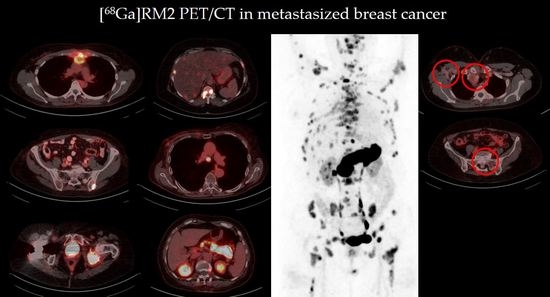
Gastrin-Releasing Peptide Receptor Antagonist [68Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Treatment of Breast Cancer by Stage. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-breast-cancer-by-stage.html (accessed on 28 August 2021).
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Singh, R.; Wang, M.; Chinchilli, V.M.; Trifiletti, D.M.; Ost, P.; Siva, S.; Meng, M.B.; Tchelebi, L.; Zaorsky, N.G. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: A systematic review and meta-analysis. JAMA Oncol. 2021, 7, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De, B.A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase ii trial. J. Clin. Oncol. 2018, 36, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The oriole phase 2 randomized clinical trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for oligometastatic prostate cancer: A prospective clinical trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020, 21, E18–E28. [Google Scholar] [CrossRef] [Green Version]
- Lecouvet, F.E.; Oprea-Lager, D.E.; Liu, Y.; Ost, P.; Bidaut, L.; Collette, L.; Deroose, C.M.; Goffin, K.; Herrmann, K.; Hoekstra, O.S.; et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: A consensus recommendation from the EORTC imaging group. Lancet Oncol. 2018, 19, e534–e545. [Google Scholar] [CrossRef]
- Ong, W.L.; Koh, T.L.; Lim, J.D.; Chao, M.; Farrugia, B.; Lau, E.; Khoo, V.; Lawrentschuk, N.; Bolton, D.; Foroudi, F. Prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT)-guided stereotactic ablative body radiotherapy for oligometastatic prostate cancer: A single-institution experience and review of the published literature. BJU Int. 2019, 124 (Suppl. S1), 9–30. [Google Scholar] [CrossRef] [PubMed]
- Hohla, F.; Schally, A.V. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle 2010, 9, 1738–1741. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Álvarez, I.; Moreno, P.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moody, T.W.; Coy, D.H.; Jensen, R.T. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides 2015, 72, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Pinski, J.; Groot, K.; Schally, A.V. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 1992, 52, 4545–4547. [Google Scholar] [PubMed]
- Nelson, J.; Donnelly, M.; Walker, B.; Gray, J.; Shaw, C.; Murphy, R.F. Bombesin stimulates proliferation of human breast cancer cells in culture. Br. J. Cancer 1991, 63, 933–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lango, M.N.; Dyer, K.F.; Lui, V.W.Y.; Gooding, W.E.; Gubish, C.; Siegfried, J.M.; Grandis, J.R. Gastrin-releasing peptide receptor-mediated autocrine growth in squamous cell carcinoma of the head and neck. J. Natl. Cancer Inst. 2002, 94, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.-C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand (125)I-D-TYR(6), beta-ALA(11), PHE(13), NLE(14) bombesin(6-14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar] [PubMed]
- Reubi, J.C.; Fleischmann, A.; Waser, B.; Rehmann, R. Concomitant vascular GRP-receptor and VEGF-receptor expression in human tumors: Molecular basis for dual targeting of tumoral vasculature. Peptides 2011, 32, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Mansi, R.; Grosu, A.L.; Schultze-Seemann, W.; Dumont-Walter, R.A.; Meyer, P.T.; Maecke, H.R.; Reubi, J.C.; Weber, W.A. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist--from mice to men. Theranostics 2014, 4, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholomä, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G.; et al. Gastrin-releasing Peptide receptor imaging in breast cancer using the receptor antagonist (68)Ga-RM2 and PET. Theranostics 2016, 6, 1641–1650. [Google Scholar] [CrossRef]
- Dalm, S.U.; Martens, J.W.M.; Sieuwerts, A.M.; van Deurzen, C.H.M.; Koelewijn, S.J.; de Blois, E.; Maina, T.; Nock, B.A.; Brunel, L.; Fehrentz, J.-A.; et al. In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer. J. Nucl. Med. 2015, 56, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Morgat, C.; MacGrogan, G.; Brouste, V.; Vélasco, V.; Sévenet, N.; Bonnefoi, H.; Fernandez, P.; Debled, M.; Hindié, E. Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: A study of 1432 primary tumors. J. Nucl. Med. 2017, 58, 1401–1407. [Google Scholar] [CrossRef]
- Morgat, C.; Schollhammer, R.; MacGrogan, G.; Barthe, N.; Vélasco, V.; Vimont, D.; Cazeau, A.-L.; Fernandez, P.; Hindié, E. Comparison of the binding of the gastrin-releasing peptide receptor (GRP-R) antagonist 68Ga-RM2 and 18F-FDG in breast cancer samples. PLoS ONE 2019, 14, e0210905. [Google Scholar] [CrossRef] [Green Version]
- Michalski, K.; Stoykow, C.; Bronsert, P.; Juhasz-Böss, I.; Meyer, P.T.; Ruf, J.; Erbes, T.; Asberger, J. Association between gastrin-releasing peptide receptor expression as assessed with [68Ga]Ga-RM2 PET/CT and histopathological tumor regression after neoadjuvant chemotherapy in primary breast cancer. Nucl. Med. Biol. 2020, 86–87, 37–43. [Google Scholar] [CrossRef]
- Clarke, R.; Tyson, J.J.; Dixon, J.M. Endocrine resistance in breast cancer--An overview and update. Mol. Cell. Endocrinol. 2015, 418, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri, R.H.A.; von, K.A.; Miller, W.R.; Smith, I.; et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef]
- Zang, J.; Mao, F.; Wang, H.; Zhang, J.; Liu, Q.; Peng, L.; Li, F.; Lang, L.; Chen, X.; Zhu, Z. 68Ga-NOTA-RM26 PET/CT in the evaluation of breast cancer: A pilot prospective study. Clin. Nucl. Med. 2018, 43, 663–669. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Z.; Gualtieri, E.E.; Parma, M.J.; Walsh, E.S.; Sebok, D.; Hsieh, Y.-L.; Tung, C.-H.; Song, X.; Griesmer, J.J.; et al. Systematic and distributed time-of-flight list mode pet reconstruction. In IEEE Nuclear Science Symposium Conference Record, Proceedings of the Nuclear Science Symposium, Medical Imaging Conference, 15th International Workshop on Room Temperature Semiconductor X- and Gamma-Ray Detectors, Special Focus Workshops, Town and Country Resort & Convention Center, San Diego, CA, USA, 29 October–4 November 2006; Phlips, B., Ed.; IEEE Operations Center: Piscataway, NJ, USA, 2006; pp. 1715–1722. ISBN 1-4244-0560-2. [Google Scholar]
- Rossi, S.; Basso, M.; Strippoli, A.; Dadduzio, V.; Cerchiaro, E.; Barile, R.; D'Argento, E.; Cassano, A.; Schinzari, G.; Barone, C. Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin. Breast Cancer 2015, 15, 307–312. [Google Scholar] [CrossRef]
- Hoefnagel, L.D.C.; van der Groep, P.; van de Vijver, M.J.; Boers, J.E.; Wesseling, P.; Wesseling, J.; van der Wall, E.; van Diest, P.J. Discordance in ERα, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann. Oncol. 2013, 24, 3017–3023. [Google Scholar] [CrossRef]
- Chen, R.; Qarmali, M.; Siegal, G.P.; Wei, S. Receptor conversion in metastatic breast cancer: Analysis of 390 cases from a single institution. Mod. Pathol. 2020, 33, 2499–2506. [Google Scholar] [CrossRef]
- Boers, J.; Venema, C.M.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Kwee, T.C.; Schuuring, E.; Martens, J.W.M.; Sjoerd, G.E.; Hospers, G.A.P.; Schröder, C.P. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur. J. Cancer 2020, 126, 11–20. [Google Scholar] [CrossRef]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist 177LuLu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Trovo, M.; Furlan, C.; Polesel, J.; Fiorica, F.; Arcangeli, S.; Giaj-Levra, N.; Alongi, F.; Del Conte, A.; Militello, L.; Muraro, E.; et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother. Oncol. 2018, 126, 177–180. [Google Scholar] [CrossRef]
- David, S.; Tan, J.; Savas, P.; Bressel, M.; Kelly, D.; Foroudi, F.; Loi, S.; Siva, S. Stereotactic ablative body radiotherapy (SABR) for bone only oligometastatic breast cancer: A prospective clinical trial. Breast 2020, 49, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Steenbruggen, T.G.; Schaapveld, M.; Horlings, H.M.; Sanders, J.; Hogewoning, S.J.; Lips, E.H.; Vrancken, P.M.T.; Kok, N.F.; Wiersma, T.; Esserman, L.; et al. Characterization of oligometastatic disease in a real-world nationwide cohort of 3447 patients with de novo metastatic breast cancer. JNCI Cancer Spectr. 2021, 5, pkab010. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Katz, A.W.; Zhang, H.; Huggins, C.F.; Aujla, K.S.; Okunieff, P. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: Some patients survive longer than a decade. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 131, 45–51. [Google Scholar] [CrossRef]
- Krug, D.; Vonthein, R.; Illen, A.; Olbrich, D.; Barkhausen, J.; Richter, J.; Klapper, W.; Schmalz, C.; Rody, A.; Maass, N.; et al. Metastases-directed radiotherapy in addition to standard systemic therapy in patients with oligometastatic breast cancer: Study protocol for a randomized controlled multi-national and multi-center clinical trial (OLIGOMA). Clin. Transl. Radiat. Oncol. 2021, 28, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Alomran, R.; White, M.; Bruce, M.; Bressel, M.; Roache, S.; Karroum, L.; Hanna, G.G.; Siva, S.; Goel, S.; David, S. Stereotactic radiotherapy for oligoprogressive ER-positive breast cancer (AVATAR). BMC Cancer 2021, 21, 303. [Google Scholar] [CrossRef] [PubMed]

| Pat. No. | Type/Side | Previous Treatment (Duration: Years) | Current Treatment (Duration: Years) | Time Since Initial Diagnosis (Years) |
|---|---|---|---|---|
| 1 | NST/left | Sentinel-LND, NAC, BCS, adj. Rtx, Tamoxifen | Tamoxifen (5) | 5 |
| 2 | ILC/right | - | Palbociclib, Letrozole (2) | 2 |
| 3 | NST/right | BCS, Sentinel-LND, adj. Rtx, Anastrozole (5) | - | 6 |
| 4 | n.a./n.a. | BSC, adj. Rtx, adj. Ctx, Letrozole (10) | - | 16 |
| 5 | ILC/right | Mastectomy, surgery of local relapse (2×), Exemestan (14) | Tamoxifen (1.5) | 32 |
| 6 | NST/right | Mastectomy, LND, adj. Rtx | Anastrozole (0.5) | 0.5 |
| 7 | NST/left | BCS, adj. Rtx, adj. Ctx, Rtx different bone metastases, Everolimus, Exemestan (5) Fulvestrant (1.5), Palbociclib, Capecitabine | Tamoxifen (0.5) | 27 |
| 8 | NST/right | BCS, Sentinel-LND, adj. Rtx | - | 12 |
| Pat. No. | Rating RM2 PET | Sites of Metastatic Disease | No. of Metastases | Current Biopsy | ||
|---|---|---|---|---|---|---|
| Site | ER Status | Time Interval to PET (Months) | ||||
| 1 | Positive | OSS, LN, HEP | 7 | HEP | >90% | 5 (after) |
| 2 | Negative | OSS, LN | >10 | Pleural effusion | 0% | 4 (after) |
| 3 | Positive | OSS | 5 | OSS | >90% | 1 (before) |
| 4 | Positive | OSS | 1 | OSS | >90% | 1 (before) |
| 5 | Negative | OSS | 4 * | Local relapse | >90% | 17 (before) |
| 6 | Positive | OSS, LN | 9 | Primary tumor | >90% | 4 (before) |
| 7 | Positive | OSS, LN, HEP | >30 | OSS | >90% | 22 (before) |
| 8 | Positive | OSS | 1 | OSS | >90% | 1 (before) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalski, K.; Kemna, L.; Asberger, J.; Grosu, A.L.; Meyer, P.T.; Ruf, J.; Sprave, T. Gastrin-Releasing Peptide Receptor Antagonist [68Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer. Cancers 2021, 13, 6106. https://doi.org/10.3390/cancers13236106
Michalski K, Kemna L, Asberger J, Grosu AL, Meyer PT, Ruf J, Sprave T. Gastrin-Releasing Peptide Receptor Antagonist [68Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer. Cancers. 2021; 13(23):6106. https://doi.org/10.3390/cancers13236106
Chicago/Turabian StyleMichalski, Kerstin, Lars Kemna, Jasmin Asberger, Anca L. Grosu, Philipp T. Meyer, Juri Ruf, and Tanja Sprave. 2021. "Gastrin-Releasing Peptide Receptor Antagonist [68Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer" Cancers 13, no. 23: 6106. https://doi.org/10.3390/cancers13236106
APA StyleMichalski, K., Kemna, L., Asberger, J., Grosu, A. L., Meyer, P. T., Ruf, J., & Sprave, T. (2021). Gastrin-Releasing Peptide Receptor Antagonist [68Ga]RM2 PET/CT for Staging of Pre-Treated, Metastasized Breast Cancer. Cancers, 13(23), 6106. https://doi.org/10.3390/cancers13236106