Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study
Abstract
:Simple Summary
Abstract
1. Introduction
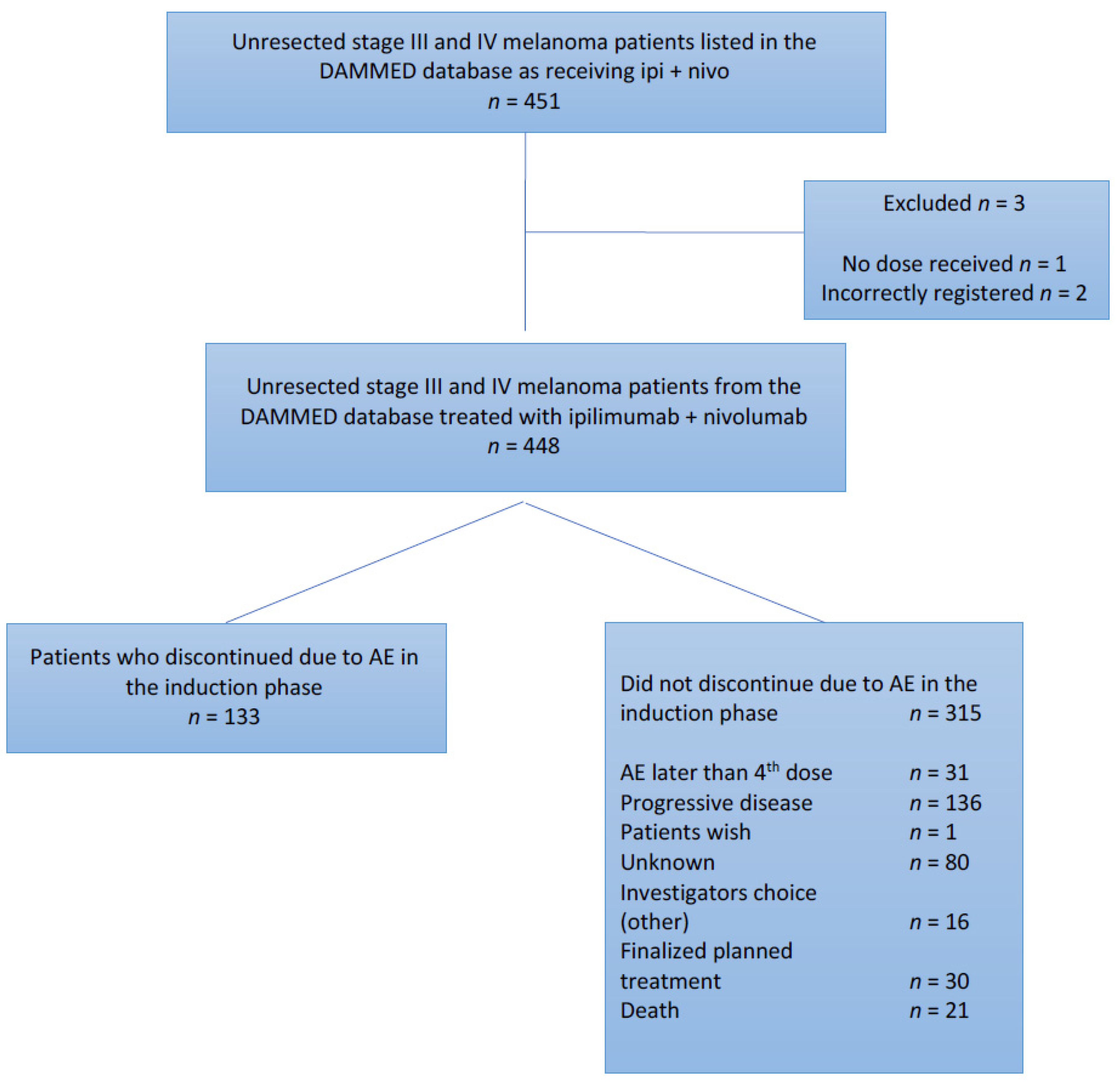
2. Materials and Methods
2.1. Patients and Study Design
2.2. Treatment Regimen
2.3. Assessments
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Objective Response Rate (ORR)
3.3. Progression-Free Survival
3.4. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. Vivo 2014, 28, 1005–1011. [Google Scholar]
- WHO Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Int. Agency Res. Cancer 2012. Available online: https://gco.iarc.fr/today/home (accessed on 2 November 2021).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. Basel Switz. 2021, 9, 63. [Google Scholar] [CrossRef]
- Lanoy, E. Epidemiology, risk factor and screening for melanoma and other skin cancers. Rev. Prat. 2014, 64, 31–36. [Google Scholar] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, L.M.; Daud, A.I. Immunotherapy for Melanoma. Semin. Cutan. Med. Surg. 2018, 37, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Hadjinicolaou, A.V.; Chiarion Sileni, V.; Rossi, C.R.; Mocellin, S. Systemic Treatments for Metastatic Cutaneous Melanoma. Cochrane Database Syst. Rev. 2018, 2, CD011123. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Cousin, S.; Italiano, A. Molecular Pathways: Immune Checkpoint Antibodies and Their Toxicities. Clin. Cancer Res. 2016, 22, 4550–4555. [Google Scholar] [CrossRef] [Green Version]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A. Immunotherapy of Melanoma. Curr. Mol. Pharmacol. 2016, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Carreau, N.A.; Pavlick, A.C. Nivolumab and Ipilimumab: Immunotherapy for Treatment of Malignant Melanoma. Future Oncol. Lond. Engl. 2019, 15, 349–358. [Google Scholar] [CrossRef]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of Melanoma: Facts and Hopes. Clin. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spain, L.; Larkin, J. Combination Immune Checkpoint Blockade with Ipilimumab and Nivolumab in the Management of Advanced Melanoma. Expert Opin. Biol. Ther. 2016, 16, 389–396. [Google Scholar] [CrossRef]
- Schadendorf, D.; Larkin, J.; Wolchok, J.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.; et al. Health-Related Quality of Life Results from the Phase III CheckMate 067 Study. Eur. J. Cancer 2017, 82, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and Safety Outcomes in Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef] [Green Version]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncology 2016, 21, 804–816. [Google Scholar] [CrossRef] [Green Version]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (IrAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Dimitrakopoulou-Strauss, A.; Larribere, L.; Hassel, J.C.; Sachpekidis, C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Khanal, S.; Zhang, H. Risk of Immune-Related Adverse Events Associated with Ipilimumab-plus-Nivolumab and Nivolumab Therapy in Cancer Patients. Ther. Clin. Risk Manag. 2019, 15, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Park, H.; Malone, D.C.; Wang, C.-Y.; Wilson, D.L.; Yeh, Y.-M.; Van Boemmel-Wegmann, S.; Lo-Ciganic, W.-H. Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2020, 3, e201611. [Google Scholar] [CrossRef]
- Ellebaek, E.; Svane, I.M.; Schmidt, H.; Haslund, C.A.; Donia, M.; Hoejberg, L.; Ruhlmann, C.; Guldbrandt, L.M.; Køhler, U.H.; Bastholt, L. The Danish Metastatic Melanoma Database (DAMMED): A Nation-Wide Platform for Quality Assurance and Research in Real-World Data on Medical Therapy in Danish Melanoma Patients. Cancer Epidemiol. 2021, 73, 101943. [Google Scholar] [CrossRef] [PubMed]
- Svane, I.M.; Schmidt, H.; Bastholt, L. Guideline for Assessment and Treatment of IrAEs. Available online: https://dsko.org/wp-content/uploads/2018/09/CheckPointInhib-Tox-2018-10-juli.pdf (accessed on 2 November 2021).
- Horiguchi, M.; Uno, H.; Wei, L.J. Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab as a Result of Adverse Events Lived Significantly Longer Than Patients Who Continued Treatment. J. Clin. Oncol. 2018, 36, 720–721. [Google Scholar] [CrossRef]
- Schadendorf, D.; Jiang, J.; Kelleher, T.; Postow, M.A. Reply to M. Horiguchi et Al. J. Clin. Oncol. 2018, 36, 721. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.J.; Lao, C.D.; et al. Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]


| Baseline Characteristics No. (%) | Patients Who Discontinued Due to irAEs in the Induction Phase n = 133 | Patients Who Did Not Discontinue Due to irAEs in the Induction Phase n = 315 |
|---|---|---|
| Gender | ||
| -Male | 69 (51.9%) | 178 (56.5%) |
| -Female | 64 (48.1%) | 137 (43.5%) |
| Age | ||
| ->65 | 77 (57.9%) | 196 (62.2%) |
| -<65 | 56 (42.0%) | 119 (38.0%) |
| M stage | ||
| -M1a | 17 (12.8%) | 36 (11.4%) |
| -M1b | 15 (11.3%) | 24 (8.0%) |
| -M1c | 63 (47.4%) | 152 (48.3%) |
| -M1d | 38 (28.5%) | 103 (32.3%) |
| LDH | ||
| -≤ULN | 58 (43.6%) | 148 (47.0%) |
| ->ULN | 66 (49.6%) | 148 (47.0%) |
| -Unknown | 9 (7.0%) | 19 (6.0%) |
| CRP | ||
| -<2x ULN | 106 (79.7%) | 212 (67.3%) |
| ->2x ULN | 16 (12.0%) | 69 (21.9%) |
| -Unknown | 11 (8.3%) | 34 (10.8%) |
| Melanoma diagnosis | ||
| -Cutaneous | 84 (63.2%) | 206 (65.4%) |
| -Mucosal | 7 (5.3%) | 12 (3.8%) |
| -Ocular | 22 (16.5%) | 48 (15.2%) |
| -Unknown primary | 20 (15.0%) | 49 (15.6%) |
| BRAF mutation | ||
| -Wild type | 72 (54.1%) | 149 (47.3%) |
| -Unknown | 3 (2.3%) | 21 (6.7%) |
| -V600E | 51 (38.3%) | 101 (32.1%) |
| -V600K | 3 (2.3%) | 13 (4.1%) |
| -V600x | 4 (3.0%) | 31 (9.8%) |
| Variation | Patients Who Discontinued Due to Adverse Events n = 133 | Patients Who Did Not Discontinue Due to Adverse Events n = 315 |
|---|---|---|
| Objective response rate No. (%) | 73 (54.9%) | 123 (39.0%) |
| Best overall response | ||
| No. (%) | ||
| CR | 33 (24.8%) | 41 (13.0%) |
| PR | 40 (30.1%) | 82 (26.0%) |
| SD | 21 (15.8%) | 34 (10.8%) |
| PD | 34 (25.6%) | 141 (44.8%) |
| Unknown * | 5 (3.7) | 17 (5.4%) |
| Median time to progression (95%CI) | 8.4 | 7.7 |
| 6.0; 12.8 | 5.2; 9.8 | |
| Median time to death ** | NR | 21.9 16.7; 37.1 |
| Variation | Univariate Analyses | Multivariate Analyses | |||||
|---|---|---|---|---|---|---|---|
| Covariates | No. of Patients | HR | 95%CI | p | HR | 95%CI | p |
| Group | |||||||
| Non-discontinuing vs. discontinuing | 315/133 | 1.19 | 0.92:1.55 | 0.19 | 1.22 | 0.93:1.60 | 0.15 |
| LDH level | |||||||
| high vs. low | 213/207 | 1.20 | 0.94:1.53 | 0.15 | - | - | - |
| CRP level | |||||||
| >2× upper level vs. low | 85/318 | 1.29 | 1.02:1.62 | 0.03 | 1.17 | 0.99:1.36 | 0.05 |
| Melanoma diagnosis | |||||||
| Cutaneous vs. other | 290/158 | 0.82 | 0.72:0.92 | 0.00 | 1.06 | 0.89:1.25 | 0.51 |
| M-status | |||||||
| M1a vs. M1b–d | 53/395 | 0.50 | 0.32:0.76 | 0.00 | 0.61 | 0.38:0.97 | 0.04 |
| M1d vs. M1a–c | 141/307 | 1.09 | 0.84:1.41 | 0.51 | 1.14 | 0.85:1.53 | 0.38 |
| Cox models for group with stratifications | |||||||
| Strata-m1a | 1.23 | 0.93:1.61 | 0.14 | ||||
| Strata-cutaneous melanoma | 1.19 | 0.91:1.57 | 0.20 | ||||
| Strata-CRP level high | 1.18 | 0.89:1.56 | 0.24 | ||||
| Covariates | No. of Patients | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | ||
| Group | |||||||
| Non-discontinuing vs. discontinuing | 315/133 | 1.45 | 1.06:1.99 | 0.02 | 1.48 | 1.07:2.07 | 0.02 |
| LDH level | |||||||
| high vs. low | 213/207 | 1.29 | 0.96:1.72 | 0.08 | 1.35 | 1.01:1.80 | 0.04 |
| CRP level | |||||||
| >2× upper level vs. low | 85/318 | 1.60 | 1.14:2.25 | 0.006 | 1.13 | 0.94:1.35 | 0.18 |
| Melanoma diagnosis | |||||||
| Cutaneous vs. other | 290/158 | 0.72 | 0.54:0.96 | 0.03 | 0.99 | 0.68:1.46 | 0.98 |
| M-status | |||||||
| M1a vs. M1b-d | 53/395 | 0.48 | 0.29:0.81 | 0.01 | 0.64 | 0.36:1.11 | 0.11 |
| M1d vs. M1a-c | 141/307 | 1.50 | 1.12:2.01 | 0.01 | 1.55 | 1.11:2.15 | 0.01 |
| Cox models for group with stratifications | |||||||
| Strata–LDH level | 1.49 | 1.08:2.08 | 0.02 | ||||
| Strata–m1d | 1.46 | 1.05:2.03 | 0.02 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, M.; Vittrup, A.S.; Bastholt, L.; Svane, I.M.; Donia, M.; Luczak, A.A.; Ruhlmann, C.H.; Guldbrandt, L.M.; Koehler, U.H.; Winther, M.L.; et al. Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study. Cancers 2021, 13, 5550. https://doi.org/10.3390/cancers13215550
Fink M, Vittrup AS, Bastholt L, Svane IM, Donia M, Luczak AA, Ruhlmann CH, Guldbrandt LM, Koehler UH, Winther ML, et al. Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study. Cancers. 2021; 13(21):5550. https://doi.org/10.3390/cancers13215550
Chicago/Turabian StyleFink, Morten, Anders Schwartz Vittrup, Lars Bastholt, Inge Marie Svane, Marco Donia, Adam A. Luczak, Christina H. Ruhlmann, Louise Mahncke Guldbrandt, Ulrich Heide Koehler, Mette Lerche Winther, and et al. 2021. "Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study" Cancers 13, no. 21: 5550. https://doi.org/10.3390/cancers13215550








