STING Signaling and Skin Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
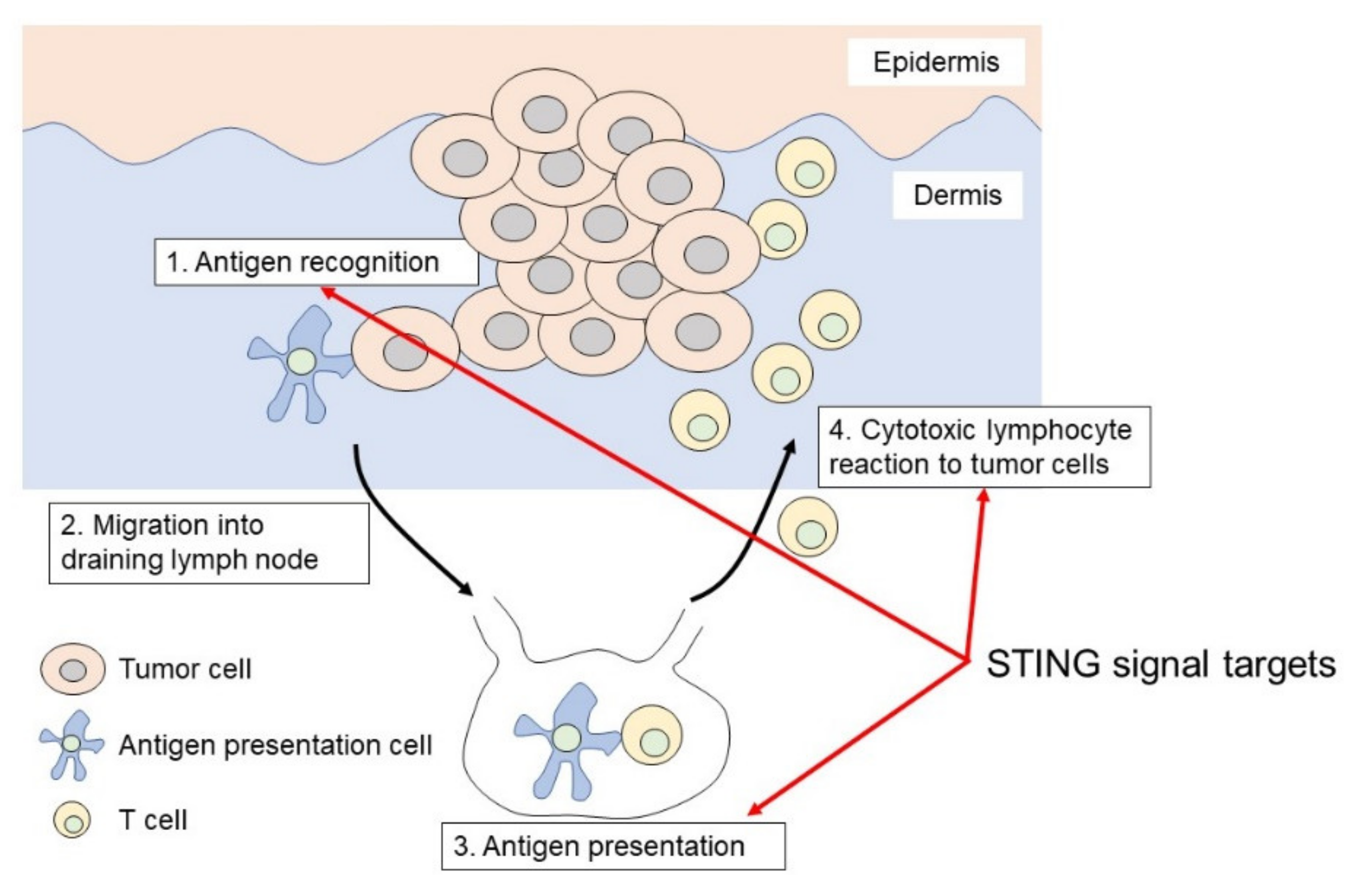
2. The Mechanism of STING Signaling
3. STING-Involved Anti-Tumor Immunity
3.1. Melanoma
3.2. Cutaneous Squamous Cell Carcinoma
3.3. Merkel Cell Carcinoma
3.4. Adult T-Cell Leukemia/Lymphoma
3.5. STING Anti-Tumor Effect Expected Skin Cancers
3.5.1. Basal Cell Carcinoma
3.5.2. Cutaneous Lymphomas
4. STING Strategy for Skin Cancers
5. Epigenetic Modification
6. Limitations and Disadvantages of STING-Mediated Anti-Tumor Immunity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- McGovern, V.J. Spontaneous regression of melanoma. Pathology 1975, 7, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [Green Version]
- Chamoto, K.; Hatae, R.; Honjo, T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.J.; Sen, G.L.; Ward, N.L.; Johnston, A.; Chun, K.; Chen, Y.; Adase, C.; Sanford, J.A.; Gao, N.; Chensee, M.; et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-β Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016, 45, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021, 6, 59. [Google Scholar] [CrossRef]
- Clifford, J.L.; Menter, D.G.; Yang, X.; Walch, E.; Zou, C.; Clayman, G.L.; Schaefer, T.S.; El-Naggar, A.K.; Lotan, R.; Lippman, S.M. Expression of protein mediators of type I interferon signaling in human squamous cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 2000, 9, 993–997. [Google Scholar]
- Karavodin, L.M.; Golub, S.H. Systemic administration of human leukocyte interferon to melanoma patients. III. Increased helper:suppressor cell ratios in melanoma patients during interferon treatment. Nat. Immun. Cell Growth Regul. 1983, 3, 193–202. [Google Scholar] [PubMed]
- Bronte, V. Tumors STING adaptive antitumor immunity. Immunity 2014, 41, 679–681. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.; Quispe-Tintaya, W.; Jahangir, A.; Asafu-Adjei, D.; Ramos, I.; Sintim, H.O.; Zhou, J.; Hayakawa, Y.; Karaolis, D.K.; Gravekamp, C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol. Res. 2014, 2, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; McAtee, C.K.; Su, X. Phase separation in immune signalling. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. cGAS-STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef]
- Wan, D.; Jiang, W.; Hao, J. Research Advances in How the cGAS-STING Pathway Controls the Cellular Inflammatory Response. Front. Immunol. 2020, 11, 615. [Google Scholar] [CrossRef]
- Corrales, L.; McWhirter, S.M.; Dubensky, T.W., Jr.; Gajewski, T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Investig. 2016, 126, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, L.T.; Chen, L.Y. Role of the cGAS-STING pathway in cancer development and oncotherapeutic approaches. EMBO Rep. 2018, 19, e46935. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Liu, D.; Li, Z.; Bian, J. Bioactive modulators targeting STING adaptor in cGAS-STING pathway. Drug. Discov. Today 2020, 25, 230–237. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Derm. 2013, 133, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Nakajima, S.; Nonomura, Y.; Otsuka, A.; Egawa, G.; Yoshimoto, T.; Nakamura, M.; Narumiya, S.; et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2019, 144, 1265–1273.e9. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Yamamoto, O.; Sawada, Y.; Egawa, G.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nakajima, S.; Miyachi, Y.; Kabashima, K. Receptor-interacting protein kinase 3 controls keratinocyte activation in a necroptosis-independent manner and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2017, 140, 619–622.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A2 facilitates IL-17A production from Vγ4+ γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683.e2. [Google Scholar] [CrossRef] [Green Version]
- Chiliveru, S.; Rahbek, S.H.; Jensen, S.K.; Jørgensen, S.E.; Nissen, S.K.; Christiansen, S.H.; Mogensen, T.H.; Jakobsen, M.R.; Iversen, L.; Johansen, C.; et al. Inflammatory cytokines break down intrinsic immunological tolerance of human primary keratinocytes to cytosolic DNA. J. Immunol. 2014, 192, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xue, X.; Tang, W.; Su, L.; Zhang, L.; Zhang, Y. Cytosolic DNA‒Mediated STING-Dependent Inflammation Contributes to the Progression of Psoriasis. J. Investig. Derm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the cGAS-STING Pathway. Front. Immunol. 2020, 11, 571334. [Google Scholar] [CrossRef]
- Orvain, C.; Lin, Y.L.; Jean-Louis, F.; Hocini, H.; Hersant, B.; Bennasser, Y.; Ortonne, N.; Hotz, C.; Wolkenstein, P.; Boniotto, M.; et al. Hair follicle stem cell replication stress drives IFI16/STING-dependent inflammation in hidradenitis suppurativa. J. Clin. Investig. 2020, 130, 3777–3790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Sawada, Y.; Nakamura, M. Immune Profile Analysis in Peripheral Blood and Tumor in Patients with Malignant Melanoma. Int. J. Mol. Sci. 2021, 22, 1957. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Sawada, Y.; Saito-Sasaki, N.; Yamamoto, K.; Yoshioka, H.; Ohmori, S.; Yoshioka, M.; Okada, E.; Nakamura, M. Profile fluctuation of peripheral blood in advanced melanoma patients treated with nivolumab. J. Derm. 2018, 45, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Mashima, E.; Inoue, A.; Sakuragi, Y.; Yamaguchi, T.; Sasaki, N.; Hara, Y.; Omoto, D.; Ohmori, S.; Haruyama, S.; Sawada, Y.; et al. Nivolumab in the treatment of malignant melanoma: Review of the literature. Onco. Targets Ther. 2015, 8, 2045–2051. [Google Scholar]
- Muñoz, N.M.; Williams, M.; Dixon, K.; Dupuis, C.; McWatters, A.; Avritscher, R.; Manrique, S.Z.; McHugh, K.; Murthy, R.; Tam, A.; et al. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J. Immunother. Cancer 2021, 9, e001800. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Xia, T.; Rabasa Capote, A.; Betancourt, D.; Barber, G.N. Extrinsic Phagocyte-Dependent STING Signaling Dictates the Immunogenicity of Dying Cells. Cancer Cell 2018, 33, 862–873.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, W.; Liang, H.; Bugno, J.; Xu, Q.; Ding, X.; Yang, K.; Fu, Y.; Weichselbaum, R.R.; Zhao, X.; Wang, L. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut 2021. [Google Scholar] [CrossRef]
- Chelvanambi, M.; Fecek, R.J.; Taylor, J.L.; Storkus, W.J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 2021, 9, e001906. [Google Scholar] [CrossRef] [PubMed]
- Chavanet, A.; Hill, K.R.; Jiménez-Andrade, Y.; Choo, M.K.; White, K.; Park, J.M. Intracellular signaling modules linking DNA damage to secretome changes in senescent melanoma cells. Melanoma Res. 2020, 30, 336–347. [Google Scholar] [CrossRef]
- Wang, Z.; Celis, E. STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol. Immunother. 2015, 64, 1057–1066. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyabe, H.; Hyodo, M.; Sato, Y.; Hayakawa, Y.; Harashima, H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release 2015, 216, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Falahat, R.; Perez-Villarroel, P.; Mailloux, A.W.; Zhu, G.; Pilon-Thomas, S.; Barber, G.N.; Mulé, J.J. STING Signaling in Melanoma Cells Shapes Antigenicity and Can Promote Antitumor T-cell Activity. Cancer Immunol. Res. 2019, 7, 1837–1848. [Google Scholar] [CrossRef]
- Falahat, R.; Berglund, A.; Putney, R.M.; Perez-Villarroel, P.; Aoyama, S.; Pilon-Thomas, S.; Barber, G.N.; Mulé, J.J. Epigenetic reprogramming of tumor cell-intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc Natl. Acad. Sci. USA 2021, 118, e2013598118. [Google Scholar] [CrossRef] [PubMed]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, K.; Takeda, Y.; Oshiumi, H.; Shime, H.; Okabe, M.; Ikawa, M.; Matsumoto, M.; Seya, T. STING in tumor and host cells cooperatively work for NK cell-mediated tumor growth retardation. Biochem. Biophys. Res. Commun. 2016, 478, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Francica, B.J.; Ghasemzadeh, A.; Desbien, A.L.; Theodros, D.; Sivick, K.E.; Reiner, G.L.; Hix Glickman, L.; Marciscano, A.E.; Sharabi, A.B.; Leong, M.L.; et al. TNFα and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors. Cancer Immunol. Res. 2018, 6, 422–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkuri, T.; Kosaka, A.; Ishibashi, K.; Kumai, T.; Hirata, Y.; Ohara, K.; Nagato, T.; Oikawa, K.; Aoki, N.; Harabuchi, Y.; et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol. Immunother. 2017, 66, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Moffett, H.F.; Stephan, S.B.; Opel, C.F.; Dumigan, A.G.; Jiang, X.; Pillarisetty, V.G.; Pillai, S.P.S.; Wittrup, K.D.; Stephan, M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Investig. 2017, 127, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, D.; Kurbatov, V.; Wang, Q.; Wang, Y.; Fang, D.; Wu, L.; Bosenberg, M.; Muzumdar, M.D.; Khan, S.; et al. 5-Fluorouracil efficacy requires anti-tumor immunity triggered by cancer-cell-intrinsic STING. EMBO J. 2021, 40, e106065. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liang, H.L.; Yu, X.; Liu, Z.; Cao, X.; Rao, E.; Huang, X.; Wang, L.; Li, L.; Bugno, J.; et al. Radiotherapy and immunotherapy converge on elimination of tumor-promoting erythroid progenitor cells through adaptive immunity. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Liang, H.; Rao, E.; Zheng, W.; Huang, X.; Deng, L.; Zhang, Y.; Yu, X.; Xu, M.; Mauceri, H.; et al. Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity 2018, 49, 490–503.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Okada, E.; Nakamura, M. Cell Adhesion Molecule 1 (CADM1) Is an Independent Prognostic Factor in Patients with Cutaneous Squamous Cell Carcinoma. Diagnostics 2021, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Nakamura, M. Daily Lifestyle and Cutaneous Malignancies. Int. J. Mol. Sci. 2021, 22, 5227. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Mashima, E.; Saito-Sasaki, N.; Nakamura, M. The Role of Cell Adhesion Molecule 1 (CADM1) in Cutaneous Malignancies. Int. J. Mol. Sci. 2020, 21, 9732. [Google Scholar] [CrossRef]
- Hayman, T.J.; Baro, M.; MacNeil, T.; Phoomak, C.; Aung, T.N.; Cui, W.; Leach, K.; Iyer, R.; Challa, S.; Sandoval-Schaefer, T.; et al. STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat. Commun. 2021, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Zhang, L.; Li, Z.L.; He, J.; Cai, T.T.; Yang, D.J.; Xie, D.R. Expression of STING and MIF in tumor infiltration lymphocytes as prognostic factors in patients with ESCC. Int. J. Clin. Exp. Pathol. 2017, 10, 10066–10074. [Google Scholar]
- Baird, J.R.; Bell, R.B.; Troesch, V.; Friedman, D.; Bambina, S.; Kramer, G.; Blair, T.C.; Medler, T.; Wu, Y.; Sun, Z.; et al. Evaluation of Explant Responses to STING Ligands: Personalized Immunosurgical Therapy for Head and Neck Squamous Cell Carcinoma. Cancer Res. 2018, 78, 6308–6319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Xiao-Feng, H.; Guan-Jun, D.; Er-Ling, H.; Sheng, C.; Ting-Ting, W.; Qin-Gang, H.; Yan-Hong, N.; Ya-Yi, H. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim. Biophys. Acta 2015, 1852, 2494–2503. [Google Scholar]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harabuchi, S.; Kosaka, A.; Yajima, Y.; Nagata, M.; Hayashi, R.; Kumai, T.; Ohara, K.; Nagato, T.; Oikawa, K.; Ohara, M.; et al. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2020, 522, 408–414. [Google Scholar] [CrossRef]
- Lu, S.; Concha-Benavente, F.; Shayan, G.; Srivastava, R.M.; Gibson, S.P.; Wang, L.; Gooding, W.E.; Ferris, R.L. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV(+) status in head and neck cancer. Oral. Oncol. 2018, 78, 186–193. [Google Scholar] [CrossRef]
- Krump, N.A.; Wang, R.; Liu, W.; Yang, J.F.; Ma, T.; You, J. Merkel Cell Polyomavirus Infection Induces an Antiviral Innate Immune Response in Human Dermal Fibroblasts. J. Virol. 2021, 95, e0221120. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kim, G.B.; Krump, N.A.; Zhou, Y.; Riley, J.L.; You, J. Selective reactivation of STING signaling to target Merkel cell carcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 13730–13739. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, L.B.; Fuji, S.; Hermine, O.; Bazarbachi, A.; Ramos, J.C.; Ratner, L.; Horwitz, S.; Fields, P.; Tanase, A.; Bumbea, H.; et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 2019, 37, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Hino, R.; Hama, K.; Ohmori, S.; Fueki, H.; Yamada, S.; Fukamachi, S.; Tajiri, M.; Kubo, R.; Yoshioka, M.; et al. Type of skin eruption is an independent prognostic indicator for adult T-cell leukemia/lymphoma. Blood 2011, 117, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Shimauchi, T.; Yamaguchi, T.; Okura, R.; Hama-Yamamoto, K.; Fueki-Yoshioka, H.; Ohmori, S.; Yamada, S.; Yoshizawa, M.; Hiromasa, K.; et al. Combination of skin-directed therapy and oral etoposide for smoldering adult T-cell leukemia/lymphoma with skin involvement. Leuk Lymphoma 2013, 54, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Sawada, Y.; Shimauchi, T. Skin manifestations of adult T-cell leukemia/lymphoma: Clinical, cytological and immunological features. J. Derm. 2014, 41, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Tomonaga, M.; Fukuda, H.; Hanada, S.; Utsunomiya, A.; Tara, M.; Sano, M.; Ikeda, S.; Takatsuki, K.; Kozuru, M.; et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br. J. Haematol. 2001, 113, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Utsunomiya, A.; Fukuda, H.; Shibata, T.; Fukushima, T.; Takatsuka, Y.; Ikeda, S.; Masuda, M.; Nagoshi, H.; Ueda, R.; et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J. Clin. Oncol. 2007, 25, 5458–5464. [Google Scholar] [CrossRef] [PubMed]
- Stanchina, M.; Soong, D.; Zheng-Lin, B.; Watts, J.M.; Taylor, J. Advances in Acute Myeloid Leukemia: Recently Approved Therapies and Drugs in Development. Cancers 2020, 12, 3225. [Google Scholar] [CrossRef] [PubMed]
- Bladé, J.; Dimopoulos, M.; Rosiñol, L.; Rajkumar, S.V.; Kyle, R.A. Smoldering (asymptomatic) multiple myeloma: Current diagnostic criteria, new predictors of outcome, and follow-up recommendations. J. Clin. Oncol. 2010, 28, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, C.K.; Chan, C.P.; Fung, S.Y.; Wang, P.H.; Wong, W.M.; Tang, H.V.; Yuen, K.S.; Chan, C.P.; Jin, D.Y.; Kok, K.H. Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation. J. Virol. 2016, 90, 3902–3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.; Hiscott, J.; van Grevenynghe, J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 2013, 14, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechner, S.A.; Wernli, M.; Harr, T.; Hahn, S.; Itin, P.; Erb, P. Regression of basal cell carcinoma by intralesional interferon-alpha treatment is mediated by CD95 (Apo-1/Fas)-CD95 ligand-induced suicide. J. Clin. Investig. 1997, 100, 2691–2696. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chi, S.; He, N.; Zhang, X.; Guicherit, O.; Wagner, R.; Tyring, S.; Xie, J. IFNalpha induces Fas expression and apoptosis in hedgehog pathway activated BCC cells through inhibiting Ras-Erk signaling. Oncogene 2004, 23, 1608–1617. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.A.; Bishop, G.A.; Lowes, M.A.; Cooke, B.; Barnetson, R.S.; Halliday, G.M. Cytokine profiles in spontaneously regressing basal cell carcinomas. Br. J. Derm. 2000, 143, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Oehadian, A.; Koide, N.; Mu, M.M.; Hassan, F.; Islam, S.; Yoshida, T.; Yokochi, T. Interferon (IFN)-beta induces apoptotic cell death in DHL-4 diffuse large B cell lymphoma cells through tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Cancer Lett. 2005, 225, 85–92. [Google Scholar] [CrossRef]
- Jumbou, O.; N’Guyen, J.M.; Tessier, M.H.; Legoux, B.; Dréno, B. Long-term follow-up in 51 patients with mycosis fungoides and Sézary syndrome treated by interferon-alfa. Br. J. Derm. 1999, 140, 427–431. [Google Scholar] [CrossRef]
- Dréno, B.; Claudy, A.; Meynadier, J.; Verret, J.L.; Souteyrand, P.; Ortonne, J.P.; Kalis, B.; Godefroy, W.Y.; Beerblock, K.; Thill, L. The treatment of 45 patients with cutaneous T-cell lymphoma with low doses of interferon-alpha 2a and etretinate. Br. J. Derm. 1991, 125, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Ishida, T.; Inagaki, A.; Ishii, T.; Ding, J.; Kusumoto, S.; Komatsu, H.; Iida, S.; Inagaki, H.; Ueda, R. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: A novel immunotherapy for aggressive/refractory Mycosis fungoides and Sezary syndrome. Clin. Cancer Res. 2007, 13, 6494–6500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupoli, S.; Barulli, S.; Guiducci, B.; Offidani, M.; Mozzicafreddo, G.; Simonacci, M.; Filosa, G.; Giacchetti, A.; Ricotti, G.; Brandozzi, G.; et al. Low dose interferon-alpha2b combined with PUVA is an effective treatment of early stage mycosis fungoides: Results of a multicenter study. Cutaneous-T Cell Lymphoma Multicenter Study Group. Haematologica 1999, 84, 809–813. [Google Scholar]
- Ständer, H.; Ständer, S.; Luger, T.; Schwarz, T. Topical interferon-beta: An additional treatment for ulcerated mycosis fungoides. Hautarzt 2001, 52, 882–884. [Google Scholar]
- Yamamoto, T.; Sasaki, G.; Sato, T.; Katayama, I.; Nishioka, K. Cytokine profile of tumor cells in mycosis fungoides: Successful treatment with intra-lesional interferon-gamma combined with chemotherapy. J. Derm. 1995, 22, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, T.; Onodera, H.; Eguchi, H.; Hanada, N.; Fukuzawa, K.; Takahashi, M.; Ishihara, K.; Ikeda, S. A patient with plaque-stage mycosis fungoides has successfully been treated with long-term administration of IFN-gamma and has been in complete remission for more than 6 years. Br. J. Derm. 1996, 134, 130–133. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, Y.; Katayama, I.; Nishioka, K. Alteration of cytokine genes and bcl-2 expression following immunotherapy with intralesional IFN-gamma in a patient with tumor-stage mycosis fungoides. Dermatology 1998, 196, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kohn, E.C.; Steis, R.G.; Sausville, E.A.; Veach, S.R.; Stocker, J.L.; Phelps, R.; Franco, S.; Longo, D.L.; Bunn, P.A.; Ihde, D.C. Phase II trial of intermittent high-dose recombinant interferon alfa-2a in mycosis fungoides and the Sézary syndrome. J. Clin. Oncol. 1990, 8, 155–160. [Google Scholar] [CrossRef]
- Foss, F.M.; Ihde, D.C.; Breneman, D.L.; Phelps, R.M.; Fischmann, A.B.; Schechter, G.P.; Linnoila, I.; Breneman, J.C.; Cotelingam, J.D.; Ghosh, B.C.; et al. Phase II study of pentostatin and intermittent high-dose recombinant interferon alfa-2a in advanced mycosis fungoides/Sézary syndrome. J. Clin. Oncol. 1992, 10, 1907–1913. [Google Scholar] [CrossRef]
- Sanli, H.; Akay, B.N.; Anadolu, R.; Ozcan, M.; Saral, S.; Akyol, A. The efficacy of vorinostat in combination with interferon alpha and extracorporeal photopheresis in late stage mycosis fungoides and Sezary syndrome. J. Drugs Derm. 2011, 10, 403–408. [Google Scholar]
- Chiarion-Sileni, V.; Bononi, A.; Fornasa, C.V.; Soraru, M.; Alaibac, M.; Ferrazzi, E.; Redelotti, R.; Peserico, A.; Monfardini, S.; Salvagno, L. Phase II trial of interferon-alpha-2a plus psolaren with ultraviolet light A in patients with cutaneous T-cell lymphoma. Cancer 2002, 95, 569–575. [Google Scholar] [CrossRef]
- Seo, N.; Tokura, Y.; Matsumoto, K.; Furukawa, F.; Takigawa, M. Tumour-specific cytotoxic T lymphocyte activity in Th2-type Sézary syndrome: Its enhancement by interferon-gamma (IFN-gamma) and IL-12 and fluctuations in association with disease activity. Clin. Exp. Immunol. 1998, 112, 403–409. [Google Scholar] [CrossRef]
- Becker, J.C.; Zur Hausen, A. Cells of origin in skin cancer. J. Investig. Derm. 2014, 134, 2491–2493. [Google Scholar] [CrossRef] [Green Version]
- Crombie, J.L.; Armand, P. Diffuse Large B-Cell Lymphoma’s New Genomics: The Bridge and the Chasm. J. Clin. Oncol. 2020, 38, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, M.H.; Nicolay, J.P.; Scarisbrick, J.J.; Zinzani, P.L. The importance of assessing blood tumour burden in cutaneous T-cell lymphoma. Br. J. Derm. 2021, 185, 19–25. [Google Scholar] [CrossRef]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Derm. 2021, 141, 484–495. [Google Scholar] [CrossRef]
- Mashima, E.; Sawada, Y.; Yamaguchi, T.; Yoshioka, H.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Okada, E.; Nakamura, M. A high expression of cell adhesion molecule 1 (CADM1) is an unfavorable prognostic factor in mycosis fungoides. Clin. Immunol. 2018, 193, 121–122. [Google Scholar] [CrossRef]
- Sawada, Y.; Sugita, K.; Kabashima, R.; Hino, R.; Nakamura, M.; Koga, C.; Tokura, Y. CD8+ CD56+ mycosis fungoides with an indolent clinical behaviour: Case report and literature review. Acta Derm. Venereol. 2010, 90, 525–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieselthier, J.S.; Koh, H.K. Sézary syndrome: Diagnosis, prognosis, and critical review of treatment options. J. Am. Acad. Derm. 1990, 22, 381–401. [Google Scholar] [CrossRef]
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1, 1600013. [Google Scholar] [CrossRef]
- Wilson, D.R.; Sen, R.; Sunshine, J.C.; Pardoll, D.M.; Green, J.J.; Kim, Y.J. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 2018, 14, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ye, Y.; Liu, L.; Sha, Q.; Wang, X.; Jiao, T.; Zhang, L.; Wang, J. The lipid platform increases the activity of STING agonists to synergize checkpoint blockade therapy against melanoma. Biomater. Sci. 2021, 9, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Miao, L.; Gao, W.; Chen, Z.; McHugh, K.J.; Sun, Y.; Tochka, Z.; Tomasic, S.; Sadtler, K.; Hyacinthe, A.; et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaaz6606. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tong, Y.; Zheng, Y.; Shi, Y.; Chen, Z.; Li, J.; Liu, X.; Zhang, D.; Yang, H. Cytosolic Delivery of Thiolated Mn-cGAMP Nanovaccine to Enhance the Antitumor Immune Responses. Small 2021, 17, e2006970. [Google Scholar] [CrossRef] [PubMed]
- Chipurupalli, S.; Ganesan, R.; Dhanabal, S.P.; Kumar, M.S.; Robinson, N. Pharmacological STING Activation Is a Potential Alternative to Overcome Drug-Resistance in Melanoma. Front. Oncol. 2020, 10, 758. [Google Scholar] [CrossRef]
- Wilski, N.A.; Stotesbury, C.; Del Casale, C.; Montoya, B.; Wong, E.; Sigal, L.J.; Snyder, C.M. STING Sensing of Murine Cytomegalovirus Alters the Tumor Microenvironment to Promote Antitumor Immunity. J. Immunol. 2020, 204, 2961–2972. [Google Scholar] [CrossRef] [PubMed]
- Ager, C.R.; Zhang, H.; Wei, Z.; Jones, P.; Curran, M.A.; Di Francesco, M.E. Discovery of IACS-8803 and IACS-8779, potent agonists of stimulator of interferon genes (STING) with robust systemic antitumor efficacy. Bioorg. Med. Chem. Lett. 2019, 29, 126640. [Google Scholar] [CrossRef]
- Melms, J.C.; Vallabhaneni, S.; Mills, C.E.; Yapp, C.; Chen, J.Y.; Morelli, E.; Waszyk, P.; Kumar, S.; Deming, D.; Moret, N.; et al. Inhibition of Haspin Kinase Promotes Cell-Intrinsic and Extrinsic Antitumor Activity. Cancer Res. 2020, 80, 798–810. [Google Scholar] [CrossRef]
- Sivick, K.E.; Desbien, A.L.; Glickman, L.H.; Reiner, G.L.; Corrales, L.; Surh, N.H.; Hudson, T.E.; Vu, U.T.; Francica, B.J.; Banda, T.; et al. Magnitude of Therapeutic STING Activation Determines CD8(+) T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2018, 25, 3074–3085.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Su, J.; Wang, J.; Liu, Z.; Wang, T. Activation of STING inhibits cervical cancer tumor growth through enhancing the anti-tumor immune response. Mol. Cell. Biochem. 2021, 476, 1015–1024. [Google Scholar] [CrossRef]
- Vonderhaar, E.P.; Barnekow, N.S.; McAllister, D.; McOlash, L.; Eid, M.A.; Riese, M.J.; Tarakanova, V.L.; Johnson, B.D.; Dwinell, M.B. STING Activated Tumor-Intrinsic Type I Interferon Signaling Promotes CXCR3 Dependent Antitumor Immunity in Pancreatic Cancer. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.H.; Kelly, R.J.; Gorbunova, A.; Omstead, A.N.; Salvitti, M.S.; Zheng, P.; Kosovec, J.E.; Lee, S.; Ayazi, S.; Babar, L.; et al. Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8+ T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget 2021, 12, 292–303. [Google Scholar] [CrossRef]
- Lu, Z.D.; Chen, Y.F.; Shen, S.; Xu, C.F.; Wang, J. Co-delivery of Phagocytosis Checkpoint Silencer and Stimulator of Interferon Genes Agonist for Synergetic Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 29424–29438. [Google Scholar] [CrossRef]
- Levy, E.S.; Chang, R.; Zamecnik, C.R.; Dhariwala, M.O.; Fong, L.; Desai, T.A. Multi-Immune Agonist Nanoparticle Therapy Stimulates Type I Interferons to Activate Antigen-Presenting Cells and Induce Antigen-Specific Antitumor Immunity. Mol. Pharm. 2021, 18, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Sansanaphongpricha, K.; Xie, Y.; Donnelly, C.R.; Luo, X.; Heath, B.R.; Zhao, X.; Bellile, E.; Hu, H.; Chen, H.; et al. Mitigating SOX2-potentiated Immune Escape of Head and Neck Squamous Cell Carcinoma with a STING-inducing Nanosatellite Vaccine. Clin. Cancer Res. 2018, 24, 4242–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Donnelly, C.R.; Gong, W.; Heath, B.R.; Hao, Y.; Donnelly, L.A.; Moghbeli, T.; Tan, Y.S.; Lin, X.; Bellile, E.; et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Investig. 2020, 130, 1635–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Gallo, R.L. Role of Epigenetics in the Regulation of Immune Functions of the Skin. J. Investig. Derm. 2021, 141, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Konno, H.; Barber, G.N. Recurrent Loss of STING Signaling in Melanoma Correlates with Susceptibility to Viral Oncolysis. Cancer Res. 2016, 76, 6747–6759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konno, H.; Yamauchi, S.; Berglund, A.; Putney, R.M.; Mulé, J.J.; Barber, G.N. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 2018, 37, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Jia, L.; Chen, D.; Chang, I.; Lake, M.; Bentolila, L.A.; Wang, C.Y. Targeting KDM4A epigenetically activates tumor-cell-intrinsic immunity by inducing DNA replication stress. Mol. Cell 2021, 81, 2148–2165.e9. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef]
- Wu, J.; Dobbs, N.; Yang, K.; Yan, N. Interferon-Independent Activities of Mammalian STING Mediate Antiviral Response and Tumor Immune Evasion. Immunity 2020, 53, 115–126.e5. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Langberg, K.; Abbas, S.; Odermatt, E.; Yerramothu, P.; Volaric, M.; Reidenbach, M.A.; Krentz, K.J.; Rubinstein, C.D.; Brautigan, D.L.; et al. A non-canonical, interferon-independent signaling activity of cGAMP triggers DNA damage response signaling. Nat. Commun. 2021, 12, 6207. [Google Scholar] [CrossRef] [PubMed]
- Zebertavage, L.K.; Alice, A.; Crittenden, M.R.; Gough, M.J. Transcriptional Upregulation of NLRC5 by Radiation Drives STING- and Interferon-Independent MHC-I Expression on Cancer Cells and T Cell Cytotoxicity. Sci. Rep. 2020, 10, 7376. [Google Scholar] [CrossRef] [PubMed]
- Tansakul, M.; Thim-Uam, A.; Saethang, T.; Makjaroen, J.; Wongprom, B.; Pisitkun, T.; Pisitkun, P. Deficiency of STING Promotes Collagen-Specific Antibody Production and B Cell Survival in Collagen-Induced Arthritis. Front. Immunol. 2020, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bax, C.; Patel, J.; Vazquez, T.; Ravishankar, A.; Bashir, M.M.; Grinnell, M.; Diaz, D.; Werth, V.P. Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis. Theranostics 2021, 11, 7144–7158. [Google Scholar] [CrossRef]
- Oda, T.; Sawada, Y.; Okada, E.; Yamaguchi, T.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Nakamura, M. Hypopituitarism and hypothyroidism following atrioventricular block during nivolumab treatment. J. Derm. 2017, 44, e144–e145. [Google Scholar] [CrossRef] [PubMed]
- Kivisäkk, P.; Alm, G.V.; Fredrikson, S.; Link, H. Neutralizing and binding anti-interferon-beta (IFN-beta) antibodies. A comparison between IFN-beta-1a and IFN-beta-1b treatment in multiple sclerosis. Eur. J. Neurol. 2000, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Xia, T.; Konno, H.; Konno, K.; Ruiz, P.; Barber, G.N. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 2014, 5, 5166. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Sawada, Y.; Nakamura, M. STING Signaling and Skin Cancers. Cancers 2021, 13, 5603. https://doi.org/10.3390/cancers13225603
Sato S, Sawada Y, Nakamura M. STING Signaling and Skin Cancers. Cancers. 2021; 13(22):5603. https://doi.org/10.3390/cancers13225603
Chicago/Turabian StyleSato, Sayaka, Yu Sawada, and Motonobu Nakamura. 2021. "STING Signaling and Skin Cancers" Cancers 13, no. 22: 5603. https://doi.org/10.3390/cancers13225603




