Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications
Abstract
:Simple Summary
Abstract
1. Introduction
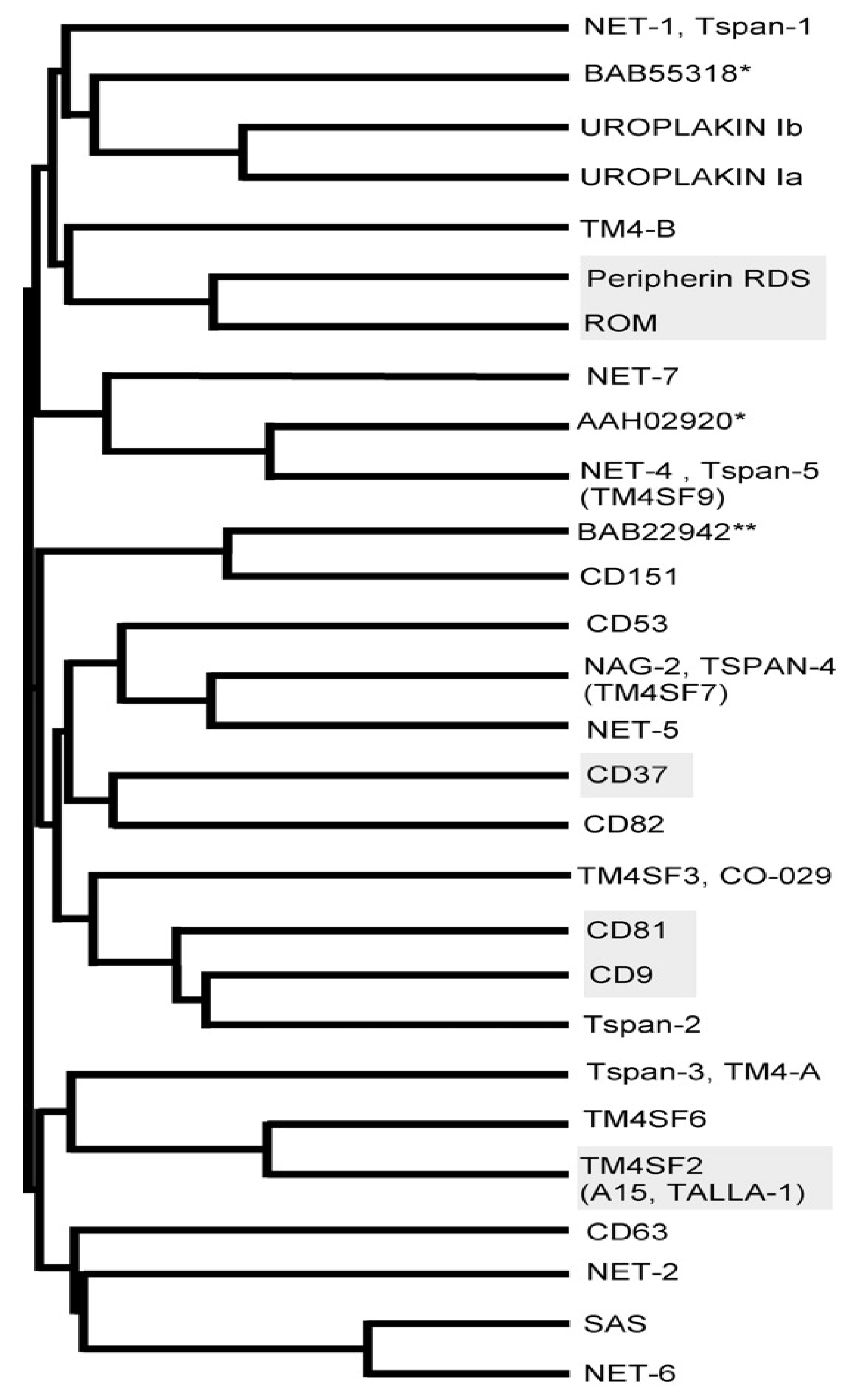
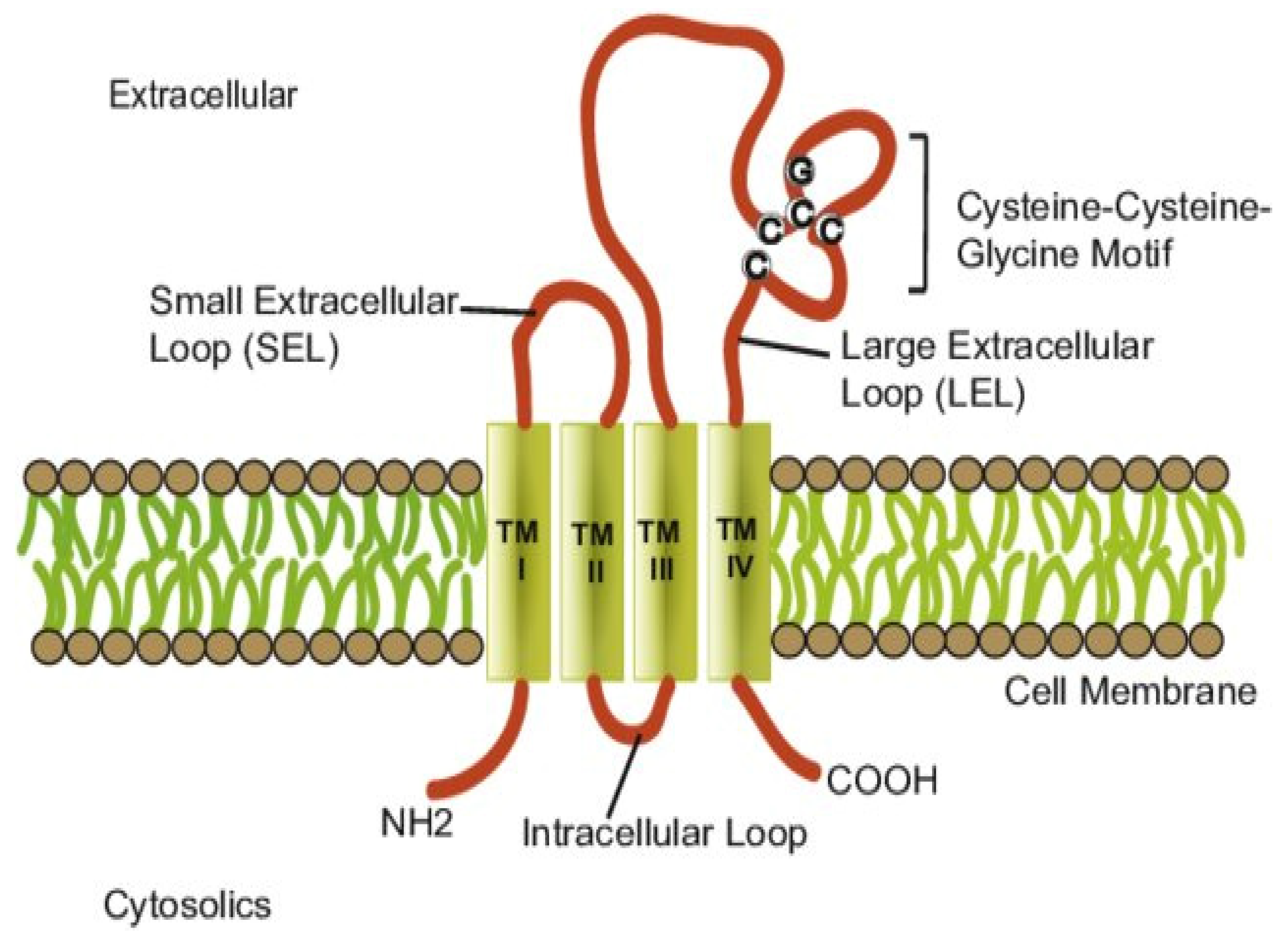
2. Structure and Function
3. General Physiology
4. Tetraspanins in Cancer: Malignancy Role
| Tetraspanin | Type of Cancer | Mechanism of Action | References |
|---|---|---|---|
| TSPAN 1 |
| Promoter of invasion but the mechanism has not been determined. | [41,42] |
| TSPAN 8 |
| TSPAN8 suppresses the motility of cells by regulating the tumor cell–matrix and cell to cell adhesion in colorectal cancer. At the same time, regulation by the E-cadherin/p120ctn complex has been found to be a promoter of invasion in colon cancer. | [43,44] |
| TSPAN 13 |
| Cell motility in breast cancer is suppressed by downregulation of the matrix metalloproteinases. | [45] |
| CD9 |
| Suppressor of cell motility in ovarian carcinoma by modifying cell adhesion on the extracellular matrix; altered integrins (β1, α2, α3β1, α5, and α6) were found due to downregulation of CD9. Suppressor in fibrosarcoma by cell motility inhibition through CD9 complexes formed with TGFα, EGFR, EWI-2, EWIF, and β1. Promoter of cell motility in breast cancer by promoting α3β1 integrin. | [46,47,48] |
| CD63 |
| Suppressor in colon cancer by regulating cell adhesion and migration. | [49] |
| CD81 |
| Promoter of cell motility by promoting α3β1 integrin in breast cancer. Promoter in histiocytic lymphoma through cell membrane structures. | [48,50] |
| CD82 |
| Suppressor in ovarian cancer by inhibiting αvβ3 integrin/vitronectin-mediated cell motility and proliferation. Suppressor in lung cancer by regulation of β1 integrin maturation. | [51,52] |
| CD151 |
| Promoter in gastric cancer in association with α3 integrins. Promoter in breast cancer in association with integrins promoting a signaling pathway (HGF/c-MET). Promoter in liver cancer by increasing Rac/Cdc42 activity. | [53,54,55] |
4.1. Tumor Progression-Promoting Tetraspanins
4.1.1. TSPAN 8
4.1.2. CD151
4.2. Tumor Progression-Suppressing Tetraspanins
4.2.1. CD9
4.2.2. CD63
4.2.3. CD82
4.3. Tetraspanins in Colorectal Cancer
5. Nanoparticles and Extracellular Vesicles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaseen, I.H.; Monk, P.N.; Partridge, L.J. Tspan2: A tetraspanin protein involved in oligodendrogenesis and cancer metastasis. Biochem. Soc. Trans. 2017, 45, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, T.; Hochheimer, N. Tetraspanins. Curr. Biol. 2020, 30, R204–R206. [Google Scholar] [CrossRef] [PubMed]
- Belov, L.; Zhou, J.; Christopherson, R.I. Cell surface markers in colorectal cancer prognosis. Int. J. Mol. Sci. 2011, 12, 78–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maecker, H.T.; Todd, S.C.; Levy, S. The tetraspanin superfamily: Molecular facilitators. FASEB J. 1997, 11, 428–442. [Google Scholar] [CrossRef]
- Le Naour, F.; André, M.; Greco, C.; Billard, M.; Sordat, B.; Emile, J.; Lanza, F.; Boucheix, C.; Rubinstein, E. Profiling of the tetraspanin web of human colon cancer cells. Mol. Cell. Proteom. 2006, 5, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemler, M.E. Specific tetraspanin functions. J. Cell Biol. 2001, 155, 1103–1108. [Google Scholar] [CrossRef]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef]
- Levy, S.; Shoham, T. Protein-protein interactions in the tetraspanin web. Physiology 2005, 20, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Sari, I.N.; Zia, M.F.; Lee, S.R.; Song, S.J.; Kwon, H.Y. Tetraspanins: Spanning from solid tumors to hematologic malignancies. Exp. Hematol. 2016, 44, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Park, C.S.; Jeoung, M.H.; Lee, W.R.; Go, N.K.; Choi, J.R.; Lee, T.S.; Shim, H.; Lee, S. Generation of a human antibody that inhibits TSPAN8-mediated invasion of metastatic colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015, 468, 774–780. [Google Scholar] [CrossRef]
- Yan, W.; Huang, J.; Zhang, Q.; Zhang, J. Role of Metastasis Suppressor KAI1/CD82 in Different Cancers. J. Oncol. 2021, 2021, 9924473. [Google Scholar] [CrossRef] [PubMed]
- Kreis, T.; Vale, R. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Sun, T.; Zhao, H.; Provet, J.; Aebi, U.; Wu, X. Formation of asymmetric unit membrane during urothelial differentiation. Mol. Biol. Rep. 1996, 23, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, S.; Westerveld, A.; De Jong, P.T.; Bleeker-Wagemakers, E.M.; Bergen, A.A. Retinitis pigmentosa: Defined from a molecular point of view. Surv. Ophthalmol. 1999, 43, 321–334. [Google Scholar] [CrossRef]
- Shaw, S.; Ginther-Lure, G.; Lksw, G. Antibodies and molecules of the 5th International Workshop of Leukocyte Differentiation Antigens. In Leukocyte Typing V: White Cell Differentiation Antigens; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Vischer, U.M.; Wagner, D.D. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood 1993, 82, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, T.; Tool, A.; van der Schoot, C.; Ginsel, L.; Onderwater, J.; Roos, D.; Verhoeven, A. Membrane surface antigen expression on neutrophils: A reappraisal of the use of surface markers for neutrophil activation. Blood 1991, 78, 1105–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucheix, C.; Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. CMLS 2001, 58, 1189–1205. [Google Scholar] [CrossRef]
- Detchokul, S.; Williams, E.; Parker, M.W.; Frauman, A.G. Tetraspanins as regulators of the tumour microenvironment: Implications for metastasis and therapeutic strategies. Br. J. Pharm. 2014, 171, 5462–5490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, A.R.; Czibener, C.; Andrews, N.W. Palmitoylation-dependent association with CD63 targets the Ca2 sensor synaptotagmin VII to lysosomes. J. Cell Biol. 2010, 191, 599–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Lee, S.; Suzuki, E.; Dugan, K.D.; Stoddard, A.; Li, H.; Chodosh, L.A.; Montell, C. A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. EMBO J. 2004, 23, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Dornier, E.; Coumailleau, F.; Ottavi, J.; Moretti, J.; Boucheix, C.; Mauduit, P.; Schweisguth, F.; Rubinstein, E. TspanC8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J. Cell. Biol. 2012, 199, 481–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, P.K.; Feest, C.; Depoil, D.; Treanor, B.; Montaner, B.; Otipoby, K.L.; Carter, R.; Justement, L.B.; Bruckbauer, A.; Batista, F.D. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 2013, 38, 461–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, M.; Muratori, C.; Corso, S.; Tenaglia, E.; Bertotti, A.; Capparuccia, L.; Trusolino, L.; Comoglio, P.M.; Tamagnone, L. The tetraspanin CD151 is required for Met-dependent signaling and tumor cell growth. J. Biol. Chem. 2010, 285, 38756–38764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odintsova, E.; Butters, T.D.; Monti, E.; Sprong, H.; van Meer, G.; Berditchevski, F. Gangliosides play an important role in the organization of CD82-enriched microdomains. Biochem. J. 2006, 400, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Sumida, Y.; Fujibayashi, A.; Fukaguchi, K.; Sanzen, N.; Nishiuchi, R.; Sekiguchi, K. The tetraspanin CD151 regulates cell morphology and intracellular signaling on laminin-511. FEBS J. 2008, 275, 3335–3351. [Google Scholar] [CrossRef]
- Anzai, N.; Lee, Y.; Youn, B.; Fukuda, S.; Kim, Y.; Mantel, C.; Akashi, M.; Broxmeyer, H.E. C-kit associated with the transmembrane 4 superfamily proteins constitutes a functionally distinct subunit in human hematopoietic progenitors. Blood J. Am. Soc. Hematol. 2002, 99, 4413–4421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissel, N.; Leroy, H.; Brethon, B.; Philippe, N.; De Botton, S.; Auvrignon, A.; Raffoux, E.; Leblanc, T.; Thomas, X.; Hermine, O. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia 2006, 20, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, N.; Wang, Z.; Ashman, L.K.; Brady-Kalnay, S.M.; Kreidberg, J.A. α3β1 integrin–CD151, a component of the cadherin–catenin complex, regulates PTPμ expression and cell–cell adhesion. J. Cell. Biol. 2003, 163, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Ablin, R.J.; Jiang, W.G.; Dvorak, H.F.; Gold, P.; Welch, D.; Kobayashi, H.; Mansel, R.E.; Pantel, K. Cancer Metastasis—Biology and Treatment; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Brakebusch, C.; Bouvard, D.; Stanchi, F.; Sakai, T.; Fassler, R.L.A. Integrins in invasive growth. J. Clin. Investig. 2002, 109, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Velling, T.; Nilsson, S.; Stefansson, A.; Johansson, S. β1-Integrins induce phosphorylation of Akt on serine 473 independently of focal adhesion kinase and Src family kinases. EMBO Rep. 2004, 5, 901–905. [Google Scholar] [CrossRef]
- Sugiura, T.; Berditchevski, F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2). J. Cell Biol. 1999, 146, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; O’Toole, T.E.; Smith, J.W.; Fransvea, E.; Ruggeri, Z.M.; Ginsberg, M.H.; Hughes, P.E.; Pampori, N.; Shattil, S.J.; Saven, A.; et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, Q.; Sharma, C.; Knoblich, K.; Hemler, M.E. Tetraspanin protein contributions to cancer. Biochem. Soc. Trans. 2011, 39, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Testa, J.E.; Brooks, P.C.; Lin, J.M.; Quigley, J.P. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999, 59, 3812–3820. [Google Scholar] [PubMed]
- Richardson, M.M.; Jennings, L.K.; Zhang, X.A. Tetraspanins and tumor progression. Clin. Exp. Metastasis 2011, 28, 261–270. [Google Scholar] [CrossRef]
- Hong, I.; Kim, Y.; Jeoung, D.; Kim, K.; Lee, H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp. Mol. Med. 2005, 37, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, D.; Zhao, R.; Li, H.; Zhu, J. Suppression of TSPAN1 by RNA interference inhibits proliferation and invasion of colon cancer cells in vitro. Tumori J. 2010, 96, 744–750. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Wei, Y.; Zhou, J.; Wu, Y.; Zhang, Y.; Qin, J.; Zhu, Y. The effect of NET-1 on the proliferation, migration and endocytosis of the SMMC-7721 HCC cell line. Oncol. Rep. 2012, 27, 1944–1952. [Google Scholar]
- Guo, Q.; Xia, B.; Zhang, F.; Richardson, M.M.; Li, M.; Zhang, J.S.; Chen, F.; Zhang, X.A. Tetraspanin CO-029 inhibits colorectal cancer cell movement by deregulating cell-matrix and cell-cell adhesions. PLoS ONE 2012, 7, e38464. [Google Scholar] [CrossRef] [Green Version]
- Greco, C.; Bralet, M.P.; Ailane, N.; Dubart-Kupperschmitt, A.; Rubinstein, E.; Le Naour, F.; Boucheix, C. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010, 70, 7674–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Sossey-Alaoui, K.; Beachy, S.H.; Geradts, J. The tetraspanin superfamily member NET-6 is a new tumor suppressor gene. J. Cancer Res. Clin. Oncol. 2007, 133, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Kato, H.; Nishimura, N.; Ishiwata, I.; Ikeda, H.; Ito, R.; Yoshiki, T.; Ishikura, H. Down-regulation of CD9 in human ovarian carcinoma cell might contribute to peritoneal dissemination: Morphologic alteration and reduced expression of beta1 integrin subsets. Cancer Res. 2005, 65, 2617–2625. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sun, Y.; Jin, Z.; Jing, X. Functional and biochemical studies of CD9 in fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 350, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Gustafson-Wagner, E.; Stipp, C.S. The CD9/CD81 tetraspanin complex and tetraspanin CD151 regulate α3β1 integrin-dependent tumor cell behaviors by overlapping but distinct mechanisms. PLoS ONE 2013, 8, e61834. [Google Scholar]
- Sordat, I.; Decraene, C.; Silvestre, T.; Petermann, O.; Auffray, C.; Piétu, G.; Sordat, B. Complementary DNA arrays identify CD63 tetraspanin and α3 integrin chain as differentially expressed in low and high metastatic human colon carcinoma cells. Lab. Investig. 2002, 82, 1715–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, R.; Guo, Q.; Xia, B.; Zhang, Y.H.; Giesert, E.E.; Levy, S.; Zheng, J.J.; Zhang, X.A. Tetraspanins regulate the protrusive activities of cell membrane. Biochem. Biophys. Res. Commun. 2011, 415, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruseva, Z.; Geiger, P.X.C.; Hutzler, P.; Kotzsch, M.; Luber, B.; Schmitt, M.; Gross, E.; Reuning, U. Tumor suppressor KAI1 affects integrin αvβ3-mediated ovarian cancer cell adhesion, motility, and proliferation. Exp. Cell Res. 2009, 315, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.K.; Lee, J.Y.; Lim, Y.; Lee, K.H.; Jo, Y. Effect of KAI1/CD82 on the β1 integrin maturation in highly migratory carcinoma cells. Biochem. Biophys. Res. Commun. 2007, 359, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Liu, Q.; Sun, Y.; Yu, J.; Xu, W. Overexpression of CD151 predicts prognosis in patients with resected gastric cancer. PLoS ONE 2013, 8, e58990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klosek, S.K.; Nakashiro, K.; Hara, S.; Goda, H.; Hasegawa, H.; Hamakawa, H. CD151 regulates HGF-stimulated morphogenesis of human breast cancer cells. Biochem. Biophys. Res. Commun. 2009, 379, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Wang, J.; Liu, W.; Zuo, H.; Qin, J.; Wang, D.; Zeng, H.; Liu, Z. CD151 promotes cancer cell metastasis via integrins α3β1 and α6β1 in vitro. Mol. Med. Rep. 2012, 6, 1226–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanetaka, K.; Sakamoto, M.; Yamamoto, Y.; Yamasaki, S.; Lanza, F.; Kanematsu, T.; Hirohashi, S. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J. Hepatol. 2001, 35, 637–642. [Google Scholar] [CrossRef]
- Claas, C.; Seiter, S.; Claas, A.; Savelyeva, L.; Schwab, M.; Zoller, M. Association between the rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulopathy. J. Cell Biol. 1998, 141, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Claas, C.; Wahl, J.; Orlicky, D.J.; Karaduman, H.; Schnölzer, M.; Kempf, T.; Zöller, M. The tetraspanin D6. 1A and its molecular partners on rat carcinoma cells. Biochem. J. 2005, 389, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadej, R.; Romanska, H.; Baldwin, G.; Gkirtzimanaki, K.; Novitskaya, V.; Filer, A.D.; Krcova, Z.; Kusinska, R.; Ehrmann, J.; Buckley, C.D. CD151 regulates tumorigenesis by modulating the communication between tumor cells and endothelium. Mol. Cancer Res. 2009, 7, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, A.; Lewis, J.; DeGryse, B.; Stuhlmann, H.; Quigley, J.P. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008, 13, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Rappa, G.; Green, T.M.; Karbanova, J.; Corbeil, D.; Lorico, A. Tetraspanin CD9 determines invasiveness and tumorigenicity of human breast cancer cells. Oncotarget 2015, 6, 7970–7991. [Google Scholar] [CrossRef]
- Jang, H.; Lee, H. A decrease in the expression of CD63 tetraspanin protein elevates invasive potential of human melanoma cells. Exp. Mol. Med. 2003, 35, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 2014, 14, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Umeda, R.; Satouh, Y.; Takemoto, M.; Nakada-Nakura, Y.; Liu, K.; Yokoyama, T.; Shirouzu, M.; Iwata, S.; Nomura, N.; Sato, K. Structural insights into tetraspanin CD9 function. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Woegerbauer, M.; Thurnher, D.; Houben, R.; Pammer, J.; Kloimstein, P.; Heiduschka, G.; Petzelbauer, P.; Erovic, B.M. Expression of the tetraspanins CD9, CD37, CD63, and CD151 in Merkel cell carcinoma: Strong evidence for a posttranscriptional fine-tuning of CD9 gene expression. Mod. Pathol. 2010, 23, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Erovic, B.M.; Pammer, J.; Hollemann, D.; Woegerbauer, M.; Geleff, S.; Fischer, M.B.; Burian, M.; Frommlet, F.; Neuchrist, C. Motility-related protein-1/CD9 expression in head and neck squamous cell carcinoma. Head Neck J. Sci. Spec. Head Neck 2003, 25, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Ovalle, S.; Gutiérrez-López, M.D.; Olmo, N.; Turnay, J.; Lizarbe, M.A.; Majano, P.; Molina-Jiménez, F.; López-Cabrera, M.; Yáñez-Mó, M.; Sánchez-Madrid, F. The tetraspanin CD9 inhibits the proliferation and tumorigenicity of human colon carcinoma cells. Int. J. Cancer 2007, 121, 2140–2152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Shin, S.; Yim, S.; Lee, K.Y.; Kang, H.; Kim, T.; Chung, Y. CD63 as a biomarker for predicting the clinical outcomes in adenocarcinoma of lung. Lung Cancer 2007, 57, 46–53. [Google Scholar] [CrossRef]
- Malik, F.A.; Sanders, A.J.; Jiang, W.G. KAI-1CD82, The molecule and clinical implication in cancer and cancer metastasis. Histol. Histopathol. 2013, 24, 519–530. [Google Scholar]
- Christgen, M.; Christgen, H.; Heil, C.; Krech, T.; Länger, F.; Kreipe, H.; Lehmann, U. Expression of KAI1/CD82 in distant metastases from estrogen receptor-negative breast cancer. Cancer Sci. 2009, 100, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Miranti, C. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene 2006, 25, 2367–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhong, L.; Feng, M.; Guo, Y. A Positive Tetraspanin 8 (TSPAN8)/beta-Catenin Regulatory Loop Enhances the Stemness of Colorectal Cancer Cells. Med. Sci. Monit. 2019, 25, 9594–9601. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Kimura, M.; Asari, S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol. Rep. 2012, 28, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Lin, S.; Lee, C.; Lin, Y.; Lee, J. Dynamic change of tetraspanin CD151 membrane protein expression in colorectal cancer patients. Cancer Investig. 2011, 29, 542–547. [Google Scholar] [CrossRef]
- Kaprio, T.; Hagstrom, J.; Andersson, L.C.; Haglund, C. Tetraspanin CD63 independently predicts poor prognosis in colorectal cancer. Histol. Histopathol. 2020, 35, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Andrijes, R. The Role of Tetraspanin 6 in Colorectal Cancer. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2019. Available online: http://etheses.bham.ac.uk/id/eprint/9145/ (accessed on 10 November 2021).
- Kogure, A.; Yoshioka, Y.; Ochiya, T. Extracellular vesicles in cancer metastasis: Potential as therapeutic targets and materials. Int. J. Mol. Sci. 2020, 21, 4463. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Achreja, A.; Iessi, E.; Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Nagrath, D.; Fais, S. The key role of extracellular vesicles in the metastatic process. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, J.S.; Bond, D.R.; Jankowski, H.; Goldie, B.J.; Burchell, R.; Naudin, C.; Smith, N.D.; Scarlett, C.J.; Larsen, M.R.; Dun, M.D. Extracellular vesicles with altered tetraspanin CD9 and CD151 levels confer increased prostate cell motility and invasion. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Manrique, P.; Serrano-Pertierra, E.; Lozano-Andrés, E.; López-Martín, S.; Matos, M.; Gutiérrez, G.; Yáñez-Mó, M.; Blanco-López, M.C. Selected Tetraspanins Functionalized Niosomes as Potential Standards for Exosome Immunoassays. Nanomaterials 2020, 10, 971. [Google Scholar] [CrossRef]
- Thapa, R.K.; Nguyen, H.T.; Jeong, J.; Kim, J.R.; Choi, H.; Yong, C.S.; Kim, J.O. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Thapa, R.K.; Shin, B.S.; Jeong, J.; Kim, J.; Yong, C.S.; Kim, J.O. CD9 monoclonal antibody-conjugated PEGylated liposomes for targeted delivery of rapamycin in the treatment of cellular senescence. Nanotechnology 2017, 28, 095101. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ou, W.; Dai Phung, C.; Nguyen, T.T.; Pham, T.T.; Poudel, K.; Gautam, M.; Nguyen, H.T.; Jeong, J.; Yong, C.S. Targeting and clearance of senescent foamy macrophages and senescent endothelial cells by antibody-functionalized mesoporous silica nanoparticles for alleviating aorta atherosclerosis. Biomaterials 2021, 269, 120677. [Google Scholar]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef]
- Tonigold, M.; Simon, J.; Estupiñán, D.; Kokkinopoulou, M.; Reinholz, J.; Kintzel, U.; Kaltbeitzel, A.; Renz, P.; Domogalla, M.P.; Steinbrink, K. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat. Nanotechnol. 2018, 13, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Syed, P.; Lehtinen, L.; Leivo, J.; Gidwani, K.; Wittfooth, S.; Pettersson, K.; Lamminmäki, U. A nanoparticle-based approach for the detection of extracellular vesicles. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Polyakov, D.; Sinitsyna, E.; Grudinina, N.; Antipchik, M.; Sakhabeev, R.; Korzhikov-Vlakh, V.; Shavlovsky, M.; Korzhikova-Vlakh, E.; Tennikova, T. Polymer Particles Bearing Recombinant LEL CD81 as Trapping Systems for Hepatitis C Virus. Pharmaceutics 2021, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]

| Tetraspanin | Overview |
|---|---|
| TSPAN8 |
|
| CD151 |
|
| Tetraspanin | Overview |
|---|---|
| CD9 |
|
| CD63 |
|
| CD82 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titu, S.; Grapa, C.M.; Mocan, T.; Balacescu, O.; Irimie, A. Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications. Cancers 2021, 13, 5662. https://doi.org/10.3390/cancers13225662
Titu S, Grapa CM, Mocan T, Balacescu O, Irimie A. Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications. Cancers. 2021; 13(22):5662. https://doi.org/10.3390/cancers13225662
Chicago/Turabian StyleTitu, Stefan, Cristiana Maria Grapa, Teodora Mocan, Ovidiu Balacescu, and Alexandru Irimie. 2021. "Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications" Cancers 13, no. 22: 5662. https://doi.org/10.3390/cancers13225662







