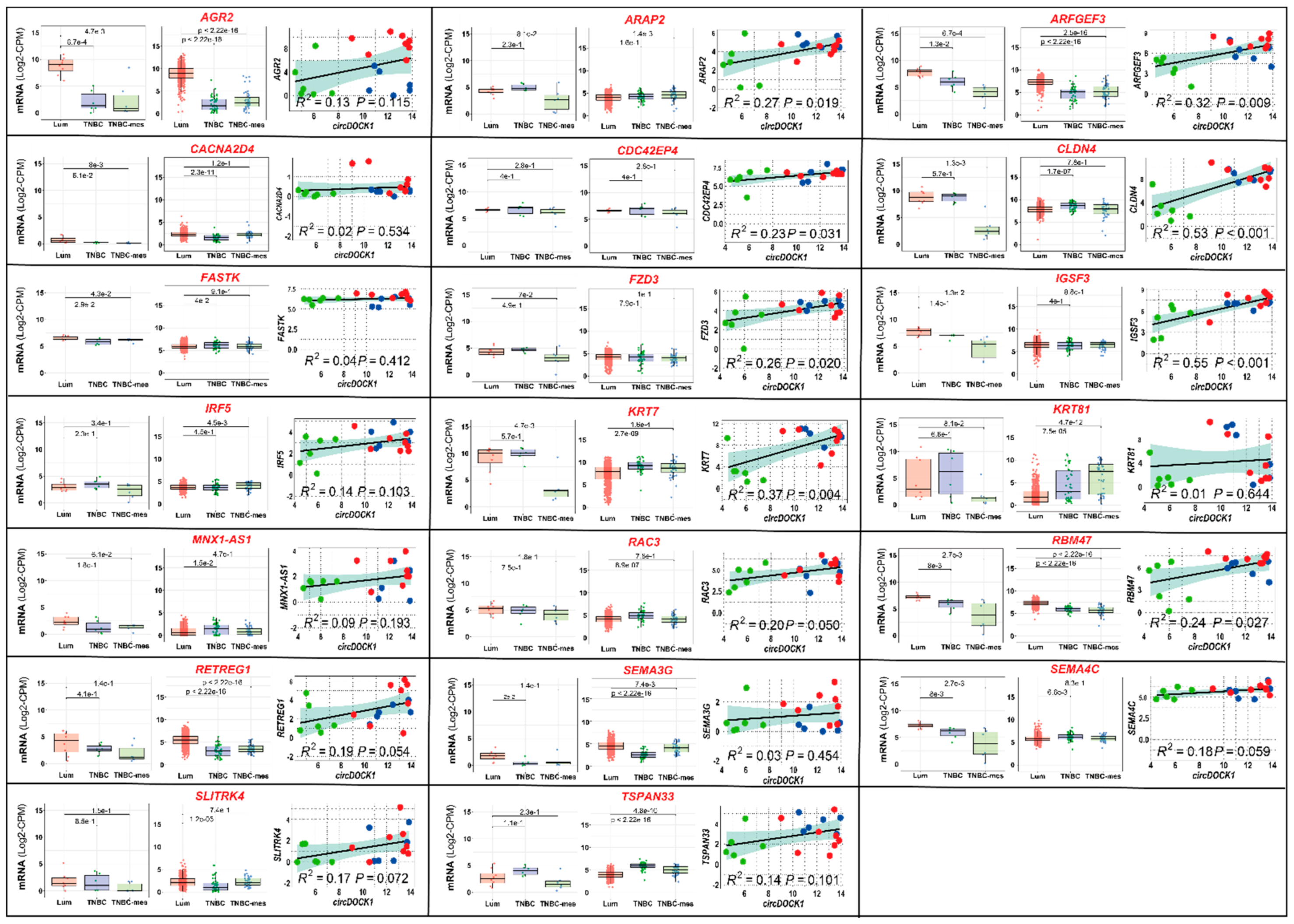
3.2. Transcriptomic Perturbations Afforded by circDOCK1-1 Overexpression in TNBC-Mes Cells
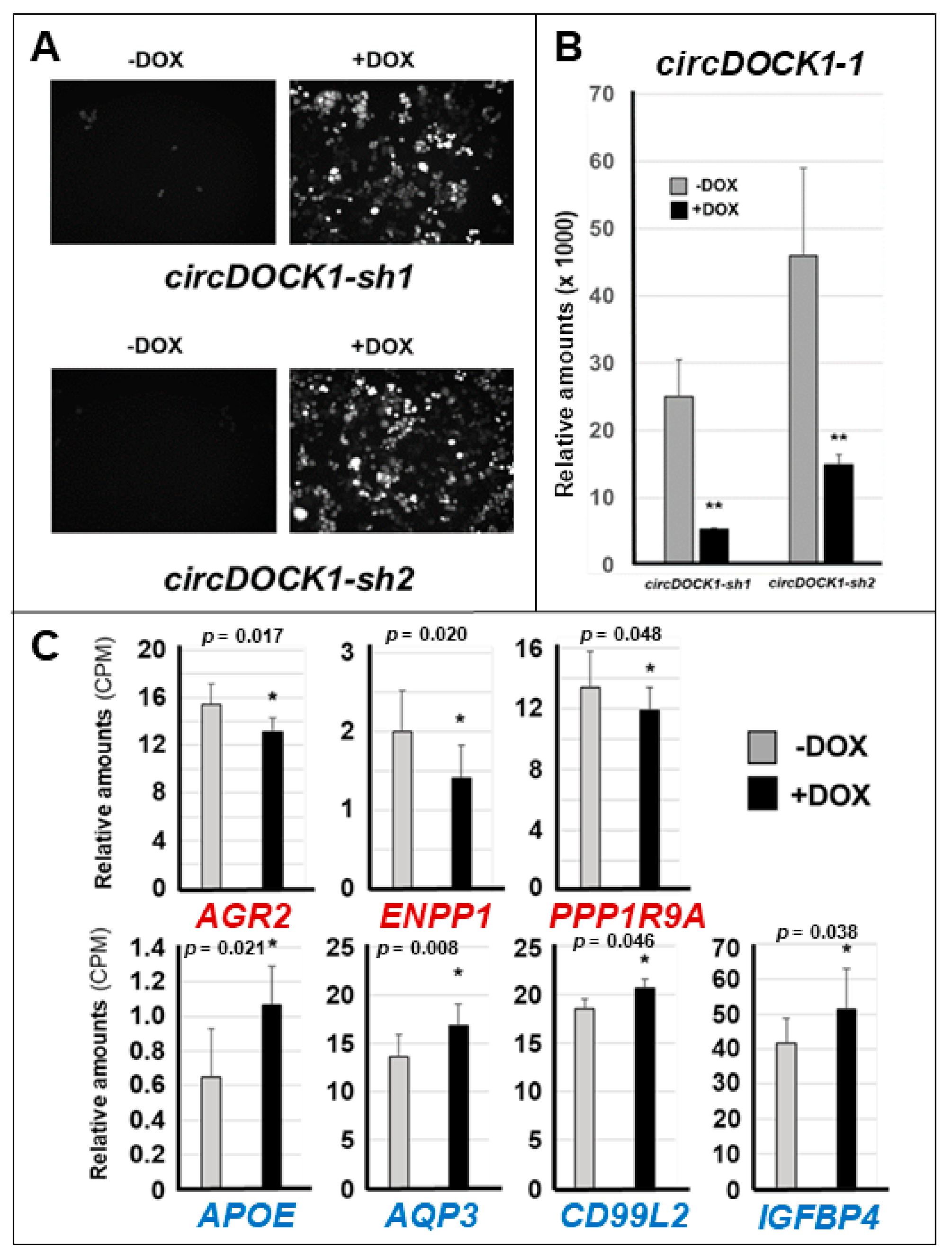
3.3. Effects of circDOCK1-1 Knockdown on the Gene Expression Profiles of Luminal CAMA-1 Cells
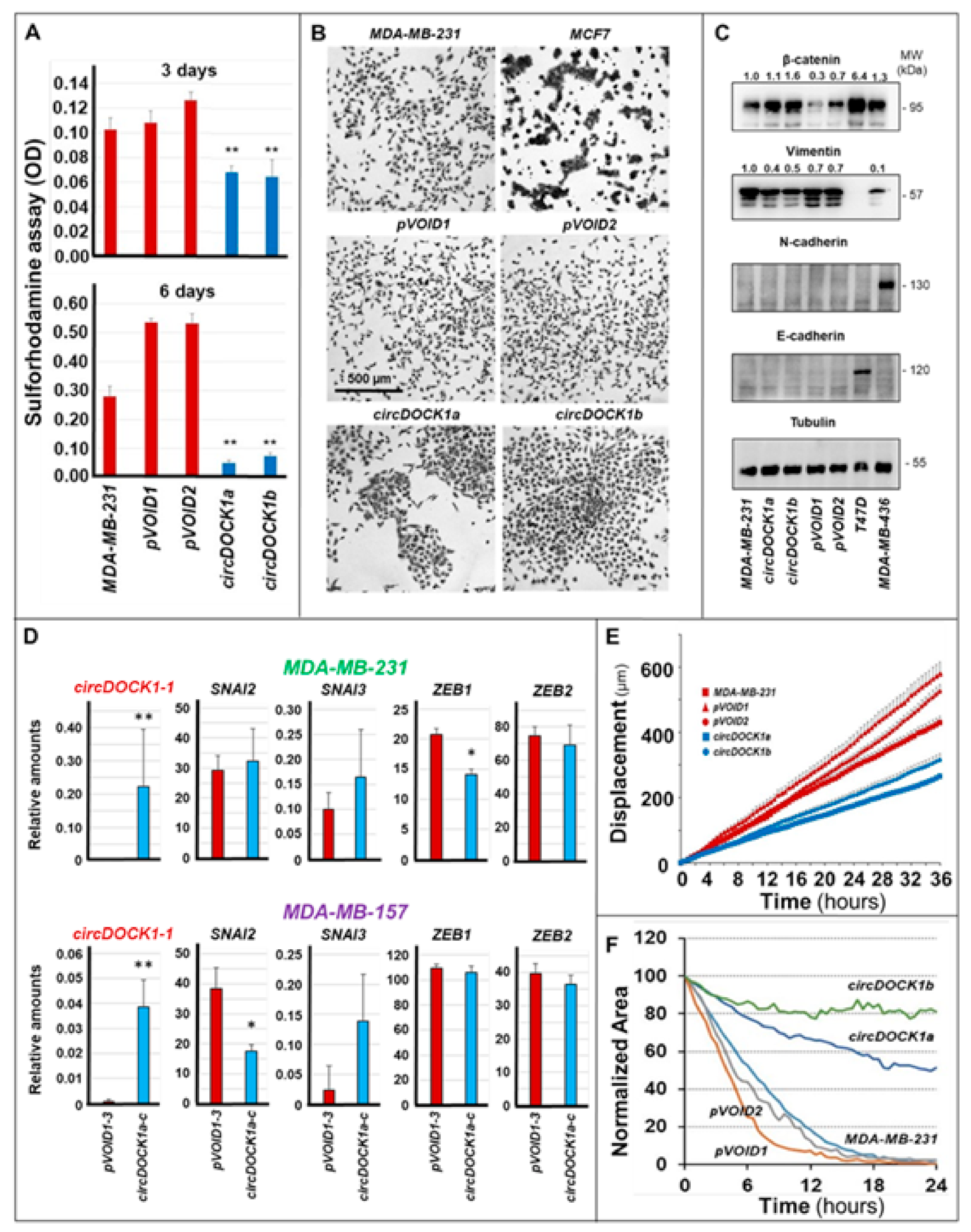
3.4. Involvement of circDOCK1-1 in the Growth, Differentiation, and Motility of Breast Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA Binding Protein Quaking Regulates Formation of CircRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Jeck, W.R.; Sharpless, N.E. Detecting and Characterizing Circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- Liu, D.; Conn, V.; Goodall, G.J.; Conn, S.J. A Highly Efficient Strategy for Overexpressing circRNAs. Methods Mol. Biol. 2018, 1724, 97–105. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Jiao, S.; Wu, S.; Huang, S.; Liu, M.; Gao, B. Advances in the Identification of Circular RNAs and Research Into circRNAs in Human Diseases. Front. Genet. 2021, 12, 665233. [Google Scholar] [CrossRef]
- Garlapati, P.; Ling, J.; Chiao, P.J.; Fu, J. Circular RNAs Regulate Cancer-Related Signaling Pathways and Serve as Potential Diagnostic Biomarkers for Human Cancers. Cancer Cell Int. 2021, 21, 317. [Google Scholar] [CrossRef]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in Cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 Circular RNA Retards Cell Cycle Progression via Forming Ternary Complexes with P21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef] [Green Version]
- Guarnerio, J.; Bezzi, M.; Jeong, J.C.; Paffenholz, S.V.; Berry, K.; Naldini, M.M.; Lo-Coco, F.; Tay, Y.; Beck, A.H.; Pandolfi, P.P. Oncogenic Role of Fusion-CircRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell 2016, 166, 1055–1056. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Du, W.W.; Li, X.; Yee, A.J.; Yang, B.B. Foxo3 Activity Promoted by Non-Coding Effects of Circular RNA and Foxo3 Pseudogene in the Inhibition of Tumor Growth and Angiogenesis. Oncogene 2016, 35, 3919–3931. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Fontemaggi, G.; Turco, C.; Esposito, G.; Di Agostino, S. New Molecular Mechanisms and Clinical Impact of CircRNAs in Human Cancer. Cancers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient MicroRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, C.; Lv, A.; Kou, C. Circular RNA circ_0010235 sponges miR-338-3p to play oncogenic role in proliferation, migration and invasion of non-small-cell lung cancer cells through modulating KIF2A. Ann. Med. 2021, 53, 693–706. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- He, X.; Xu, T.; Hu, W.; Tan, Y.; Wang, D.; Wang, Y.; Zhao, C.; Yi, Y.; Xiong, M.; Lv, W.; et al. Circular RNAs: Their Role in the Pathogenesis and Orchestration of Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 647736. [Google Scholar] [CrossRef]
- Zeng, Y.; Zou, Y.; Gao, G.; Zheng, S.; Wu, S.; Xie, X.; Tang, H. The biogenesis, function and clinical significance of circular RNAs in breast cancer. Cancer Biol. Med. 2021. [Google Scholar] [CrossRef]
- Prat, A.; Perou, C.M. Deconstructing the Molecular Portraits of Breast Cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast Cancer as an Example of Tumour Heterogeneity and Tumour Cell Plasticity during Malignant Progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Rosenberg, P.S.; Prat, A.; Perou, C.M.; Sherman, M.E. How Many Etiological Subtypes of Breast Cancer: Two, Three, Four, or More? J. Natl. Cancer Inst. 2014, 106, dju165. [Google Scholar] [CrossRef] [Green Version]
- Guido, L.P.; Gomez-Fernandez, C. Advances in the Molecular Taxonomy of Breast Cancer. Arch. Med. Res. 2020, 51, 777–783. [Google Scholar] [CrossRef]
- Neumann, D.P.; Goodall, G.J.; Gregory, P.A. Regulation of Splicing and Circularisation of RNA in Epithelial Mesenchymal Plasticity. Semin. Cell Dev. Biol. 2018, 75, 50–60. [Google Scholar] [CrossRef]
- Bolis, M.; Paroni, G.; Fratelli, M.; Vallerga, A.; Guarrera, L.; Zanetti, A.; Kurosaki, M.; Garattini, S.K.; Gianni’, M.; Lupi, M.; et al. All-Trans Retinoic Acid Stimulates Viral Mimicry, Interferon Responses and Antigen Presentation in Breast-Cancer Cells. Cancers 2020, 12, 1169. [Google Scholar] [CrossRef]
- Paroni, G.; Zanetti, A.; Barzago, M.M.; Kurosaki, M.; Guarrera, L.; Fratelli, M.; Troiani, M.; Ubezio, P.; Bolis, M.; Vallerga, A.; et al. Retinoic Acid Sensitivity of Triple-Negative Breast Cancer Cells Characterized by Constitutive Activation of the Notch1 Pathway: The Role of Rarβ. Cancers 2020, 12, E3027. [Google Scholar] [CrossRef]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An Introduction to the Wound Healing Assay Using Live-Cell Microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Wingett, S.W.; Andrews, S. FastQ Screen: A Tool for Multi-Genome Mapping and Quality Control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kiyokawa, E.; Tanaka, S.; Nagashima, K.; Gotoh, N.; Shibuya, M.; Kurata, T.; Matsuda, M. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell. Biol. 1996, 16, 1770–1776. [Google Scholar] [CrossRef] [Green Version]
- Kiyokawa, E.; Hashimoto, Y.; Kobayashi, S.; Sugimura, H.; Kurata, T.; Matsuda, M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998, 12, 3331–3336. [Google Scholar] [CrossRef] [Green Version]
- Côté, J.F.; Vuori, K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002, 115, 4901–4913. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT Signaling Cross-Talk and Gene Regulatory Networks by Single-Cell RNA Sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118. [Google Scholar] [CrossRef]
- Cai, J.; Guan, H.; Fang, L.; Yang, Y.; Zhu, X.; Yuan, J.; Wu, J.; Li, M. MicroRNA-374a Activates Wnt/β-Catenin Signaling to Promote Breast Cancer Metastasis. J. Clin. Investig. 2013, 123, 566–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Y.; Cao, M.; Liu, Y.; He, Y.; Zhang, G.; Du, Y.; Gao, F.; Yang, C. Hyaluronan Synthase 2 (HAS2) Regulates Cell Phenotype and Invadopodia Formation in Luminal-like Breast Cancer Cells. Mol. Cell. Biochem. 2021, 476, 3383–3391. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in Cancer and Its Potential as a Molecular Target for Cancer Therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, A.-P.; Lièvre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Chakraborty, S.; Mazumdar, M.; Ghosh, S.; Mukherjee, S.; Manna, A.; Mohanty, S.; Nakka, K.K.; Joshi, S.; De, A.; et al. Inhibition of Epithelial to Mesenchymal Transition by E-Cadherin up-Regulation via Repression of Slug Transcription and Inhibition of E-Cadherin Degradation: Dual Role of Scaffold/Matrix Attachment Region-Binding Protein 1 (SMAR1) in Breast Cancer Cells. J. Biol. Chem. 2014, 289, 25431–25444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, Z.; Xu, C.; Zhou, Z.; Zhu, Z.; You, T. HMGA2 Induces Transcription Factor Slug Expression to Promote Epithelial-to-Mesenchymal Transition and Contributes to Colon Cancer Progression. Cancer Lett. 2014, 355, 130–140. [Google Scholar] [CrossRef]
- Leong, K.G.; Niessen, K.; Kulic, I.; Raouf, A.; Eaves, C.; Pollet, I.; Karsan, A. Jagged1-Mediated Notch Activation Induces Epithelial-to-Mesenchymal Transition through Slug-Induced Repression of E-Cadherin. J. Exp. Med. 2007, 204, 2935–2948. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-W.; Jian, X.; Yoon, H.-Y.; Randazzo, P.A. ARAP2 Signals through Arf6 and Rac1 to Control Focal Adhesion Morphology. J. Biol. Chem. 2013, 288, 5849–5860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, X.; Hameed, M.; Mirani, N.; Pimenta, E.M.; Anari, J.; Barnes, B.J. Loss of Interferon Regulatory Factor 5 (IRF5) Expression in Human Ductal Carcinoma Correlates with Disease Stage and Contributes to Metastasis. Breast Cancer Res. 2011, 13, R111. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, E.; Barnes, B.J. A Conserved Region within Interferon Regulatory Factor 5 Controls Breast Cancer Cell Migration through a Cytoplasmic and Transcription-Independent Mechanism. Mol. Cancer 2015, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Strebhardt, K.; Pasquale, E.B.; Shen, T.-L.; Guan, J.-L.; Wang, B. Inhibition of Integrin-Mediated Cell Adhesion but Not Directional Cell Migration Requires Catalytic Activity of EphB3 Receptor Tyrosine Kinase: Role of Rho family small GTPases. J. Biol. Chem. 2005, 280, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.-D.; Li, G.; Feng, Y.-X.; Zhao, J.-S.; Li, J.-J.; Sun, Z.-J.; Shi, S.; Deng, Y.-Z.; Xu, J.-F.; Zhu, Y.-Q.; et al. EphB3 Is Overexpressed in Non-Small-Cell Lung Cancer and Promotes Tumor Metastasis by Enhancing Cell Survival and Migration. Cancer Res. 2011, 71, 1156–1166. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Huang, O.; Jiang, M.; Xie, Z.; Chen, D.; Zhang, X. Prognostic Value of Ephrin B Receptors in Breast Cancer: An Online Survival Analysis Using the Microarray Data of 3554 patients. Oncol. Lett. 2019, 18, 742–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galligan, J.J.; Petersen, D.R. The Human Protein Disulfide Isomerase Gene Family. Hum. Genomics 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Lacambra, M.D.; Tsang, J.Y.S.; Ni, Y.-B.; Chan, S.-K.; Tan, P.H.; Tse, G.M. Anterior Gradient 2 Is a Poor Outcome Indicator in Luminal Breast Cancer. Ann. Surg. Oncol. 2015, 22, 3489–3496. [Google Scholar] [CrossRef]
- Zheng, W.; Rosenstiel, P.; Huse, K.; Sina, C.; Valentonyte, R.; Mah, N.; Zeitlmann, L.; Grosse, J.; Ruf, N.; Nürnberg, P.; et al. Evaluation of AGR2 and AGR3 as Candidate Genes for Inflammatory Bowel Disease. Genes Immun. 2006, 7, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Fu, A.K.; Ip, N.Y. APOE Signaling in Neurodegenerative Diseases: An Integrative Approach Targeting APOE Coding and Noncoding Variants for Disease Intervention. Curr. Opin. Neurobiol. 2021, 69, 58–67. [Google Scholar] [CrossRef]
- Xing, L.; Wen, J.-G.; Frøkiær, J.; Djurhuus, J.C.; Nørregaard, R. Ontogeny of the Mammalian Kidney: Expression of Aquaporins 1, 2, 3, and 4. World J. Pediatr. 2014, 10, 306–312. [Google Scholar] [CrossRef]
- Huang, R.Y.-J.; Kuay, K.T.; Tan, T.Z.; Asad, M.; Tang, H.M.; Ng, A.H.C.; Ye, J.; Chung, V.Y.; Thiery, J.P. Functional Relevance of a Six Mesenchymal Gene Signature in Epithelial-Mesenchymal Transition (EMT) Reversal by the Triple Angiokinase Inhibitor, Nintedanib (BIBF1120). Oncotarget 2015, 6, 22098–22113. [Google Scholar] [CrossRef] [Green Version]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Bezzi, M.; Guarnerio, J.; Pandolfi, P.P. A circular twist on microRNA regulation. Cell Res. 2017, 27, 1401–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-L.; Xu, L.-L.; Wang, F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the miR-138 targets TERT and PD-L1. Cell Biol. Int. 2017, 41, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Guo, X.; Chen, J.; Li, C.; Chen, M.; Liu, L.; Zhang, X.; Zu, X. Circular RNA DOCK1 promotes bladder carcinoma progression via modulating circDOCK1/hsa-miR-132-3p/Sox5 signalling pathway. Cell Prolif. 2019, 52, e12614. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Circular RNA | Circ ID | Location | Exons (no) | Size (nt) | HMLE (Mean-Counts) | HMLE-mes (Mean-Counts) | HMLE-mes/HMLE (Fold Change) |
|---|---|---|---|---|---|---|---|
| circPVRL3 | hsa_circ_0066776 | chr3: 110830876-110845182 | 2-5 (4) | 909 | ND (0;0) | 51 (68;34) | ND |
| circSHPRH | hsa_circ_0001649 | chr6: 146209155-146216113 | 2-5 (4) | 440 | ND (0;0) | 18 (21;16) | ND |
| circVEGFC | hsa_circ_0004496 | chr4: 177632652-177650900 | 4-6 (3) | 557 | 5 (11;0) | 65 (91;39) | 13.0 |
| circBMPR2 | hsa_circ_0003218 | chr2: 203329531-203332412 | 2-3 (2) | 342 | 21 (37;5) | 102 (133;72) | 4.9 |
| circCCNB1 | hsa_circ_0001495 | chr5: 68470703-68471364 | 6-7 (2) | 378 | 20 (24;16) | 90 (114;66) | 4.5 |
| circSLC8A1 | hsa_circ_0000994 | chr2: 40655612-40657444 | 9 (1) | 1832 | 67 (52;82) | 271 (206;337) | 4.0 |
| circASXL1 | hsa_circ_0001136 | chr20: 30954186-30956926 | 2-3 (2) | 195 | 101 (134;69) | 375 (643;107) | 3.7 |
| circSMARCA5 | hsa_circ_0001445 | chr4: 144464661-144465125 | 15-16 (2) | 269 | 47 (72;22) | 174 (154;195) | 3.7 |
| circATXN2 | hsa_circ_0002457 | chr12:111990083-111993723 | 21-24 (4) | 320 | 21 (35;8) | 68 (85;51) | 3.2 |
| circRTN4 | hsa_circ_0001006 | chr2: 55209650-55214834 | 5-6 (2) | 347 | 48 (47;49) | 152 (152;152) | 3.2 |
| circSETD3 | hsa_circ_0000567 | chr14: 99924615-99932150 | 8-12 (5) | 683 | 90 (115;65) | 256 (209;304) | 2.8 |
| circDOCK1-1 | hsa_circ_0020397 | chr10:128768965-128926028 | 2-27 (26) | 2738 | 172 (211;134) | 7 (12;3) | 0.04 |
| circGNB1 | hsa_circ_0008702 | chr1: 1747194-1770677 | 8-11 (4) | 298 | 38 (36;40) | 13 (19;8) | 0.3 |
| circFGD6 | hsa_circ_0141065 | chr12: 95602618-95605043 | 20 (1) | 2425 | 66 (57;74) | 27 (29;25) | 0.4 |
| circTNFRSF21 | hsa_circ_0001610 | chr6: 47251673-47254331 | 4-5 (2) | 1147 | 58 (65;52) | 27 (22; 32) | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurosaki, M.; Terao, M.; Liu, D.; Zanetti, A.; Guarrera, L.; Bolis, M.; Gianni’, M.; Paroni, G.; Goodall, G.J.; Garattini, E. A DOCK1 Gene-Derived Circular RNA Is Highly Expressed in Luminal Mammary Tumours and Is Involved in the Epithelial Differentiation, Growth, and Motility of Breast Cancer Cells. Cancers 2021, 13, 5325. https://doi.org/10.3390/cancers13215325
Kurosaki M, Terao M, Liu D, Zanetti A, Guarrera L, Bolis M, Gianni’ M, Paroni G, Goodall GJ, Garattini E. A DOCK1 Gene-Derived Circular RNA Is Highly Expressed in Luminal Mammary Tumours and Is Involved in the Epithelial Differentiation, Growth, and Motility of Breast Cancer Cells. Cancers. 2021; 13(21):5325. https://doi.org/10.3390/cancers13215325
Chicago/Turabian StyleKurosaki, Mami, Mineko Terao, Dawei Liu, Adriana Zanetti, Luca Guarrera, Marco Bolis, Maurizio Gianni’, Gabriela Paroni, Gregory J. Goodall, and Enrico Garattini. 2021. "A DOCK1 Gene-Derived Circular RNA Is Highly Expressed in Luminal Mammary Tumours and Is Involved in the Epithelial Differentiation, Growth, and Motility of Breast Cancer Cells" Cancers 13, no. 21: 5325. https://doi.org/10.3390/cancers13215325
APA StyleKurosaki, M., Terao, M., Liu, D., Zanetti, A., Guarrera, L., Bolis, M., Gianni’, M., Paroni, G., Goodall, G. J., & Garattini, E. (2021). A DOCK1 Gene-Derived Circular RNA Is Highly Expressed in Luminal Mammary Tumours and Is Involved in the Epithelial Differentiation, Growth, and Motility of Breast Cancer Cells. Cancers, 13(21), 5325. https://doi.org/10.3390/cancers13215325









