Experimental Validation of the MRcollar: An MR Compatible Applicator for Deep Heating in the Head and Neck Region
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
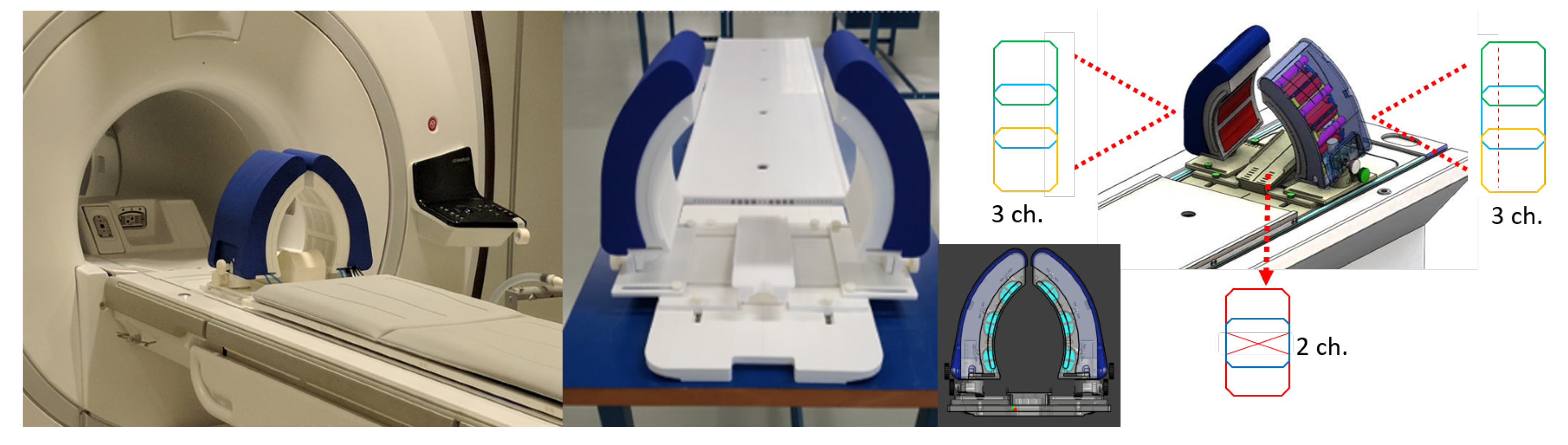
2.1. The MRcollar Design
2.2. Phantoms
2.3. Antenna Characterization Measurements, Heating and Focusing Steering Capabilities
2.4. MR-Compatibility Measurements
3. Results
3.1. Reflection and Cross-Coupling Measurements
3.2. Heating, Steering Capabilities and Focus-Size
3.3. MR-Compatibility Measurements
4. Discussion
4.1. EM Compatibility
4.2. Heating Capabilities
4.3. MR Compatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordóñez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Klingbiel, D.; Gómez, S.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperth. 2016, 32, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; van der Zee, J.; van Tienhoven, G.; Kok, H.P.; Rasch, C.R.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1023–1038. [Google Scholar] [CrossRef] [Green Version]
- Amichetti, M.; Romano, M.; Busana, L.; Bolner, A.; Fellin, G.; Pani, G.; Tomio, L.; Valdagni, R. Hyperfractionated radiation in combination with local hyperthermia in the treatment of advanced squamous cell carcinoma of the head and neck: A phase I–II study. Radiother. Oncol. 1997, 45, 155–158. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, S.; Fu, Z.; Hu, Q.; Wang, L.E.I.; Piao, Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int. J. Hyperth. 2011, 27, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Verduijn, G.M.; de Wee, E.M.; Rijnen, Z.; Togni, P.; Hardillo, J.A.U.; Ten Hove, I.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma—A feasibility study. Int. J. Hyperth. 2018, 34, 994–1001. [Google Scholar] [CrossRef]
- Feasibility, SAR Distribution and Clinical Outcome upon Re-Irradiation and Deep Hyperthermia Using the Hypercollar3D in Head and Neck Cancer Patients. Available online: https://programm.conventus.de/index.php?id=icho2021&tx_coprogramm_programm%5Bprogramm%5D=106&tx_coprogramm_programm%5Bsession%5D=14&tx_coprogramm_programm%5BcurrentPage%5D=&tx_coprogramm_programm%5Baction%5D=programm&tx_coprogramm_programm%5Bcontroller%5D=Source&cHash=861da91e121ea43a7bf09765137e7589 (accessed on 28 September 2021).
- Zschaeck, S.; Weingärtner, J.; Ghadjar, P.; Wust, P.; Mehrhof, F.; Kalinauskaite, G.; Ehrhardt, V.H.; Hartmann, V.; Tinhofer, I.; Heiland, M.; et al. Fever range whole body hyperthermia for re-irradiation of head and neck squamous cell carcinomas: Final results of a prospective study. Oral Oncol. 2021, 116, 105240. [Google Scholar] [CrossRef]
- Paulides, M.; Bakker, J.; Neufeld, E.; van der Zee, J.; Jansen, P.; Levendag, P.; van Rhoon, G. The HYPERcollar: A novel applicator for hyperthermia in the head and neck. Int. J. Hyperth. 2007, 23, 567–576. [Google Scholar] [CrossRef]
- Togni, P.; Rijnen, Z.; Numan, W.C.M.; Verhaart, R.F.; Bakker, J.F.; Van Rhoon, G.C.; Paulides, M.M. Electromagnetic redesign of the HYPERcollar applicator: Toward improved deep local head-and-neck hyperthermia. Phys. Med. Biol. 2013, 58, 5997. [Google Scholar] [CrossRef]
- Rijnen, Z.; Togni, P.; Roskam, R.; van de Geer, S.G.; Goossens, R.H.M.; Paulides, M.M. Quality and comfort in head and neck hyperthermia: A redesign according to clinical experience and simulation studies. Int. J. Hyperth. 2015, 31, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status quo and directions in deep head and neck hyperthermia. Radiat. Oncol. 2016, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Drizdal, T.; Sumser, K.; Bellizzi, G.G.; Fiser, O.; Vrba, J.; Rhoon, G.C.V.; Yeo, D.T.B.; Paulides, M.M. Simulation guided design of the MRcollar: A MR compatible applicator for deep heating in the head and neck region. Int. J. Hyperth. 2021, 38, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther. Onkol. 2011, 187, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trefná, H.D.; Crezee, J.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Nadobny, J.; Hartmann, J.; Lomax, N.; Abdel-Rahman, S.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials. Strahlenther. Onkol. 2017, 193, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Dobšíček Trefná, H.; Schmidt, M.; Van Rhoon, G.; Kok, H.; Gordeyev, S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 276–293. [Google Scholar] [CrossRef] [Green Version]
- Verhaart, R.F.; Verduijn, G.M.; Fortunati, V.; Rijnen, Z.; van Walsum, T.; Veenland, J.F.; Paulides, M.M. Accurate 3D temperature dosimetry during hyperthermia therapy by combining invasive measurements and patient-specific simulations. Int. J. Hyperth. 2015, 31, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Aklan, B.; Zilles, B.; Paprottka, P.; Manz, K.; Pfirrmann, M.; Santl, M.; Abdel-Rahman, S.; Lindner, L. Regional deep hyperthermia: Quantitative evaluation of predicted and direct measured temperature distributions in patients with high-risk extremity soft-tissue sarcoma. Int. J. Hyperth. 2019, 169–184. [Google Scholar] [CrossRef]
- Sebeke, L.C.; Rademann, P.; Maul, A.C.; Yeo, S.Y.; Castillo Gómez, J.D.; Deenen, D.A.; Schmidt, P.; de Jager, B.; Heemels, W.; Grüll, H.; et al. Visualization of thermal washout due to spatiotemporally heterogenous perfusion in the application of a model-based control algorithm for MR-HIFU mediated hyperthermia. Int. J. Hyperth. 2021, 38, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, L.; Wlodarczyk, W.; Nadobny, J.; Weihrauch, M.; Gellermann, J.; Wust, P. Non-invasive magnetic resonance thermography during regional hyperthermia. Int. J. Hyperth. 2010, 26, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Adibzadeh, F.; Sumser, K.; Curto, S.; Yeo, D.T.B.; Shishegar, A.A.; Paulides, M.M. Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia. Int. J. Hyperth. 2020, 37, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical performance and future potential of magnetic resonance thermometry in hyperthermia. Cancers 2021, 13, 31. [Google Scholar] [CrossRef]
- Numan, W.C.M.; Hofstetter, L.W.; Kotek, G.; Bakker, J.F.; Fiveland, E.W.; Houston, G.C.; Kudielka, G.; Yeo, D.T.B.; Paulides, M.M. Exploration of MR-guided head and neck hyperthermia by phantom testing of a modified prototype applicator for use with proton resonance frequency shift thermometry. Int. J. Hyperth. 2014, 30, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Oezerdem, C.; Hoffmann, W.; van de Lindt, T.; Periquito, J.; Ji, Y.; Ghadjar, P.; Budach, V.; Wust, P.; Niendorf, T. Thermal magnetic resonance: Physics considerations and electromagnetic field simulations up to 23.5 Tesla (1 GHz). Radiat. Oncol. 2015, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Oberacker, E.; Kuehne, A.; Wang, S.; Eigentler, T.W.; Grass, E.; Niendorf, T. Multi-channel RF supervision module for thermal magnetic resonance based cancer therapy. Cancers 2021, 13, 1001. [Google Scholar] [CrossRef]
- Sumser, K.; Bellizzi, G.G.; Forner, R.; Drizdal, T.; Tamames, J.A.H.; Van Rhoon, G.C.; Paulides, M.M. Dual-function MR-guided hyperthermia: An innovative integrated approach and experimental demonstration of proof of principle. IEEE Trans. Biomed. Eng. 2020, 68, 712–717. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Sumser, K.; Van Rhoon, G.C.; Forner, R.; Paulides, M.M. Feasibility of Integrating an MR Receive Coil Array into the MRcollar. In Proceedings of the 2020 XXXIIIrd General Assembly and Scientific Symposium of the International Union of Radio Science, Rome, Italy, 29 August–5 September 2020; pp. 1–4. [Google Scholar]
- Paulides, M.M.; Mestrom, R.M.C.; Salim, G.; Adela, B.B.; Numan, W.C.M.; Drizdal, T.; Yeo, D.T.B.; Smolders, A.B. A printed Yagi–Uda antenna for application in magnetic resonance thermometry guided microwave hyperthermia applicators. Phys. Med. Biol. 2017, 62, 1831. [Google Scholar] [CrossRef]
- Fink, M. Time reversal of ultrasonic fields. I. Basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 555–566. [Google Scholar] [CrossRef]
- Bakker, J.F.; Paulides, M.M.; Westra, A.H.; Schippers, H.; Van Rhoon, G.C. Design and test of a 434 MHz multi-channel amplifier system for targeted hyperthermia applicators. Int. J. Hyperth. 2010, 26, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Harms, W.B.; Mutic, S.; Purdy, J.A. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998, 25, 656–661. [Google Scholar] [CrossRef]
- Goerner, F.L.; Clarke, G.D. Measuring signal-to-noise ratio in partially parallel imaging MRI. Med Phys. 2011, 38, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Poorter, J.D.; Wagter, C.D.; Deene, Y.D.; Thomsen, C.; Ståhlberg, F.; Achten, E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: In vivo results in human muscle. Magn. Reson. Med. 1995, 33, 74–81. [Google Scholar] [CrossRef]
- Paulides, M.M.; Bakker, J.F.; van Rhoon, G.C. Electromagnetic head-and-neck hyperthermia applicator: Experimental phantom verification and FDTD model. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 612–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulides, M.M.; Wielheesen, D.H.M.; Van Der Zee, J.; Van Rhoon, G.C. Assessment of the local SAR distortion by major anatomical structures in a cylindrical neck phantom. Int. J. Hyperth. 2005, 21, 125–140. [Google Scholar] [CrossRef]
- Bucci, O.M.; Gennarelli, C.; Savarese, C. Representation of electromagnetic fields over arbitrary surfaces by a finite and nonredundant number of samples. IEEE Trans. Antennas Propag. 1998, 46, 351–359. [Google Scholar] [CrossRef]
- Kok, H.P.; Van Haaren, P.M.A.; Van de Kamer, J.B.; Wiersma, J.; Van Dijk, J.D.P.; Crezee, J. High-resolution temperature-based optimization for hyperthermia treatment planning. Phys. Med. Biol. 2005, 50, 3127. [Google Scholar] [CrossRef]
- Rijnen, Z.; Bakker, J.F.; Canters, R.A.M.; Togni, P.; Verduijn, G.M.; Levendag, P.C.; Van Rhoon, G.C.; Paulides, M.M. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int. J. Hyperth. 2013, 29, 181–193. [Google Scholar] [CrossRef]
- Bellizzi, G.G.; Drizdal, T.; van Rhoon, G.C.; Crocco, L.; Isernia, T.; Paulides, M.M. The potential of constrained SAR focusing for hyperthermia treatment planning: Analysis for the head & neck region. Phys. Med. Biol. 2018, 64, 015013. [Google Scholar] [PubMed]
- Cappiello, G.; Mc Ginley, B.; Elahi, M.A.; Drizdal, T.; Paulides, M.M.; Glavin, M.; O’Halloran, M.; Jones, E. Differential evolution optimization of the SAR distribution for head and neck hyperthermia. IEEE Trans. Biomed. Eng. 2017, 64, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Maass, P.; Wust, P.; Seebass, M. A fast algorithm to find optimal controls of multiantenna applicators in regional hyperthermia. Phys. Med. Biol. 2001, 46, 2503. [Google Scholar] [CrossRef]
- Kuehne, A.; Oberacker, E.; Waiczies, H.; Niendorf, T. Solving the time-and frequency-multiplexed problem of constrained radiofrequency induced hyperthermia. Cancers 2020, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Adibzadeh, F.; Paulides, M.M.; van Rhoon, G.C. SAR thresholds for electromagnetic exposure using functional thermal dose limits. Int. J. Hyperth. 2018, 34, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Sumser, K.; Bellizzi, G.G.; Van Rhoon, G.C.; Paulides, M.M. The potential of adjusting water bolus liquid properties for economic and precise MR thermometry guided radiofrequency hyperthermia. Sensors 2020, 20, 2946. [Google Scholar] [CrossRef] [PubMed]





| Topic | Phantom | Measurement Method |
|---|---|---|
| Antenna characterization | Cylindrical Split Phantom | Antenna reflection (S) and cross coup- ling (S) measurements by the VNA. |
| Heating ability | ||
| SAR | Cylindrical Split Phantom | Maximum temperature increase in the first 60 s measured by the fiber optic probes and scaled using the specific heat capacity of the phantom as well as heating duration. |
| Focus Size and steering | Cylindrical Split Phantom | Differential temperature maps from an IR camera before and after 180 s of heating. Focus size was defined as length and width of the 50% iso-SAR contour. This experiment was repeated for three different focus locations with two identical phantoms. |
| MR compatibility | ||
| MR SNR | Cylindrical Phantom | The dual image subtraction method was performed using a fast gradient echo sequence. |
| Heating in MRI | Cylindrical Split Phantom | MR images were acquired before and after the 480 s of heating window. Temperature change calculated by PRFS method. |
| Variable | Gamma10 | Gamma5 |
|---|---|---|
| Central Focus | 88% | 72% |
| X-steering | 98% | 74% |
| Z-steering | 99% | 79% |
| MRT | 99% | 92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumser, K.; Drizdal, T.; Bellizzi, G.G.; Hernandez-Tamames, J.A.; van Rhoon, G.C.; Paulides, M.M. Experimental Validation of the MRcollar: An MR Compatible Applicator for Deep Heating in the Head and Neck Region. Cancers 2021, 13, 5617. https://doi.org/10.3390/cancers13225617
Sumser K, Drizdal T, Bellizzi GG, Hernandez-Tamames JA, van Rhoon GC, Paulides MM. Experimental Validation of the MRcollar: An MR Compatible Applicator for Deep Heating in the Head and Neck Region. Cancers. 2021; 13(22):5617. https://doi.org/10.3390/cancers13225617
Chicago/Turabian StyleSumser, Kemal, Tomas Drizdal, Gennaro G. Bellizzi, Juan A. Hernandez-Tamames, Gerard C. van Rhoon, and Margarethus Marius Paulides. 2021. "Experimental Validation of the MRcollar: An MR Compatible Applicator for Deep Heating in the Head and Neck Region" Cancers 13, no. 22: 5617. https://doi.org/10.3390/cancers13225617







