Radionuclide-Based Imaging of Breast Cancer: State of the Art
Abstract
:Simple Summary
Abstract
1. Introduction
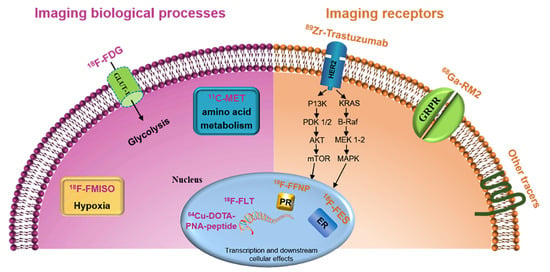
2. Imaging Biological Processes of Breast Cancer
2.1. Imaging Glucose Metabolism (18F-FDG)
2.2. Imaging Amino Acid Metabolism
2.3. Imaging Cell Proliferation
2.4. Imaging Hypoxia
2.5. Imaging Cellular Transmembrane Electrical Potential
3. Imaging Receptors in Breast Cancer
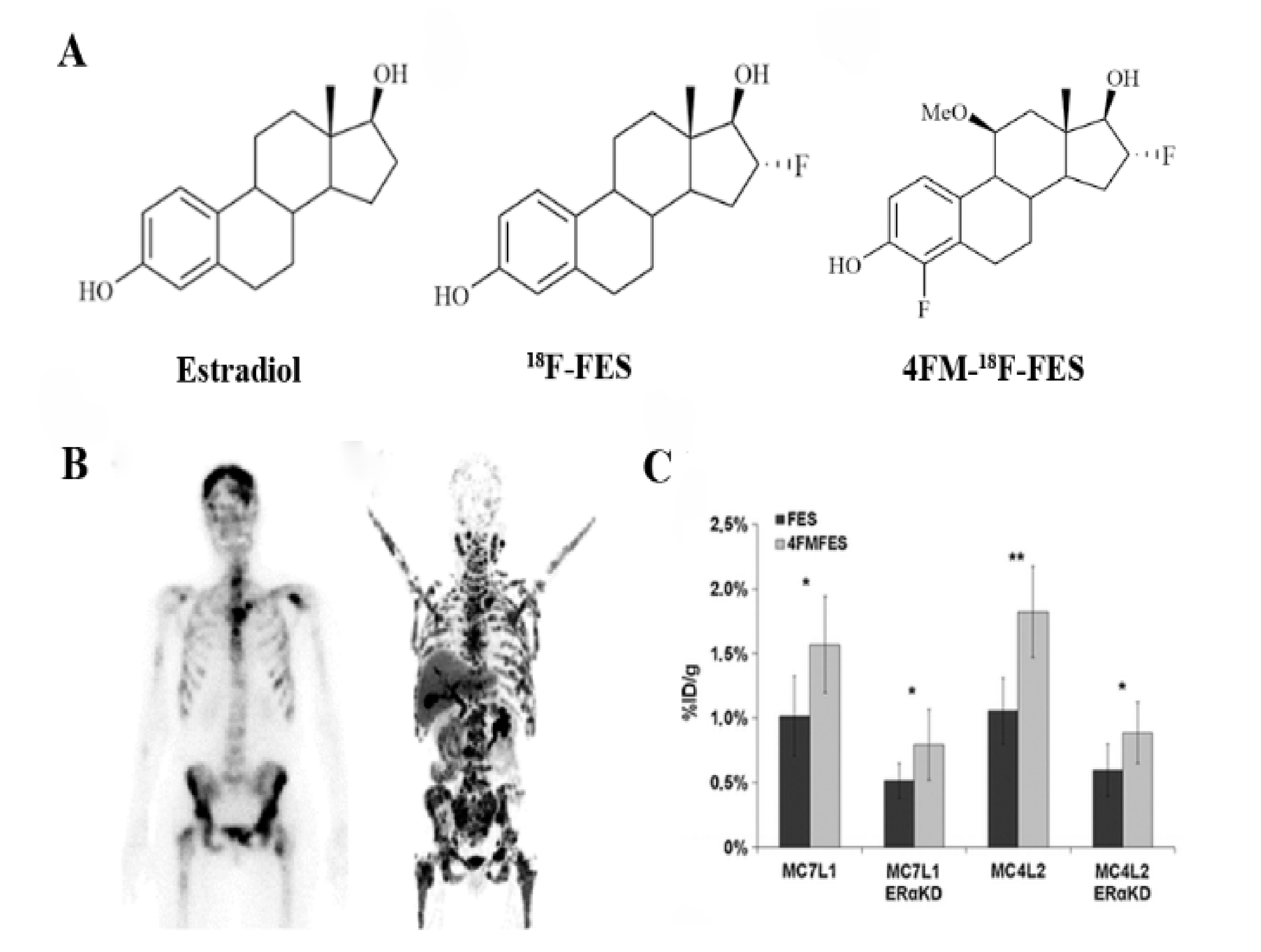
3.1. Targeting Estrogen Receptor (ER)
3.2. Targeting Progesterone Receptor (PR)
3.3. Targeting HER2
3.4. Targeting Gastrin-Releasing Peptide Receptor (GRPR)
3.5. Other Receptors
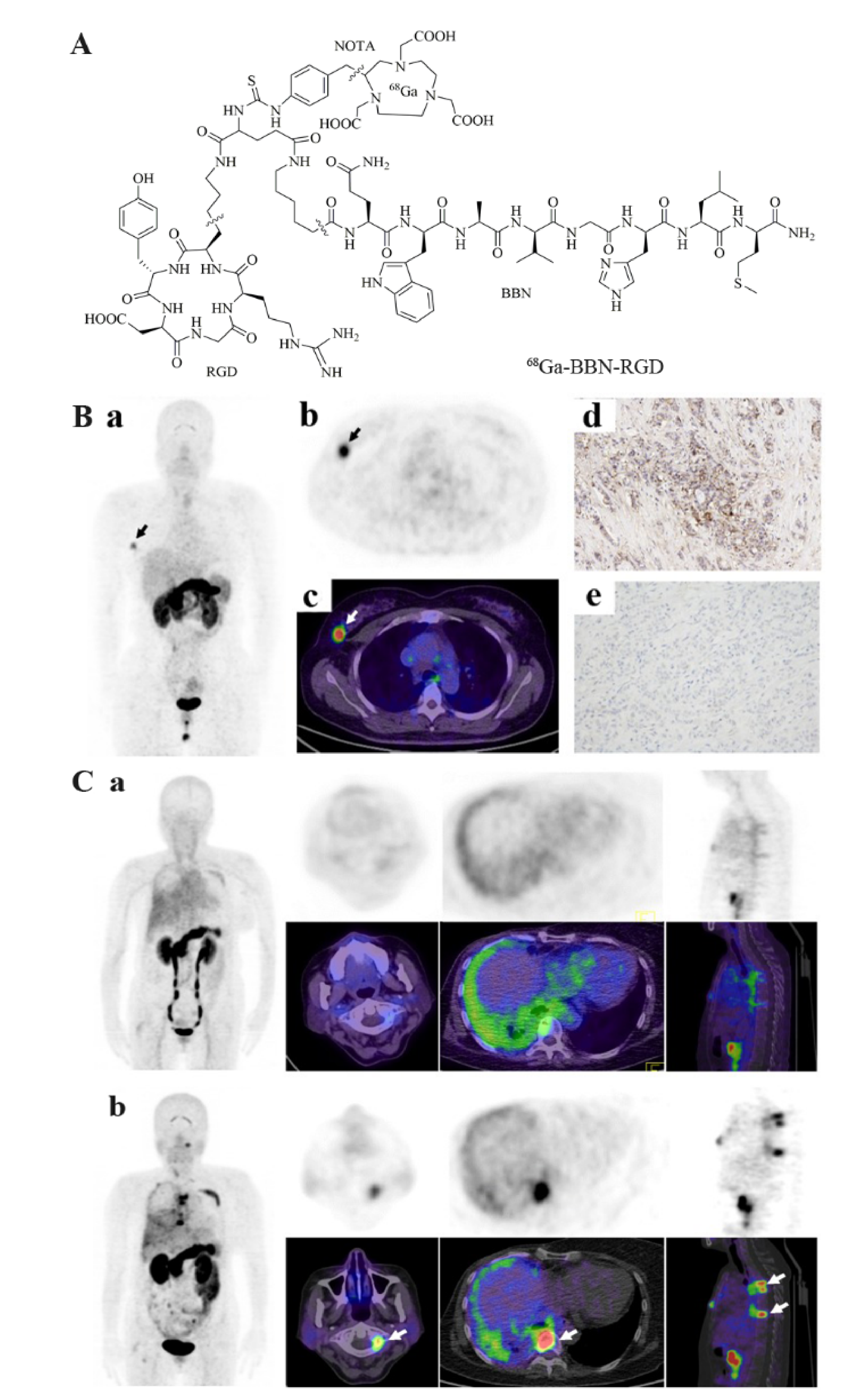
3.6. Dual Receptor-Targeted Molecular Imaging
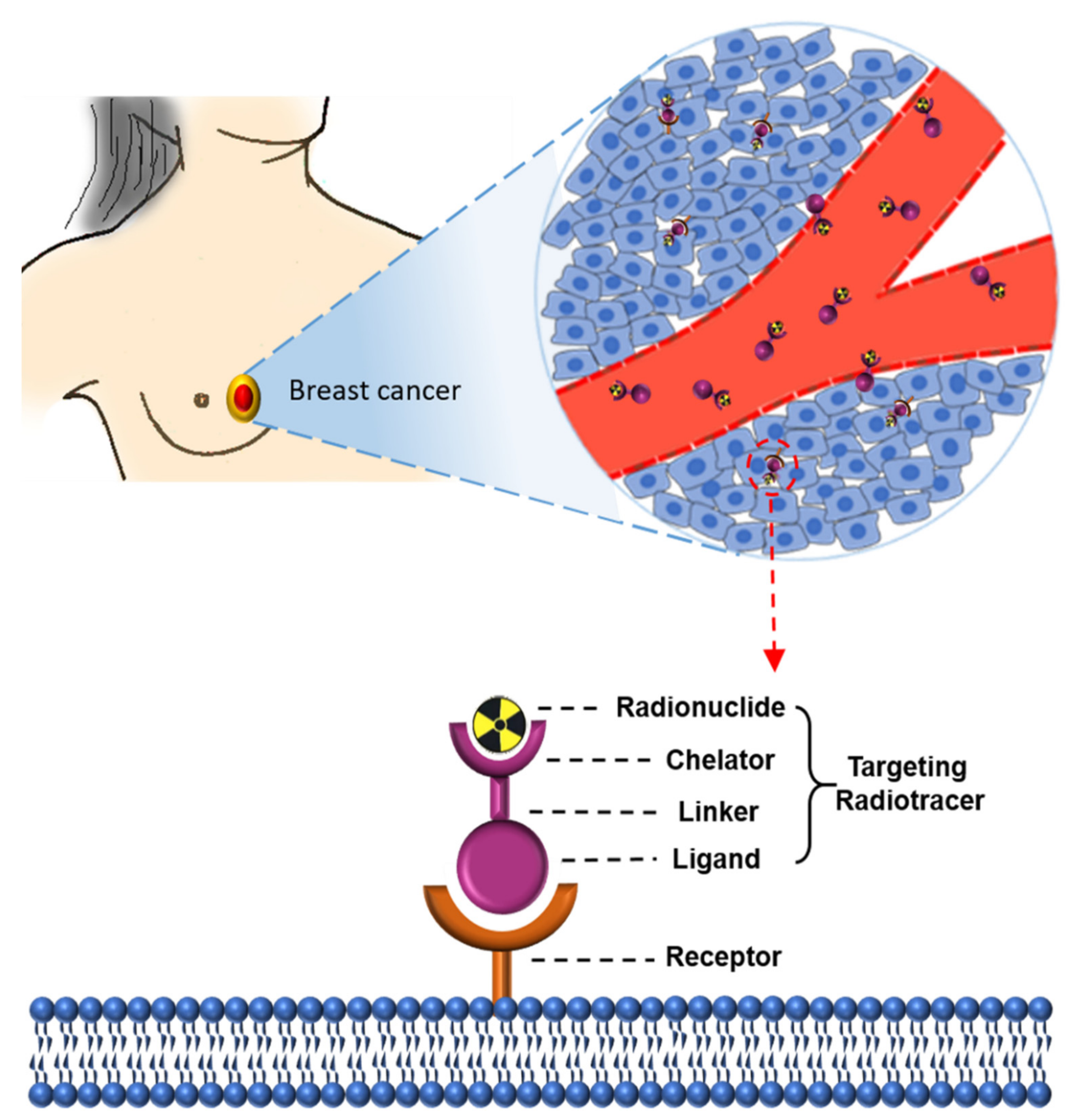
4. Biomaterial-Based Probes for Imaging of Breast Cancer
4.1. Membrane-Based Imaging Probes
4.2. Exosome-Based Imaging Probes
4.3. Peptide Nucleic Acid-Based Imaging Probes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, R.; Sosabowski, J.; Vigor, K.; Chester, K.; Meyer, T. Developments in single photon emission computed tomography and PET-based HER2 molecular imaging for breast cancer. Expert Rev. Anticancer Ther. 2013, 13, 359–373. [Google Scholar] [CrossRef]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT imaging in oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast cancer diagnosis: Imaging techniques and biochemical markers. J. Cell. Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef]
- Lindholm, P.; Lapela, M.; Nagren, K.; Lehikoinen, P.; Minn, H.; Jyrkkio, S. Preliminary study of carbon-11 methionine PET in the evaluation of early response to therapy in advanced breast cancer. Nucl. Med. Commun. 2009, 30, 30–36. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Goldman, D.A.; Corben, A.; Lyashchenko, S.K.; Gonen, M.; Lewis, J.S.; Dickler, M. Prospective Clinical Trial of (18)F-Fluciclovine PET/CT for Determining the Response to Neoadjuvant Therapy in Invasive Ductal and Invasive Lobular Breast Cancers. J. Nucl. Med. 2017, 58, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Kenny, L.M.; Al-Nahhas, A.; Aboagye, E.O. Novel PET biomarkers for breast cancer imaging. Nucl. Med. Commun. 2011, 32, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.N.; Manavaki, R.; Blower, P.J.; West, C.; Williams, K.J.; Harris, A.L.; Domarkas, J.; Lord, S.; Baldry, C.; Gilbert, F.J. Imaging tumour hypoxia with positron emission tomography. Br. J. Cancer 2015, 112, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehdashti, F.; Mortimer, J.E.; Siegel, B.A.; Griffeth, L.K.; Bonasera, T.J.; Fusselman, M.J.; Detert, D.D.; Cutler, P.D.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J. Nucl. Med. 1995, 36, 1766–1774. [Google Scholar]
- Webster, R.; Didier, E.; Harris, P.; Siegel, N.; Stadler, J.; Tilbury, L.; Smith, D. PEGylated proteins: Evaluation of their safety in the absence of definitive metabolism studies. Drug Metab. Dispos. 2007, 35, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Dehdashti, F.; McGuire, A.H.; Van Brocklin, H.F.; Siegel, B.A.; Andriole, D.P.; Griffeth, L.K.; Pomper, M.G.; Katzenellenbogen, J.A.; Welch, M.J. Assessment of 21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone as a positron-emitting radiopharmaceutical for the detection of progestin receptors in human breast carcinomas. J. Nucl. Med. 1991, 32, 1532–1537. [Google Scholar] [PubMed]
- Wu, X.; You, L.; Zhang, D.; Gao, M.; Li, Z.; Xu, D.; Zhang, P.; Huang, L.; Zhuang, R.; Wu, H.; et al. Synthesis and preliminary evaluation of a (18) F-labeled ethisterone derivative [(18) F]EAEF for progesterone receptor targeting. Chem. Biol. Drug Des. 2017, 89, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lub-de Hooge, M.N.; Kosterink, J.G.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.; de Jong, S.; Jager, P.L.; de Vries, E.G. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Chereau, E.; Durand, L.; Frati, A.; Prignon, A.; Talbot, J.N.; Rouzier, R. Correlation of immunohistopathological expression of somatostatin receptor-2 in breast cancer and tumor detection with 68Ga-DOTATOC and 18F-FDG PET imaging in an animal model. Anticancer Res. 2013, 33, 3015–3019. [Google Scholar]
- Zhao, Y.; Detering, L.; Sultan, D.; Cooper, M.L.; You, M.; Cho, S.; Meier, S.L.; Luehmann, H.; Sun, G.; Rettig, M.; et al. Gold Nanoclusters Doped with (64)Cu for CXCR4 Positron Emission Tomography Imaging of Breast Cancer and Metastasis. ACS Nano 2016, 10, 5959–5970. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, F.; Liu, C.; Yin, L.; Zhang, Y.; Zhang, Y.; Lan, X. Evaluation of (99m)Tc-HYNIC-VCAM-1(scFv) as a Potential Qualitative and Semiquantitative Probe Targeting Various Tumors. Contrast Media Mol. Imaging 2018, 2018, 7832805. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ma, Q.; Chen, M.; Chen, B.; Wen, Q.; Jia, B.; Wang, F.; Sun, B.; Gao, S. An exploratory study on 99mTc-RGD-BBN peptide scintimammography in the assessment of breast malignant lesions compared to 99mTc-3P4-RGD2. PLoS ONE 2015, 10, e0123401. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Dong, C.; Cui, L.; Jin, X.; Jia, B.; Zhu, Z.; Li, F.; Wang, F. 99mTc-labeled RGD-BBN peptide for small-animal SPECT/CT of lung carcinoma. Mol. Pharm. 2012, 9, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, H.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Li, H.; Hou, Y.; Gao, M.; Lan, X. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater. Sci. 2020, 8, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Good, L.; Nielsen, P.E. Progress in developing PNA as a gene-targeted drug. Antisense Nucleic Acid Drug Dev. 1997, 7, 431–437. [Google Scholar] [CrossRef]
- Paudyal, B.; Zhang, K.; Chen, C.P.; Wampole, M.E.; Mehta, N.; Mitchell, E.P.; Gray, B.D.; Mattis, J.A.; Pak, K.Y.; Thakur, M.L.; et al. Determining efficacy of breast cancer therapy by PET imaging of HER2 mRNA. Nucl. Med. Biol. 2013, 40, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Wang, M.; Dong, Y.; Xu, B.; Chen, J.; Ding, Y.; Qiu, S.; Li, L.; Karamfilova Zaharieva, E.; Zhou, X.; et al. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020, 11, 145. [Google Scholar] [CrossRef]
- Huang, S.C.; Phelps, M.E.; Hoffman, E.J.; Sideris, K.; Selin, C.J.; Kuhl, D.E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am. J. Physiol. 1980, 238, E69–E82. [Google Scholar] [CrossRef] [Green Version]
- Reivich, M.; Alavi, A.; Wolf, A.; Fowler, J.; Russell, J.; Arnett, C.; MacGregor, R.R.; Shiue, C.Y.; Atkins, H.; Anand, A.; et al. Glucose metabolic rate kinetic model parameter determination in humans: The lumped constants and rate constants for [18F]fluorodeoxyglucose and [11C]deoxyglucose. J. Cereb. Blood Flow Metab. 1985, 5, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Juweid, M.E.; Cheson, B.D. Positron-emission tomography and assessment of cancer therapy. N. Engl. J. Med. 2006, 354, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Kung, B.T.; Seraj, S.M.; Zadeh, M.Z.; Rojulpote, C.; Kothekar, E.; Ayubcha, C.; Ng, K.S.; Ng, K.K.; Au-Yong, T.K.; Werner, T.J.; et al. An update on the role of (18)F-FDG-PET/CT in major infectious and inflammatory diseases. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 255–273. [Google Scholar]
- Yararbas, U.; Cetin, N.; Yeniay, L.; Argon, A.M. The value of 18F-FDG PET/CT imaging in breast cancer staging. Bosn. J. Basic Med. Sci. 2018, 18, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, F.; Harbeck, N.; Fallowfield, L.; Kyriakides, S.; Senkus, E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii11–vii19. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Senda, M.; Jinnouchi, S.; Terauchi, T.; Yoshida, T.; Inoue, T. Detection of breast cancer in an FDG-PET cancer screening program: Results of a nationwide Japanese survey. Clin. Breast Cancer 2015, 15, e139–e146. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Treglia, G.; Orlando, E.; Dognini, L.; Giovanella, L.; Sadeghi, R.; Giubbini, R. Prevalence and clinical significance of incidental F18-FDG breast uptake: A systematic review and meta-analysis. Jpn. J. Radiol. 2014, 32, 59–68. [Google Scholar] [CrossRef]
- Boers, J.; de Vries, E.F.J.; Glaudemans, A.; Hospers, G.A.P.; Schröder, C.P. Application of PET Tracers in Molecular Imaging for Breast Cancer. Curr. Oncol. Rep. 2020, 22, 85. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef]
- Lu, X.R.; Qu, M.M.; Zhai, Y.N.; Feng, W.; Gao, Y.; Lei, J.Q. Diagnostic role of 18F-FDG PET/MRI in the TNM staging of breast cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4328–4337. [Google Scholar] [CrossRef]
- Han, S.; Choi, J.Y. Impact of 18F-FDG PET, PET/CT, and PET/MRI on Staging and Management as an Initial Staging Modality in Breast Cancer: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2021, 46, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.; Schirrmeister, H.; Kuhn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.R.; Park, J.S.; Kang, K.W.; Cho, N.; Chang, J.M.; Bae, M.S.; Kim, W.H.; Lee, S.H.; Kim, M.Y.; Kim, J.Y.; et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur. Radiol. 2014, 24, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Fukushima, K.; Miyoshi, Y.; Nishimukai, A.; Hirota, S.; Igarashi, Y.; Katsuura, T.; Maruyama, K.; Hirota, S. Association between (1)(8)F-FDG uptake and molecular subtype of breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; McDonald, E.; Lynch, M.C.; Mankoff, D. Using nuclear medicine imaging in clinical practice: Update on PET to guide treatment of patients with metastatic breast cancer. Oncology 2014, 28, 424–430. [Google Scholar] [PubMed]
- Mghanga, F.P.; Lan, X.; Bakari, K.H.; Li, C.; Zhang, Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: A meta-analysis. Clin. Breast Cancer 2013, 13, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, J.Y. Prognostic value of (18)F-FDG PET and PET/CT for assessment of treatment response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2020, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Caldarella, C.; Treglia, G.; Giordano, A. Diagnostic performance of dedicated positron emission mammography using fluorine-18-fluorodeoxyglucose in women with suspicious breast lesions: A meta-analysis. Clin. Breast Cancer 2014, 14, 241–248. [Google Scholar] [CrossRef]
- Gebhart, G.; Gamez, C.; Holmes, E.; Robles, J.; Garcia, C.; Cortes, M.; de Azambuja, E.; Fauria, K.; Van Dooren, V.; Aktan, G.; et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: Results from Neo-ALTTO. J. Nucl. Med. 2013, 54, 1862–1868. [Google Scholar] [CrossRef] [Green Version]
- Humbert, O.; Berriolo-Riedinger, A.; Cochet, A.; Gauthier, M.; Charon-Barra, C.; Guiu, S.; Desmoulins, I.; Toubeau, M.; Dygai-Cochet, I.; Coutant, C.; et al. Prognostic relevance at 5 years of the early monitoring of neoadjuvant chemotherapy using (18)F-FDG PET in luminal HER2-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 416–427. [Google Scholar] [CrossRef]
- Schwarz-Dose, J.; Untch, M.; Tiling, R.; Sassen, S.; Mahner, S.; Kahlert, S.; Harbeck, N.; Lebeau, A.; Brenner, W.; Schwaiger, M.; et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J. Clin. Oncol. 2009, 27, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.; Noh, W.C.; Park, J.; Park, J.A.; Kim, H.A.; Kim, E.K.; Park, K.W.; Lee, S.S.; You, E.Y.; Kim, K.M.; et al. The combination of FDG PET and dynamic contrast-enhanced MRI improves the prediction of disease-free survival in patients with advanced breast cancer after the first cycle of neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1852–1860. [Google Scholar] [CrossRef]
- Dawood, S.; Merajver, S.D.; Viens, P.; Vermeulen, P.B.; Swain, S.M.; Buchholz, T.A.; Dirix, L.Y.; Levine, P.H.; Lucci, A.; Krishnamurthy, S.; et al. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann. Oncol. 2011, 22, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Even-Sapir, E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): Accumulated data from four years of experience with PET/CT. Semin. Nucl. Med. 2007, 37, 206–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mac Manus, M.P.; Hicks, R.J.; Matthews, J.P.; McKenzie, A.; Rischin, D.; Salminen, E.K.; Ball, D.L. Positron Emission Tomography Is Superior to Computed Tomography Scanning for Response-Assessment After Radical Radiotherapy or Chemoradiotherapy in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2003, 21, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Kostakoglu, L.; Goldsmith, S.J. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J. Nucl. Med. 2003, 44, 224–239. [Google Scholar]
- Breillout, F.; Antoine, E.; Poupon, M.F. Methionine dependency of malignant tumors: A possible approach for therapy. J. Natl. Cancer Inst. 1990, 82, 1628–1632. [Google Scholar] [CrossRef]
- Leskinen-Kallio, S.; Nagren, K.; Lehikoinen, P.; Ruotsalainen, U.; Joensuu, H. Uptake of 11C-methionine in breast cancer studied by PET. An association with the size of S-phase fraction. Br. J. Cancer 1991, 64, 1121–1124. [Google Scholar] [CrossRef] [Green Version]
- Huovinen, R.; Leskinen-Kallio, S.; Nagren, K.; Lehikoinen, P.; Ruotsalainen, U.; Teras, M. Carbon-11-methionine and PET in evaluation of treatment response of breast cancer. Br. J. Cancer 1993, 67, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Westlin, J.E.; Ahlstrom, H.; Lilja, A.; Langstrom, B.; Bergh, J. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: A method for early therapy evaluation? J. Clin. Oncol. 1995, 13, 1470–1477. [Google Scholar] [CrossRef]
- Inoue, T.; Kim, E.E.; Wong, F.C.; Yang, D.J.; Bassa, P.; Wong, W.H.; Korkmaz, M.; Tansey, W.; Hicks, K.; Podoloff, D.A. Comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine PET in detection of malignant tumors. J. Nucl. Med. 1996, 37, 1472–1476. [Google Scholar] [PubMed]
- Harris, S.M.; Davis, J.C.; Snyder, S.E.; Butch, E.R.; Vavere, A.L.; Kocak, M.; Shulkin, B.L. Evaluation of the biodistribution of 11C-methionine in children and young adults. J. Nucl. Med. 2013, 54, 1902–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derlon, J.M.; Bourdet, C.; Bustany, P.; Chatel, M.; Theron, J.; Darcel, F.; Syrota, A. [11C]L-methionine uptake in gliomas. Neurosurgery 1989, 25, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, M.; Shirane, R.; Itoh, J.; Sato, K.; Katakura, R.; Yoshimoto, T.; Hatazawa, J.; Itoh, M.; Ido, T. The accumulation of 11C-methionine in cerebral glioma patients studied with PET. Acta Neurochir. 1990, 104, 8–12. [Google Scholar] [CrossRef]
- Langen, K.J.; Broer, S. Molecular transport mechanisms of radiolabeled amino acids for PET and SPECT. J. Nucl. Med. 2004, 45, 1435–1436. [Google Scholar]
- Wagner, C.A.; Lang, F.; Broer, S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 2001, 281, C1077–C1093. [Google Scholar] [CrossRef]
- McConathy, J.; Yu, W.; Jarkas, N.; Seo, W.; Schuster, D.M.; Goodman, M.M. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med. Res. Rev. 2012, 32, 868–905. [Google Scholar] [CrossRef]
- McConathy, J.; Goodman, M.M. Non-natural amino acids for tumor imaging using positron emission tomography and single photon emission computed tomography. Cancer Metastasis Rev. 2008, 27, 555–573. [Google Scholar] [CrossRef]
- Laverman, P.; Boerman, O.C.; Corstens, F.H.; Oyen, W.J. Fluorinated amino acids for tumour imaging with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 681–690. [Google Scholar] [CrossRef]
- Jager, P.L.; Vaalburg, W.; Pruim, J.; de Vries, E.G.; Langen, K.J.; Piers, D.A. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. J. Nucl. Med. 2001, 42, 432–445. [Google Scholar]
- Morana, G.; Puntoni, M.; Garre, M.L.; Massollo, M.; Lopci, E.; Naseri, M.; Severino, M.; Tortora, D.; Rossi, A.; Piccardo, A. Ability of (18)F-DOPA PET/CT and fused (18)F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Metellus, P.; Sala, Q.; Colin, C.; Bialecki, E.; Taieb, D.; Chinot, O.; Figarella-Branger, D.; Guedj, E. IDH mutation is paradoxically associated with higher (18)F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.L.; Benz, M.R.; Buck, A.K.; Phelps, M.E.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neur. Oncol. 2014, 16, 603–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helali, M.; Addeo, P.; Heimburger, C.; Detour, J.; Goichot, B.; Bachellier, P.; Namer, I.J.; Taieb, D.; Imperiale, A. Carbidopa-assisted (18)F-fluorodihydroxyphenylalanine PET/CT for the localization and staging of non-functioning neuroendocrine pancreatic tumors. Ann. Nucl. Med. 2016, 30, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Deroose, C.M.; Hindie, E.; Kebebew, E.; Goichot, B.; Pacak, K.; Taieb, D.; Imperiale, A. Molecular Imaging of Gastroenteropancreatic Neuroendocrine Tumors: Current Status and Future Directions. J. Nucl. Med. 2016, 57, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knie, B.; Plotkin, M.; Zschieschang, P.; Prasad, V.; Moskopp, D. A family with pheochromocytoma-paraganglioma inherited tumour syndrome. Serial 18F-DOPA PET/CT investigations. Nuklearmedizin 2016, 55, 34–40. [Google Scholar] [CrossRef]
- Feral, C.C.; Tissot, F.S.; Tosello, L.; Fakhry, N.; Sebag, F.; Pacak, K.; Taieb, D. (18)F-fluorodihydroxyphenylalanine PET/CT in pheochromocytoma and paraganglioma: Relation to genotype and amino acid transport system L. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 812–821. [Google Scholar] [CrossRef]
- Heimburger, C.; Veillon, F.; Taieb, D.; Goichot, B.; Riehm, S.; Petit-Thomas, J.; Averous, G.; Cavalcanti, M.; Hubele, F.; Chabrier, G.; et al. Head-to-head comparison between (18)F-FDOPA PET/CT and MR/CT angiography in clinically recurrent head and neck paragangliomas. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 979–987. [Google Scholar] [CrossRef]
- Chopra, A. [18F]6-fluoro-3-O-methyl-L-3,4-dihydroxyphenylalanine; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004.
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J.O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Tscherpel, C.; Dunkl, V.; Ceccon, G.; Stoffels, G.; Judov, N.; Rapp, M.; Meyer, P.T.; Kops, E.R.; Ermert, J.; Fink, G.R.; et al. The use of O-(2-18F-fluoroethyl)-L-tyrosine PET in the diagnosis of gliomas located in the brainstem and spinal cord. Neuro. Oncol. 2017, 19, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Liu, X.; Li, J.; Yao, H.; Yuan, S. Fluorine-18 labeled amino acids for tumor PET/CT imaging. Oncotarget 2017, 8, 60581–60588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; McConathy, J. Radiolabeled amino acids for oncologic imaging. J. Nucl. Med. 2013, 54, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Inoue, Y.; Fujimoto, H.; Yonese, J.; Tanabe, K.; Fukasawa, S.; Inoue, T.; Saito, S.; Ueno, M.; Otaka, A. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate cancer: Multicenter Phase IIb clinical trial. Jpn. J. Clin. Oncol. 2016, 46, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoup, T.M.; Olson, J.; Hoffman, J.M.; Votaw, J.; Eshima, D.; Eshima, L.; Camp, V.M.; Stabin, M.; Votaw, D.; Goodman, M.M. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J. Nucl. Med. 1999, 40, 331–338. [Google Scholar] [PubMed]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Borghesi, M.; Ambrosini, V.; Zanoni, L.; Gentile, G.; Vagnoni, V.; Romagnoli, D.; Martorana, G.; et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: A prospective study in 28 patients. Clin. Genitourin. Cancer 2014, 12, 106–110. [Google Scholar] [CrossRef]
- Schiavina, R.; Concetti, S.; Brunocilla, E.; Nanni, C.; Borghesi, M.; Gentile, G.; Cevenini, M.; Bianchi, L.; Molinaroli, E.; Fanti, S.; et al. First case of 18F-FACBC PET/CT-guided salvage retroperitoneal lymph node dissection for disease relapse after radical prostatectomy for prostate cancer and negative 11C-choline PET/CT: New imaging techniques may expand pioneering approaches. Urol. Int. 2014, 92, 242–245. [Google Scholar] [CrossRef]
- Brunocilla, E.; Schiavina, R.; Nanni, C.; Borghesi, M.; Cevenini, M.; Molinaroli, E.; Vagnoni, V.; Castellucci, P.; Ceci, F.; Fanti, S.; et al. First case of 18F-FACBC PET/CT-guided salvage radiotherapy for local relapse after radical prostatectomy with negative 11C-Choline PET/CT and multiparametric MRI: New imaging techniques may improve patient selection. Arch. Ital. Urol. Androl. 2014, 86, 239–240. [Google Scholar] [CrossRef] [Green Version]
- Nanni, C.; Zanoni, L.; Pultrone, C.; Schiavina, R.; Brunocilla, E.; Lodi, F.; Malizia, C.; Ferrari, M.; Rigatti, P.; Fonti, C.; et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: Results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1601–1610. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S. Evaluation of Prostate Cancer with Radiolabeled Amino Acid Analogs. J. Nucl. Med. 2016, 57, 61s–66s. [Google Scholar] [CrossRef] [Green Version]
- Schuster, D.M.; Nieh, P.T.; Jani, A.B.; Amzat, R.; Bowman, F.D.; Halkar, R.K.; Master, V.A.; Nye, J.A.; Odewole, O.A.; Osunkoya, A.O.; et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: Results of a prospective clinical trial. J. Urol. 2014, 191, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Nanni, C.; Schiavina, R.; Boschi, S.; Ambrosini, V.; Pettinato, C.; Brunocilla, E.; Martorana, G.; Fanti, S. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Votaw, J.R.; Nieh, P.T.; Yu, W.; Nye, J.A.; Master, V.; Bowman, F.D.; Issa, M.M.; Goodman, M.M. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007, 48, 56–63. [Google Scholar] [PubMed]
- Zhou, R.; Pantel, A.R.; Li, S.; Lieberman, B.P.; Ploessl, K.; Choi, H.; Blankemeyer, E.; Lee, H.; Kung, H.F.; Mach, R.H.; et al. [(18)F](2S,4R)4-Fluoroglutamine PET Detects Glutamine Pool Size Changes in Triple-Negative Breast Cancer in Response to Glutaminase Inhibition. Cancer Res. 2017, 77, 1476–1484. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Jenni, S.; Colovic, M.; Merkens, H.; Poleschuk, C.; Rodrigo, I.; Miao, Q.; Johnson, B.F.; Rishel, M.J.; Sossi, V.; et al. (18)F-5-Fluoroaminosuberic Acid as a Potential Tracer to Gauge Oxidative Stress in Breast Cancer Models. J. Nucl. Med. 2017, 58, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.M.; Morton, C.A.; Johnson, B.F.; Yang, H.; Rishel, M.J.; Lee, B.D.; Miao, Q.; Pabba, C.; Yapp, D.T.; Schaffer, P. Functional imaging of oxidative stress with a novel PET imaging agent, 18F-5-fluoro-L-aminosuberic acid. J. Nucl. Med. 2014, 55, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulaner, G.A.; Goldman, D.A.; Gonen, M.; Pham, H.; Castillo, R.; Lyashchenko, S.K.; Lewis, J.S.; Dang, C. Initial Results of a Prospective Clinical Trial of 18F-Fluciclovine PET/CT in Newly Diagnosed Invasive Ductal and Invasive Lobular Breast Cancers. J. Nucl. Med. 2016, 57, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Tade, F.I.; Cohen, M.A.; Styblo, T.M.; Odewole, O.A.; Holbrook, A.I.; Newell, M.S.; Savir-Baruch, B.; Li, X.; Goodman, M.M.; Nye, J.A.; et al. Anti-3-18F-FACBC (18F-Fluciclovine) PET/CT of Breast Cancer: An Exploratory Study. J. Nucl. Med. 2016, 57, 1357–1363. [Google Scholar] [CrossRef] [Green Version]
- McConathy, J. 18F-Fluciclovine (FACBC) and Its Potential Use for Breast Cancer Imaging. J. Nucl. Med. 2016, 57, 1329–1330. [Google Scholar] [CrossRef] [Green Version]
- Kenny, L.M.; Aboagye, E.O.; Price, P.M. Positron emission tomography imaging of cell proliferation in oncology. Clin. Oncol. 2004, 16, 176–185. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Shields, A.F.; Link, J.M.; Graham, M.M.; Muzi, M.; Peterson, L.M.; Eary, J.F.; Krohn, K.A. Kinetic analysis of 2-[11C]thymidine PET imaging studies: Validation studies. J. Nucl. Med. 1999, 40, 614–624. [Google Scholar] [PubMed]
- Mach, R.H.; Dehdashti, F.; Wheeler, K.T. PET Radiotracers for Imaging the Proliferative Status of Solid Tumors. PET Clin. 2009, 4, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bading, J.R.; Shields, A.F. Imaging of cell proliferation: Status and prospects. J. Nucl. Med. 2008, 49 (Suppl. S2), 64s–80s. [Google Scholar] [CrossRef] [Green Version]
- Linden, H.M.; Dehdashti, F. Novel methods and tracers for breast cancer imaging. Semin. Nucl. Med. 2013, 43, 324–329. [Google Scholar] [CrossRef]
- Kenny, L. The Use of Novel PET Tracers to Image Breast Cancer Biologic Processes Such as Proliferation, DNA Damage and Repair, and Angiogenesis. J. Nucl. Med. 2016, 57 (Suppl. S1), 89S–95S. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Lin, L.; Snider, J.; Prat, A.; Parker, J.S.; Luo, J.; DeSchryver, K.; Allred, D.C.; et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 2011, 29, 2342–2349. [Google Scholar] [CrossRef] [Green Version]
- Pio, B.S.; Park, C.K.; Pietras, R.; Hsueh, W.A.; Satyamurthy, N.; Pegram, M.D.; Czernin, J.; Phelps, M.E.; Silverman, D.H. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol. Imaging Biol. 2006, 8, 36–42. [Google Scholar] [CrossRef]
- Kenny, L.; Coombes, R.C.; Vigushin, D.M.; Al-Nahhas, A.; Shousha, S.; Aboagye, E.O. Imaging early changes in proliferation at 1 week post chemotherapy: A pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sloan, A.; Mangner, T.J.; Vaishampayan, U.; Muzik, O.; Collins, J.M.; Douglas, K.; Shields, A.F. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 15–22. [Google Scholar] [CrossRef]
- Karakashev, S.V.; Reginato, M.J. Progress toward overcoming hypoxia-induced resistance to solid tumor therapy. Cancer Manag. Res. 2015, 7, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Generali, D.; Symmans, W.F.; Berruti, A.; Fox, S.B. Predictive immunohistochemical biomarkers in the context of neoadjuvant therapy for breast cancer. J. Natl. Cancer Inst. Monogr. 2011, 2011, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.R.; Chakraborty, G.; Wang, C.H.; Rockne, R.; Harpold, H.L.; Muzi, M.; Adamsen, T.C.; Krohn, K.A.; Spence, A.M. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J. Nucl. Med. 2009, 50, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Lei, L.; Xu, J.; Sun, Y.; Zhang, Y.; Wang, X.; Pan, L.; Shao, Z.; Zhang, Y.; Liu, G. 18F-fluoromisonidazole PET/CT: A potential tool for predicting primary endocrine therapy resistance in breast cancer. J. Nucl. Med. 2013, 54, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Halmos, G.B.; Bruine de Bruin, L.; Langendijk, J.A.; van der Laan, B.F.; Pruim, J.; Steenbakkers, R.J. Head and neck tumor hypoxia imaging by 18F-fluoroazomycin-arabinoside (18F-FAZA)-PET: A review. Clin. Nucl. Med. 2014, 39, 44–48. [Google Scholar] [CrossRef]
- Postema, E.J.; McEwan, A.J.; Riauka, T.A.; Kumar, P.; Richmond, D.A.; Abrams, D.N.; Wiebe, L.I. Initial results of hypoxia imaging using 1-alpha-D: -(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA). Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1565–1573. [Google Scholar] [CrossRef]
- Hu, M.; Xing, L.; Mu, D.; Yang, W.; Yang, G.; Kong, L.; Yu, J. Hypoxia imaging with 18F-fluoroerythronitroimidazole integrated PET/CT and immunohistochemical studies in non-small cell lung cancer. Clin. Nucl. Med. 2013, 38, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Terasaki, K.; Sasaki, T.; Fujiwara, S.; Matsuura, H.; Ogasawara, K.; Sera, K.; Yamada, N.; Uesugi, N.; Sugai, T.; et al. Standardized uptake value in high uptake area on positron emission tomography with 18F-FRP170 as a hypoxic cell tracer correlates with intratumoral oxygen pressure in glioblastoma. Mol. Imaging Biol. 2014, 16, 127–135. [Google Scholar] [CrossRef]
- Zegers, C.M.; van Elmpt, W.; Wierts, R.; Reymen, B.; Sharifi, H.; Ollers, M.C.; Hoebers, F.; Troost, E.G.; Wanders, R.; van Baardwijk, A.; et al. Hypoxia imaging with [(1)(8)F]HX4 PET in NSCLC patients: Defining optimal imaging parameters. Radiother. Oncol. 2013, 109, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Lohith, T.G.; Kudo, T.; Demura, Y.; Umeda, Y.; Kiyono, Y.; Fujibayashi, Y.; Okazawa, H. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. J. Nucl. Med. 2009, 50, 1948–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehdashti, F.; Grigsby, P.W.; Lewis, J.S.; Laforest, R.; Siegel, B.A.; Welch, M.J. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2008, 49, 201–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, D.W.; Dehdashti, F.; Grigsby, P.W.; Malyapa, R.S.; Myerson, R.J.; Picus, J.; Ritter, J.; Lewis, J.S.; Welch, M.J.; Siegel, B.A. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: A pilot study. Dis. Colon Rectum 2008, 51, 1641–1648. [Google Scholar] [CrossRef] [Green Version]
- Tateishi, K.; Tateishi, U.; Sato, M.; Yamanaka, S.; Kanno, H.; Murata, H.; Inoue, T.; Kawahara, N. Application of 62Cu-diacetyl-bis (N4-methylthiosemicarbazone) PET imaging to predict highly malignant tumor grades and hypoxia-inducible factor-1alpha expression in patients with glioma. AJNR Am. J. Neuroradiol. 2013, 34, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Taillefer, R. Clinical applications of 99mTc-sestamibi scintimammography. Semin. Nucl. Med. 2005, 35, 100–115. [Google Scholar] [CrossRef]
- Khalkhali, I.; Baum, J.K.; Villanueva-Meyer, J.; Edell, S.L.; Hanelin, L.G.; Lugo, C.E.; Taillefer, R.; Freeman, L.M.; Neal, C.E.; Scheff, A.M.; et al. (99m)Tc sestamibi breast imaging for the examination of patients with dense and fatty breasts: Multicenter study. Radiology 2002, 222, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Ferretti, G.; Povolato, M.; Marzola, M.C.; Zucchetta, P.; Geatti, O.; Brandes, A.A.; Bui, F. Accuracy of technetium-99m sestamibi scintimammography and X-ray mammography in premenopausal women with suspected breast cancer. Eur. J. Nucl. Med. 2001, 28, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, E.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Buscombe, J.; Chatal, J.F.; Maffioli, L.; Moncayo, R.; Mortelmans, L.; Reske, S.N. Breast scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 107–114. [Google Scholar] [CrossRef]
- Spanu, A.; Dettori, G.; Nuvoli, S.; Porcu, A.; Falchi, A.; Cottu, P.; Solinas, M.E.; Scanu, A.M.; Chessa, F.; Madeddu, G. (99)mTc-tetrofosmin SPET in the detection of both primary breast cancer and axillary lymph node metastasis. Eur. J. Nucl. Med. 2001, 28, 1781–1794. [Google Scholar] [CrossRef]
- Spanu, A.; Schillaci, O.; Meloni, G.B.; Porcu, A.; Cottu, P.; Nuvoli, S.; Falchi, A.; Chessa, F.; Solinas, M.E.; Madeddu, G. The usefulness of 99mTc-tetrofosmin SPECT scintimammography in the detection of small size primary breast carcinomas. Int. J. Oncol. 2002, 21, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Spanu, A. Use of tomographic nuclear medicine procedures, SPECT and pinhole SPECT, with cationic lipophilic radiotracers for the evaluation of axillary lymph node status in breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2004, 31 (Suppl. S1), S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Sanna, D.; Chessa, F.; Farris, A.; Nuvoli, S.; Madeddu, G. The usefulness of Tc-99m-tetrofosmin SPECT/CT in the detection of residual tumors and axillary lymph node metastases in breast cancer patients following neoadjuvant therapy. Clin. Nucl. Med. 2011, 36, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Farris, A.; Chessa, F.; Sanna, D.; Pittalis, M.; Manca, A.; Madeddu, G. Planar scintimammography and SPECT in neoadjuvant chemo or hormonotherapy response evaluation in locally advanced primary breast cancer. Int. J. Oncol. 2008, 32, 1275–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanu, A.; Chessa, F.; Meloni, G.B.; Sanna, D.; Cottu, P.; Manca, A.; Nuvoli, S.; Madeddu, G. The role of planar scintimammography with high-resolution dedicated breast camera in the diagnosis of primary breast cancer. Clin. Nucl. Med. 2008, 33, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Cottu, P.; Manca, A.; Chessa, F.; Sanna, D.; Madeddu, G. Scintimammography with dedicated breast camera in unifocal and multifocal/multicentric primary breast cancer detection: A comparative study with SPECT. Int. J. Oncol. 2007, 31, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Spanu, A.; Sanna, D.; Chessa, F.; Cottu, P.; Manca, A.; Madeddu, G. Breast scintigraphy with breast-specific γ-camera in the detection of ductal carcinoma in situ: A correlation with mammography and histologic subtype. J. Nucl. Med. 2012, 53, 1528–1533. [Google Scholar] [CrossRef] [Green Version]
- Spanu, A.; Sanna, D.; Chessa, F.; Manca, A.; Cottu, P.; Fancellu, A.; Nuvoli, S.; Madeddu, G. The clinical impact of breast scintigraphy acquired with a breast specific γ-camera (BSGC) in the diagnosis of breast cancer: Incremental value versus mammography. Int. J. Oncol. 2012, 41, 483–489. [Google Scholar] [CrossRef]
- Rhodes, D.J.; Hruska, C.B.; Conners, A.L.; Tortorelli, C.L.; Maxwell, R.W.; Jones, K.N.; Toledano, A.Y.; O’Connor, M.K. Journal club: Molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR Am. J. Roentgenol. 2015, 204, 241–251. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, M.K. Molecular breast imaging: An emerging modality for breast cancer screening. Breast Cancer Manag. 2015, 4, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhan, H.; Sun, D. Comparison of (99m)Tc-MIBI scintigraphy, ultrasound, and mammography for the diagnosis of BI-RADS 4 category lesions. BMC Cancer 2020, 20, 463. [Google Scholar] [CrossRef]
- Nuvoli, S.; Galassi, S.; Gelo, I.; Rocchitta, G.; Fancellu, A.; Serra, P.A.; Madeddu, G.; Spanu, A. The role of molecular breast imaging in predicting complete tumor response to treatment and residual tumor extent following neoadjuvant therapy. Oncol. Rep. 2018, 39, 2055–2062. [Google Scholar] [CrossRef]
- Piwnica-Worms, D.; Chiu, M.L.; Budding, M.; Kronauge, J.F.; Kramer, R.A.; Croop, J.M. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res. 1993, 53, 977–984. [Google Scholar] [PubMed]
- Ballinger, J.R.; Bannerman, J.; Boxen, I.; Firby, P.; Hartman, N.G.; Moore, M.J. Technetium-99m-tetrofosmin as a substrate for P-glycoprotein: In vitro studies in multidrug-resistant breast tumor cells. J. Nucl. Med. 1996, 37, 1578–1582. [Google Scholar]
- Del Vecchio, S.; Salvatore, M. 99mTc-MIBI in the evaluation of breast cancer biology. Eur. J. Nucl. Med. Mol. Imaging 2004, 31 (Suppl. S1), S88–S96. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Semenenko, I.; Meir, M.; Eyal, S. Molecular Imaging of Membrane Transporters’ Activity in Cancer: A Picture is Worth a Thousand Tubes. AAPS J. 2015, 17, 788–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Hong, S.P.; Lee, J.H.; Moon, S.H.; Cho, Y.S.; Jung, K.H.; Lee, J.; Lee, K.H. 99mTc-MIBI uptake as a marker of mitochondrial membrane potential in cancer cells and effects of MDR1 and verapamil. PLoS ONE 2020, 15, e0228848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordobes, M.D.; Starzec, A.; Delmon-Moingeon, L.; Blanchot, C.; Kouyoumdjian, J.C.; Prévost, G.; Caglar, M.; Moretti, J.L. Technetium-99m-sestamibi uptake by human benign and malignant breast tumor cells: Correlation with mdr gene expression. J. Nucl. Med. 1996, 37, 286–289. [Google Scholar] [PubMed]
- Gustafsson, J.A.; Warner, M. Estrogen receptor beta in the breast: Role in estrogen responsiveness and development of breast cancer. J. Steroid Biochem. Mol. Biol. 2000, 74, 245–248. [Google Scholar] [CrossRef]
- Bange, J.; Zwick, E.; Ullrich, A. Molecular targets for breast cancer therapy and prevention. Nat. Med. 2001, 7, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Rozeboom, B.; Dey, N.; De, P. ER+ metastatic breast cancer: Past, present, and a prescription for an apoptosis-targeted future. Am. J. Cancer Res. 2019, 9, 2821–2831. [Google Scholar]
- McGuire, W.L.; Clark, G.M. Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N. Engl. J. Med. 1992, 326, 1756–1761. [Google Scholar] [CrossRef]
- Clarke, R.B.; Howell, A.; Potten, C.S.; Anderson, E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997, 57, 4987–4991. [Google Scholar]
- Fuqua, S.A.; Cui, Y. Estrogen and progesterone receptor isoforms: Clinical significance in breast cancer. Breast Cancer Res. Treat. 2004, 87 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Akazawa, K.; Kamigaki, S.; Ueda, S.; Yanagisawa, T.; Inoue, T.; Yamamura, J.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Prognostic significance of intra-tumoral estradiol level in breast cancer patients. Cancer Lett. 2004, 216, 115–121. [Google Scholar] [CrossRef]
- Amir, E.; Miller, N.; Geddie, W.; Freedman, O.; Kassam, F.; Simmons, C.; Oldfield, M.; Dranitsaris, G.; Tomlinson, G.; Laupacis, A.; et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 2012, 30, 587–592. [Google Scholar] [CrossRef]
- Cardoso, F.; Costa, A.; Norton, L.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.H.; Bergh, J.; Biganzoli, L.; Blackwell, K.L.; et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014, 23, 489–502. [Google Scholar] [CrossRef]
- Hoefnagel, L.D.; van de Vijver, M.J.; van Slooten, H.J.; Wesseling, P.; Wesseling, J.; Westenend, P.J.; Bart, J.; Seldenrijk, C.A.; Nagtegaal, I.D.; Oudejans, J.; et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010, 12, R75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoubina, E.V.; Smith, P.G. Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J. Urol. 2003, 169, 382–385. [Google Scholar] [CrossRef]
- Pettersson, K.; Gustafsson, J.A. Role of estrogen receptor beta in estrogen action. Annu. Rev. Physiol. 2001, 63, 165–192. [Google Scholar] [CrossRef]
- Dehdashti, F.; Flanagan, F.L.; Mortimer, J.E.; Katzenellenbogen, J.A.; Welch, M.J.; Siegel, B.A. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur. J. Nucl. Med. 1999, 26, 51–56. [Google Scholar] [CrossRef]
- Seimbille, Y.; Rousseau, J.; Benard, F.; Morin, C.; Ali, H.; Avvakumov, G.; Hammond, G.L.; van Lier, J.E. 18F-labeled difluoroestradiols: Preparation and preclinical evaluation as estrogen receptor-binding radiopharmaceuticals. Steroids 2002, 67, 765–775. [Google Scholar] [CrossRef]
- Kurland, B.F.; Peterson, L.M.; Lee, J.H.; Linden, H.M.; Schubert, E.K.; Dunnwald, L.K.; Link, J.M.; Krohn, K.A.; Mankoff, D.A. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J. Nucl. Med. 2011, 52, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Salem, K.; Kumar, M.; Kloepping, K.C.; Michel, C.J.; Yan, Y.; Fowler, A.M. Determination of binding affinity of molecular imaging agents for steroid hormone receptors in breast cancer. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 119–126. [Google Scholar]
- Gemignani, M.L.; Patil, S.; Seshan, V.E.; Sampson, M.; Humm, J.L.; Lewis, J.S.; Brogi, E.; Larson, S.M.; Morrow, M.; Pandit-Taskar, N. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J. Nucl. Med. 2013, 54, 1697–1702. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Kiyono, Y.; Tsujikawa, T.; Kurokawa, T.; Okazawa, H.; Kotsuji, F. Additional value of 16alpha-[18F]fluoro-17beta-oestradiol PET for differential diagnosis between uterine sarcoma and leiomyoma in patients with positive or equivocal findings on [18F]fluorodeoxyglucose PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1824–1831. [Google Scholar] [CrossRef]
- Peterson, L.M.; Mankoff, D.A.; Lawton, T.; Yagle, K.; Schubert, E.K.; Stekhova, S.; Gown, A.; Link, J.M.; Tewson, T.; Krohn, K.A. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J. Nucl. Med. 2008, 49, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Dehdashti, F.; Mortimer, J.E.; Trinkaus, K.; Naughton, M.J.; Ellis, M.; Katzenellenbogen, J.A.; Welch, M.J.; Siegel, B.A. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res. Treat. 2009, 113, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankoff, D.A.; Tewson, T.J.; Eary, J.F. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES). Nucl. Med. Biol. 1997, 24, 341–348. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Neto, C.; Ribeiro Morais, G.; Thiemann, T. Steroid receptor ligands for breast cancer targeting: An insight into their potential role as PET imaging agents. Curr. Med. Chem. 2013, 20, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Phoenix, S.; Ouellet, R.; Langlois, R.; van Lier, J.E.; Turcotte, E.E.; Benard, F.; Lecomte, R. Assessment of the novel estrogen receptor PET tracer 4-fluoro-11beta-methoxy-16alpha-[(18)F]fluoroestradiol (4FMFES) by PET imaging in a breast cancer murine model. Mol. Imaging Biol. 2013, 15, 625–632. [Google Scholar] [CrossRef]
- Xu, D.; Zhuang, R.; You, L.; Guo, Z.; Wang, X.; Peng, C.; Zhang, D.; Zhang, P.; Wu, H.; Pan, W.; et al. (18)F-labeled estradiol derivative for targeting estrogen receptor-expressing breast cancer. Nucl. Med. Biol. 2018, 59, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Feng, H.; Li, C.; Qin, C.; Song, Y.; Zhang, Y.; Lan, X. 99mTc-labeled estradiol as an estrogen receptor probe: Preparation and preclinical evaluation. Nucl. Med. Biol. 2016, 43, 89–96. [Google Scholar] [CrossRef]
- Van Kruchten, M.; Glaudemans, A.W.; de Vries, E.F.; Beets-Tan, R.G.; Schroder, C.P.; Dierckx, R.A.; de Vries, E.G.; Hospers, G.A. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J. Nucl. Med. 2012, 53, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Chi, D.Y.; Dence, C.S.; Welch, M.J.; Katzenellenbogen, J.A. Synthesis and biodistribution of fluorine-18-labeled fluorocyclofenils for imaging the estrogen receptor. Nucl. Med. Biol. 2007, 34, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Moon, B.S.; Carlson, K.E.; Katzenellenbogen, J.A.; Choi, T.H.; Chi, D.Y.; Kim, J.Y.; Cheon, G.J.; Koh, H.Y.; Lee, K.C.; An, G. Synthesis and evaluation of aryl-substituted diarylpropionitriles, selective ligands for estrogen receptor beta, as positron-emission tomographic imaging agents. Bioorg. Med. Chem. 2009, 17, 3479–3488. [Google Scholar] [CrossRef]
- Lee, J.H.; Peters, O.; Lehmann, L.; Dence, C.S.; Sharp, T.L.; Carlson, K.E.; Zhou, D.; Jeyakumar, M.; Welch, M.J.; Katzenellenbogen, J.A. Synthesis and biological evaluation of two agents for imaging estrogen receptor beta by positron emission tomography: Challenges in PET imaging of a low abundance target. Nucl. Med. Biol. 2012, 39, 1105–1116. [Google Scholar] [CrossRef] [Green Version]
- Skaddan, M.B.; Wüst, F.R.; Jonson, S.; Syhre, R.; Welch, M.J.; Spies, H.; Katzenellenbogen, J.A. Radiochemical synthesis and tissue distribution of Tc-99m-labeled 7alpha-substituted estradiol complexes. Nucl. Med. Biol. 2000, 27, 269–278. [Google Scholar] [CrossRef]
- Nayak, T.K.; Hathaway, H.J.; Ramesh, C.; Arterburn, J.B.; Dai, D.; Sklar, L.A.; Norenberg, J.P.; Prossnitz, E.R. Preclinical development of a neutral, estrogen receptor-targeted, tridentate 99mTc(I)-estradiol-pyridin-2-yl hydrazine derivative for imaging of breast and endometrial cancers. J. Nucl. Med. 2008, 49, 978–986. [Google Scholar] [CrossRef] [Green Version]
- McKenna, N.J.; O’Malley, B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002, 108, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.; Gano, L.; Morais, G.R.; Thiemann, T.; Oliveira, M.C. Progesterone receptor targeting with radiolabelled steroids: An approach in predicting breast cancer response to therapy. J. Steroid Biochem. Mol. Biol. 2013, 137, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef]
- Natrajan, R.; Weigelt, B.; Mackay, A.; Geyer, F.C.; Grigoriadis, A.; Tan, D.S.; Jones, C.; Lord, C.J.; Vatcheva, R.; Rodriguez-Pinilla, S.M.; et al. An integrative genomic and transcriptomic analysis reveals molecular pathways and networks regulated by copy number aberrations in basal-like, HER2 and luminal cancers. Breast Cancer Res. Treat. 2010, 121, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Regan, M.M.; Maiorano, E.; Mastropasqua, M.G.; Dell’Orto, P.; Rasmussen, B.B.; Raffoul, J.; Neven, P.; Orosz, Z.; Braye, S.; et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J. Clin. Oncol. 2007, 25, 3846–3852. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.; Tripathy, D. Aromatase inhibitors: Rationale and use in breast cancer. Annu. Rev. Med. 2005, 56, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.B.; Anderson, E.; Howell, A. Steroid receptors in human breast cancer. Trends Endocrinol. Metab. 2004, 15, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Pomper, M.G.; Katzenellenbogen, J.A.; Welch, M.J.; Brodack, J.W.; Mathias, C.J. 21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone: Synthesis and target tissue selective uptake of a progestin receptor based radiotracer for positron emission tomography. J. Med. Chem. 1988, 31, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.S.; Bonasera, T.A.; Chi, D.Y.; Welch, M.J.; Katzenellenbogen, J.A. 6 alpha-[18F]fluoroprogesterone: Synthesis via halofluorination-oxidation, receptor binding and tissue distribution. Nucl. Med. Biol. 1995, 22, 635–642. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhou, H.B.; Dence, C.S.; Carlson, K.E.; Welch, M.J.; Katzenellenbogen, J.A. Development of [F-18]fluorine-substituted Tanaproget as a progesterone receptor imaging agent for positron emission tomography. Bioconjug. Chem. 2010, 21, 1096–1104. [Google Scholar] [CrossRef]
- Dehdashti, F.; Laforest, R.; Gao, F.; Aft, R.L.; Dence, C.S.; Zhou, D.; Shoghi, K.I.; Siegel, B.A.; Katzenellenbogen, J.A.; Welch, M.J. Assessment of progesterone receptors in breast carcinoma by PET with 21-18F-fluoro-16alpha,17alpha-[(R)-(1′-alpha-furylmethylidene)dioxy]-19-norpregn- 4-ene-3,20-dione. J. Nucl. Med. 2012, 53, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Fowler, A.M.; Chan, S.R.; Sharp, T.L.; Fettig, N.M.; Zhou, D.; Dence, C.S.; Carlson, K.E.; Jeyakumar, M.; Katzenellenbogen, J.A.; Schreiber, R.D.; et al. Small-Animal PET of Steroid Hormone Receptors Predicts Tumor Response to Endocrine Therapy Using a Preclinical Model of Breast Cancer. J. Nucl. Med. 2012, 53, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The trastuzumab era: Current and upcoming targeted HER2+ breast cancer therapies. Am. J. Cancer Res. 2020, 10, 1045–1067. [Google Scholar] [PubMed]
- Velikyan, I.; Schweighöfer, P.; Feldwisch, J.; Seemann, J.; Frejd, F.Y.; Lindman, H.; Sörensen, J. Diagnostic HER2-binding radiopharmaceutical, [(68)Ga]Ga-ABY-025, for routine clinical use in breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 12–23. [Google Scholar]
- Capala, J.; Bouchelouche, K. Molecular imaging of HER2-positive breast cancer: A step toward an individualized ‘image and treat’ strategy. Curr. Opin. Oncol. 2010, 22, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.J.; Krueger, J.S.; Landis, N.D.; Eberhard, D.A.; Young, G.D.; Schmechel, S.C.; Lange, H. Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab. Investig. J. Technical Methods Pathol. 2012, 92, 1342–1357. [Google Scholar] [CrossRef] [Green Version]
- Ejlertsen, B.; Jensen, M.B.; Nielsen, K.V.; Balslev, E.; Rasmussen, B.B.; Willemoe, G.L.; Hertel, P.B.; Knoop, A.S.; Mouridsen, H.T.; Brunner, N. HER2, TOP2A, and TIMP-1 and responsiveness to adjuvant anthracycline-containing chemotherapy in high-risk breast cancer patients. J. Clin. Oncol. 2010, 28, 984–990. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Muller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Smith-Jones, P.M.; Solit, D.; Afroze, F.; Rosen, N.; Larson, S.M. Early tumor response to Hsp90 therapy using HER2 PET: Comparison with 18F-FDG PET. J. Nucl. Med. 2006, 47, 793–796. [Google Scholar]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET Imaging of HER2-Positive Lesions in Patients With Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Yang, M.; Gao, H.; Zhou, Y.; Ma, Y.; Quan, Q.; Lang, L.; Chen, K.; Niu, G.; Yan, Y.; Chen, X. F-Labeled GRPR Agonists and Antagonists: A Comparative Study in Prostate Cancer Imaging. Theranostics 2011, 1, 220–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar] [PubMed]
- Carlucci, G.; Kuipers, A.; Ananias, H.J.; de Paula Faria, D.; Dierckx, R.A.; Helfrich, W.; Rink, R.; Moll, G.N.; de Jong, I.J.; Elsinga, P.H. GRPR-selective PET imaging of prostate cancer using [(18)F]-lanthionine-bombesin analogs. Peptides 2015, 67, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pourghiasian, M.; Liu, Z.; Pan, J.; Zhang, Z.; Colpo, N.; Lin, K.S.; Perrin, D.M.; Benard, F. (18)F-AmBF3-MJ9: A novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg. Med. Chem. 2015, 23, 1500–1506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, D.; Lang, L.; Zhu, Z.; Wang, L.; Wu, P.; Niu, G.; Li, F.; Chen, X. 68Ga-NOTA-Aca-BBN(7-14) PET/CT in Healthy Volunteers and Glioma Patients. J. Nucl. Med. 2016, 57, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Wiele, C.; Phonteyne, P.; Pauwels, P.; Goethals, I.; Van den Broecke, R.; Cocquyt, V.; Dierckx, R.A. Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry. J. Nucl. Med. 2008, 49, 260–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.; Baquero-Buitrago, J.; Ben Youss Gironda, Z.; Wadghiri, Y.Z.; Reiner, T.; Boada, F.E.; Carlucci, G. Noninvasive PET Imaging of CDK4/6 Activation in Breast Cancer. J. Nucl. Med. 2020, 61, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F. Dual-targeted molecular probes for cancer imaging. Curr. Pharm. Biotechnol. 2010, 11, 610–619. [Google Scholar] [CrossRef]
- Lucente, E.; Liu, H.; Liu, Y.; Hu, X.; Lacivita, E.; Leopoldo, M.; Cheng, Z. Novel (64)Cu Labeled RGD(2)-BBN Heterotrimers for PET Imaging of Prostate Cancer. Bioconjug. Chem. 2018, 29, 1595–1604. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.B.; Cao, Q.; Liu, S.; Wang, F.; Chen, X. Small-animal PET of tumors with (64)Cu-labeled RGD-bombesin heterodimer. J. Nucl. Med. 2009, 50, 1168–1177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Mao, F.; Niu, G.; Peng, L.; Lang, L.; Li, F.; Ying, H.; Wu, H.; Pan, B.; Zhu, Z.; et al. (68)Ga-BBN-RGD PET/CT for GRPR and Integrin alphavbeta3 Imaging in Patients with Breast Cancer. Theranostics 2018, 8, 1121–1130. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Y.; Liu, S.; Wang, F.; Chen, X. (18)F, (64)Cu, and (68)Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug. Chem. 2009, 20, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Gai, Y.; Jiang, Y.; Long, Y.; Sun, L.; Liu, Q.; Qin, C.; Zhang, Y.; Zeng, D.; Lan, X. Evaluation of an Integrin α(v)β(3) and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Mol. Pharm. 2020, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.; Sun, S.; Lee, S.; Bodner, J.; Li, Z.; Huang, Y.; Moores, S.L.; Marquez-Nostra, B. Development of [(89)Zr]ZrDFO-amivantamab bispecific to EGFR and c-MET for PET imaging of triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Meng, Q.F.; Bu, L.L.; Cai, B.; Huang, Q.; Sun, Z.J.; Zhang, W.F.; Li, A.; Guo, S.S.; Liu, W.; et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Ying, M.; Zhuang, J.; Wei, X.; Zhang, X.; Zhang, Y.; Jiang, Y.; Dehaini, D.; Chen, M.; Gu, S.; Gao, W.; et al. Remote-Loaded Platelet Vesicles for Disease-Targeted Delivery of Therapeutics. Adv. Funct. Mater. 2018, 28, 1801032. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H.; et al. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef]
- Fang, H.; Li, M.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Zhang, X.; Ye, M.; Zhang, Y.; Gao, M.; et al. Ultra-sensitive Nanoprobe Modified with Tumor Cell Membrane for UCL/MRI/PET Multimodality Precise Imaging of Triple-Negative Breast Cancer. Nano-Micro. Lett. 2020, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.S.; Jeong, J.M.; et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci. Rep. 2015, 5, 15636. [Google Scholar] [CrossRef]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, D.; Szigeti, K. Radiolabeling of Extracellular Vesicles with (99m)Tc for Quantitative In Vivo Imaging Studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Faruqu, F.N.; Wang, J.T.; Xu, L.; McNickle, L.; Chong, E.M.; Walters, A.; Gurney, M.; Clayton, A.; Smyth, L.A.; Hider, R.; et al. Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice—A Novel and Universal Approach. Theranostics 2019, 9, 1666–1682. [Google Scholar] [CrossRef]
- Shi, S.; Li, T.; Wen, X.; Wu, S.Y.; Xiong, C.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.; Sood, A.K.; et al. Copper-64 Labeled PEGylated Exosomes for In Vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjug. Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Aruva, M.R.; Qin, W.; Zhu, W.; Duffy, K.T.; Sauter, E.R.; Thakur, M.L.; Wickstrom, E. External imaging of CCND1 cancer gene activity in experimental human breast cancer xenografts with 99mTc-peptide-peptide nucleic acid-peptide chimeras. J. Nucl. Med. 2004, 45, 2070–2082. [Google Scholar] [PubMed]
- Jiang, Y.; Gai, Y.; Long, Y.; Liu, Q.; Liu, C.; Zhang, Y.; Lan, X. Application and Evaluation of [(99m)Tc]-Labeled Peptide Nucleic Acid Targeting MicroRNA-155 in Breast Cancer Imaging. Mol. Imaging 2020, 19, 1536012120916124. [Google Scholar] [CrossRef] [PubMed]



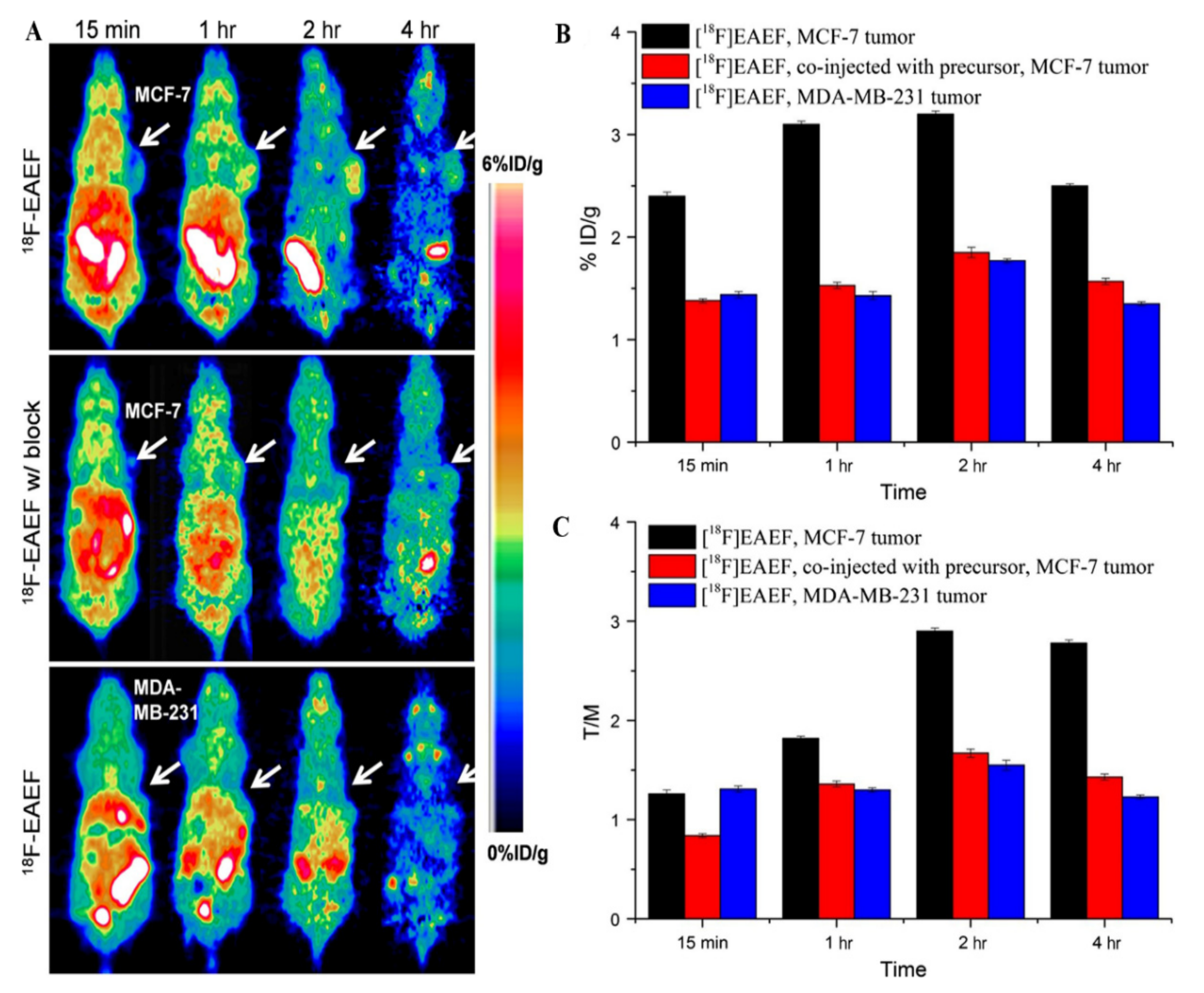


| Receptor Type | Target | Imaging Modality | Imaging Targeting Agents | Examples | Characteristics | Limitations | Ref. |
|---|---|---|---|---|---|---|---|
| Imaging biological processes | Glycolysis | PET | Glucose analog | 18F-FDG | 1. Reflects cellular glycolysis 2. Has been applied in breast cancer screening, staging, molecular subtype determination, and treatment monitoring | 1. Not supposed to diagnose inflammatory breast cancer 2. Limited spatial resolution | [6] |
| Amino acid transporter | PET | Methionine | 11C-MET | Can be used for predicting the early treatment response | Short half-life of 11C | [7] | |
| Leucine analog | 18F-fluciclovine | 1. Long half-life 2. Detects bone, lung, brain, and axillary nodal metastases | Limitations in detecting liver metastases | [8] | |||
| Cellular proliferation | PET | Thymidine analog | 18F-FLT | Can visualize the status of cell proliferation and avoid the false positive results occurring in 18F-FDG imaging | Physiological uptake occurs in highly proliferative tissues | [9] | |
| Hypoxia | PET | Small molecules | 18F-FMISO | Evaluation of tumor hypoxia in vivo | 1. Slow clearance from the blood 2. Modest hypoxic-to-normoxic ratio and limited contrast images | [10] | |
| Imaging receptors | ER | PET | Estradiol | 18F-FES | The sensitivity and specificity of 18F-FES for tumor detection were 69–100% and 80–100% | 1. Lack of precise SUV (standardized uptake value) thresholds to distinguish specific uptake from nonspecific uptake 2. Less selectivity for ERα and ERβ | [11] |
| SPECT | Estradiol | 99mTc-DTPA-estradiol | Satisfactory labeling efficiency and stability | High background/liver uptake | [12] | ||
| PR | PET | Progestin | 18F-FENP | A high binding affinity for PR | 1. High lipophilicity and metabolic liability led to increased adipose tissue and liver uptake 2. Low target/background ratio | [13] | |
| Progestin | 18F-FFNP | Specifically binds to PR with high affinity and high selectivity | Small sample size | [14] | |||
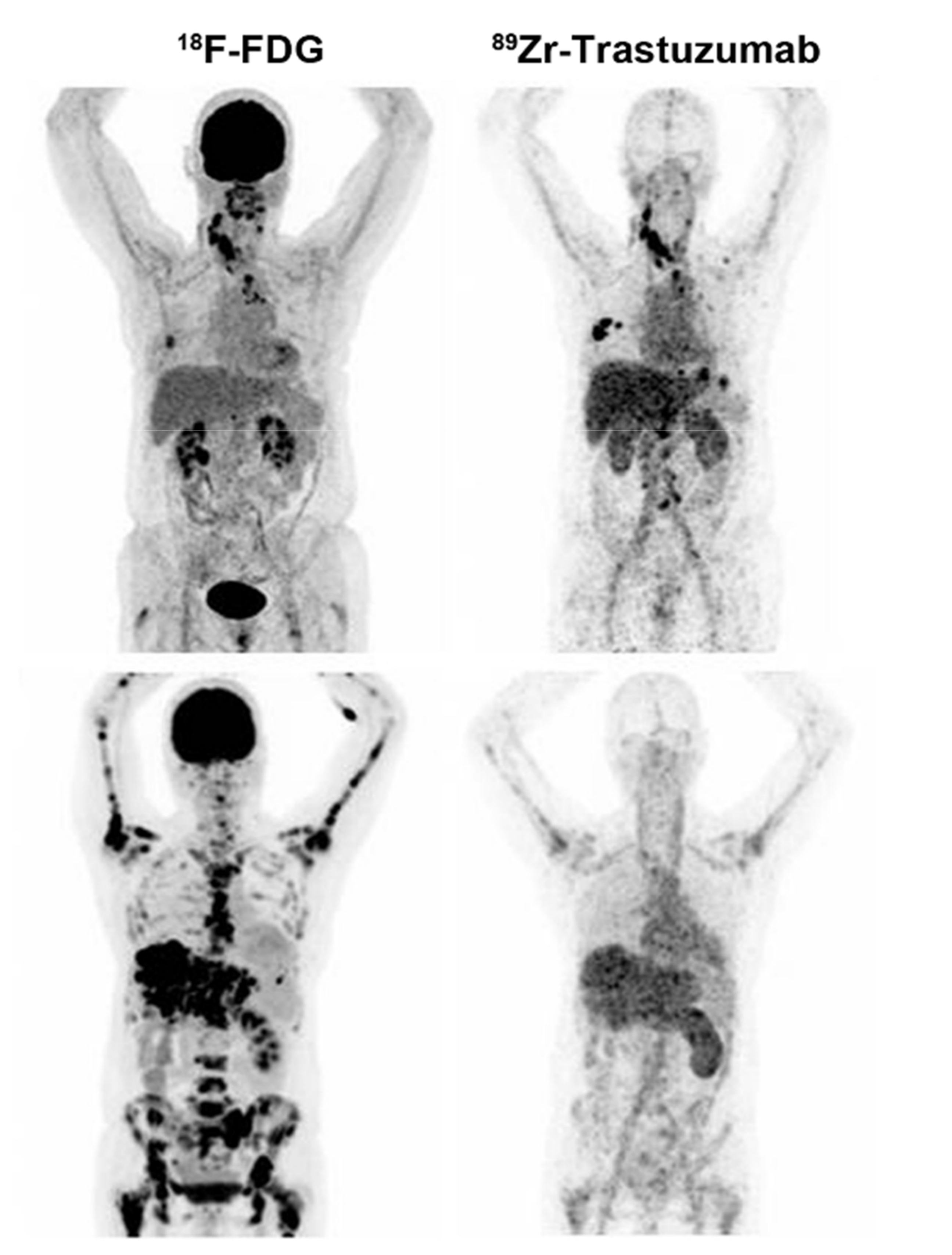
| HER2 | PET | Antibody | 89Zr-trastuzumab | 1. Radiolabeling efficiency: 77.6 ± 3.9% 2. Radiochemical purity: 98.1 ± 1.1% | 1. Low sensitivity 2. Liver and spleen had higher uptake | [15] | |
| Antibody fragments | 68Ga-DOTA-F(ab’)2-trastuzumab | Can identify HER2 downregulation by Hsp90 inhibition | Lack of sufficient sensitivity for clinical use | [16] | |||
| SPECT | Antibody | 111In-DPTA-trastuzumab | 1. High stability 2. High labeling yields 3. Maintains immunoreactivity and internalization properties | 1. Low sensitivity 2. Low tumor-to-blood ratio 3. Liver, kidney, and spleen had tracer uptake | [17] | ||
| Other receptors | PET | Antibody | 89Zr-labeled atezolizumab (targeting PD-L1) | Can help assess the PD-L1 status and clinical response predictions | 1. A small patient population 2. No tumor biopsies that were immunohistochemically highly PD-L1 positive | [18] | |
| PET | Peptide | 68Ga-DOTA-TOC (targeting SSTR) | Could be used for the detection of breast tumors not detected with 18F-FDG | [19] | |||
| PET | CXCR4 antagonist | 64Cu-AuNCs-AMD3100 (targeting CXCR4) | 1. Flexible and straightforward preparation 2. High radiolabeling specific activity 3. Sensitive and accurate detection of CXCR4 | The ability to determine tumor progression and burden needs further improvement | [20] | ||
| SPECT | scFv | 99mTc-HYNIC-VCAM-1scFv (targeting VCAM-1) | The probe can reach the tumor site quickly based on the high tissue penetrability of small antibody fragments | High activity in blood and liver | [21] | ||
| Dual receptor targeted | PET | Peptide | 64Cu-NOTA-RGD-BBN (targeting αvβ3 and GRPR) | Favorable in vivo kinetics and enhanced tumor uptake | Did not set other controls such as the RAD-bombesin heterodimer and RGD-scramble bombesin heterodimer | [22] | |
| PET | Peptide | 68Ga-NGR-RGD (targeting αvβ3 and CD13) | Dual receptor-targeting tracers showed higher binding avidities, targeting efficiency, and longer tumor retention time | The uptake of 68Ga-NGR-RGD in tumors is still relatively low | [23] | ||
| Biomaterial-based probes | Membrane | PET | Cancer cell membrane | CCm-UCNPs | 1. Exhibited homologous targeting and immune escaping abilities 2. Can be used for ultra-sensitive in vivo UCL/MRI/PET multimodality precise imaging of triple-negative breast cancer (TNBC) | [24] | |
| PET | Red blood cell membrane | RBC-UCNPs | 1. The combination of a pre-targeting strategy and in vivo click chemistry successfully realized 4T1 tumor PET imaging by short half-life nuclide-labeled biomimetic nanoparticles 2. The inserted FA was used to increase the tumor-targeting ability of RBC-UCNPs | [25] | |||
| Exosomes | PET | Exosome | 64Cu-NOTA-exosome-PEG | 1. One of the first examples of radiolabeling and in vivo PET imaging of exosomes 2. PEGylation reduced hepatic clearance of exosomes 3. Exhibited enhanced tumor uptake and imaging capacity | The radiolabeling yield of NOTA−exosome−PEG was slightly lower than that of NOTA−exosome | [26] | |
| Peptide nucleic acid | SPECT | PNA | 99mTc-CCND1 antisense probe | Establish the proof of principle for identifying oncogene activity in breast cancer xenografts | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, Z.; Yuan, L.; Fan, K.; Zhang, Y.; Cai, W.; Lan, X. Radionuclide-Based Imaging of Breast Cancer: State of the Art. Cancers 2021, 13, 5459. https://doi.org/10.3390/cancers13215459
Li H, Liu Z, Yuan L, Fan K, Zhang Y, Cai W, Lan X. Radionuclide-Based Imaging of Breast Cancer: State of the Art. Cancers. 2021; 13(21):5459. https://doi.org/10.3390/cancers13215459
Chicago/Turabian StyleLi, Huiling, Zhen Liu, Lujie Yuan, Kevin Fan, Yongxue Zhang, Weibo Cai, and Xiaoli Lan. 2021. "Radionuclide-Based Imaging of Breast Cancer: State of the Art" Cancers 13, no. 21: 5459. https://doi.org/10.3390/cancers13215459