Fracture Risk Evaluation of Bone Metastases: A Burning Issue
Abstract
:Simple Summary
Abstract
1. Introduction
2. Main Pathophysiological and Clinical Features of Bone Metastases
3. Bone Metastases: New Clinical Insights
- (1)
- A personalized medicine based on the molecular diagnosis of the tumor. Molecular diagnosis of the tumor has enabled refining of the histological classification and has revealed considerable variations of overall survival among molecular subgroups. For instance, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutated adenocarcinoma lung cancer are associated with a poorer prognosis than wild type adenocarcinoma [22]. Variations within the histological type have also been observed for bone affinity; for example, Epidermal Growth Factor Receptor (EGFR)-mutated lung adenocarcinoma have a higher bone affinity than the one with ALK translocation [23,24,25,26]. Tumor molecular diagnosis used to be restricted to primary tumors and soft metastases, and is now routinely available for bone metastases [27].
- (2)
- The advent of targeted therapy and immunotherapy have provoked a considerable increase in life expectancy, even for patients whose cancers have spread to distant parts of the body (stage IV). For example, gefitinib in lung cancer has drastically improved life expectancy [28]. Similarly, pembrolizumab has also improved life expectancy in lung cancer [29], even in stage IV metastatic cancers. Both these examples highlight that prognosis is prolonged far beyond the historical prognosis of synchronous bone metastatic lung adenocarcinoma [30]. Thus, more and more patients stabilize for a long period of time, which raises new questions about profit and loss balance for anti-resorptive agents and dose-intensity treatments. Indeed, bone metastatic patients in anti-resorptive agent phase III trials were treated during 24 months, however long-term data are still not available, while this clinical situation is becoming common. Furthermore, de-escalation studies are ongoing. Bisphosphonate studies have shown that after an initial monthly regimen, it is possible to space out the injections [31,32,33,34]. Data about denosumab, a monoclonal antibody and not a pyrophosphate analogue, are very scarce. Moreover, it is already known that soon after denosumab suspension, a bone remodeling flare occurs; this flare is conceptually not desirable for patients as it exposes them to a benign fracture cascade [35,36,37], highlighting the importance of blocking bone remodeling at the end of denosumab sequence using a powerful bisphosphonate. Interestingly, recent ESMO guidelines have evolved and propose a first switch toward a personalized bone antiresorptive agent prescription after an initial phase of 3–6 months of dose-dense monthly infusions [38].
- (3)
- The observation of the high lability (transition from lytic to sclerotic aspect) of bone metastases with the use of targeted therapies. Indeed, it is amazing to observe how quickly a highly osteolytic lesion responding well to anti-hormonal treatment or to targeted therapies such as EGFR inhibitor treatment, may condense, within a short period of time [39]. A synergistic effect has also been observed in combination with Rankl inhibition [40].
- (4)
4. Current Fracture Risk Evaluation of the Tumoral Bone
4.1. Bone Metastasis Cartography
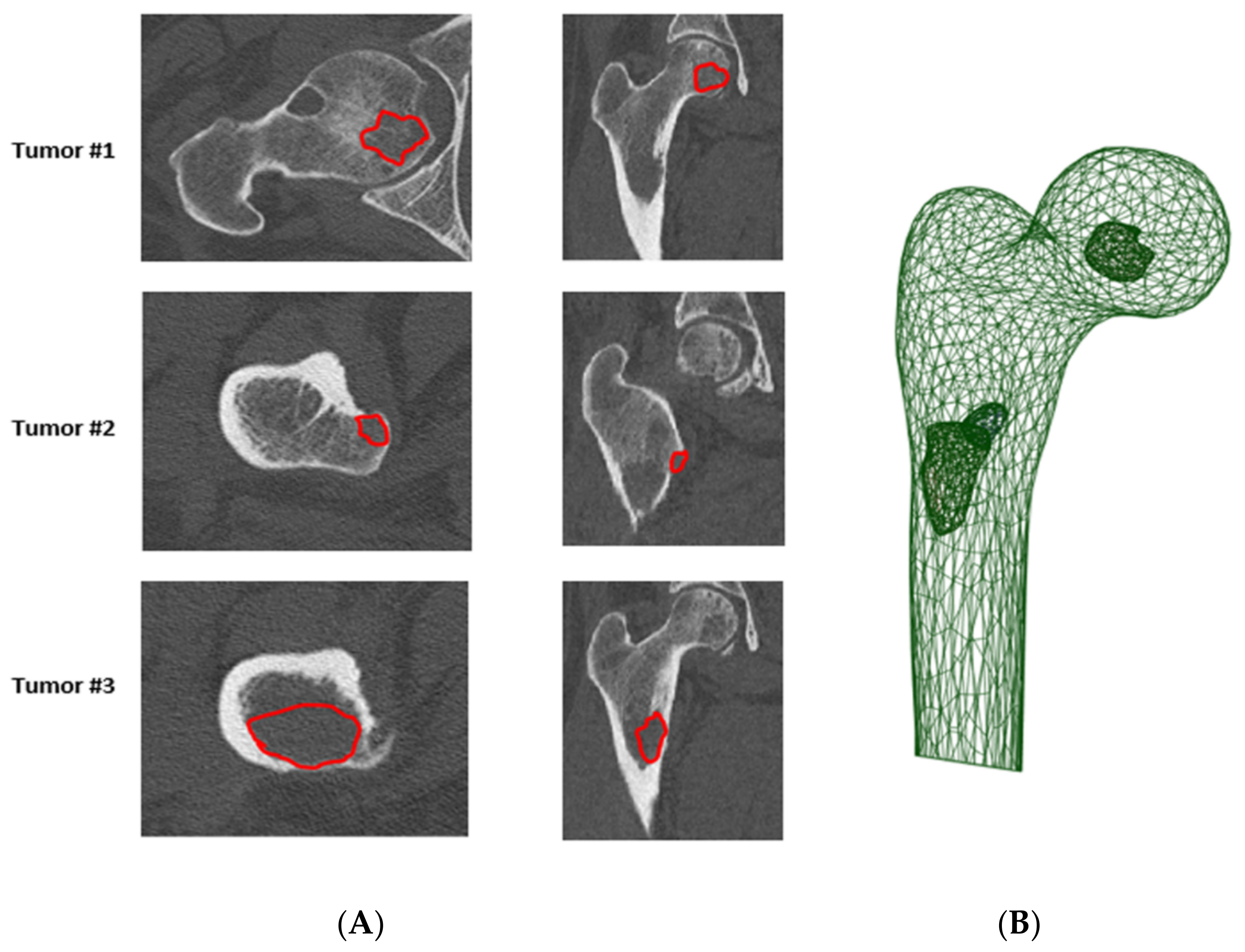
4.2. Local Evaluation of Bone Metastasis
4.3. Bone Metastatic Fracture Risk Scores and Their Limit
5. Emerging Tools
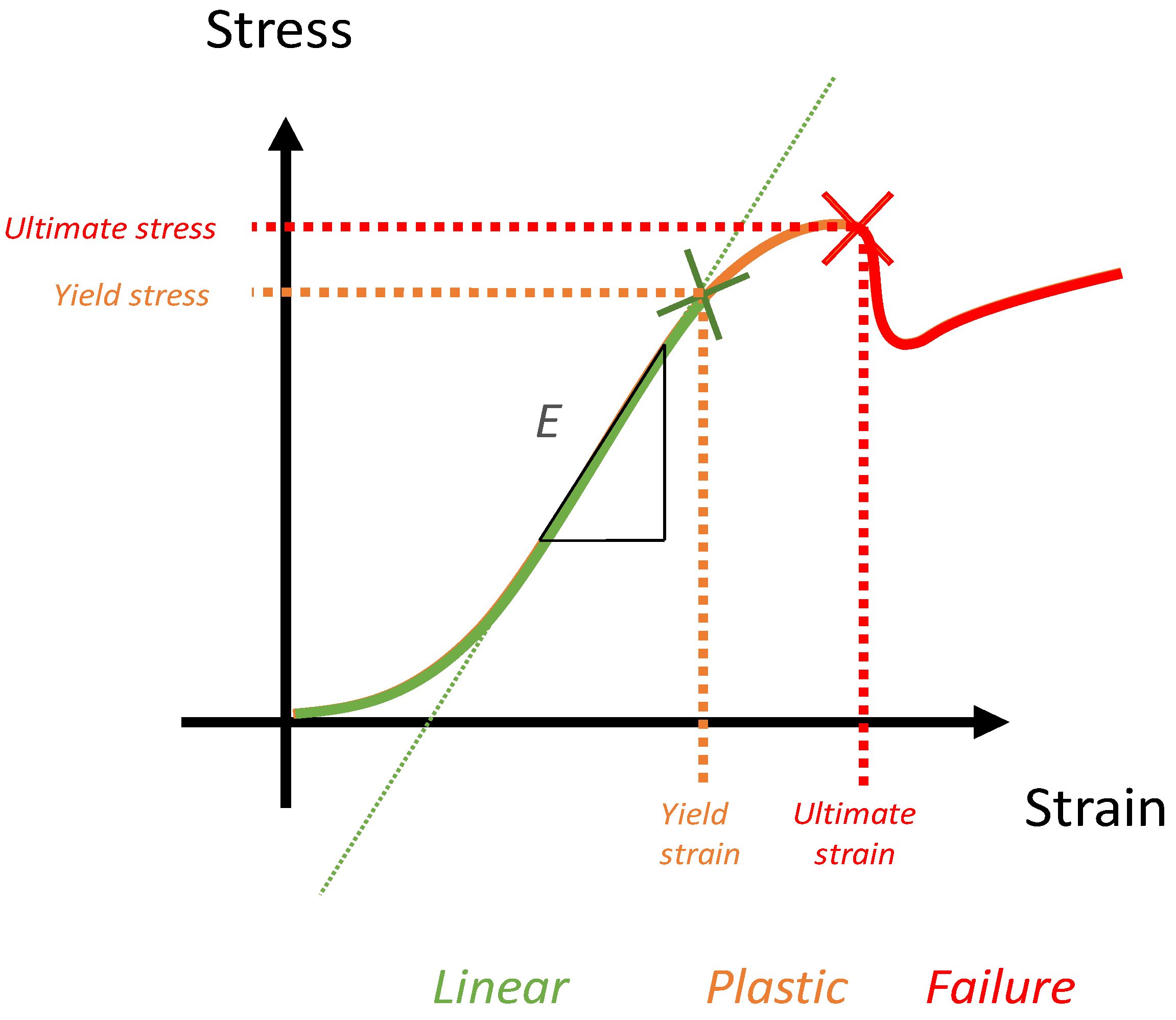
5.1. Key Concept of Biomechanics and Numerical Simulation
5.2. Femoral Fracture Risk Assessment Using Numerical Simulation
5.3. Vertebral Fracture Risk Assessment Using Numerical Simulation
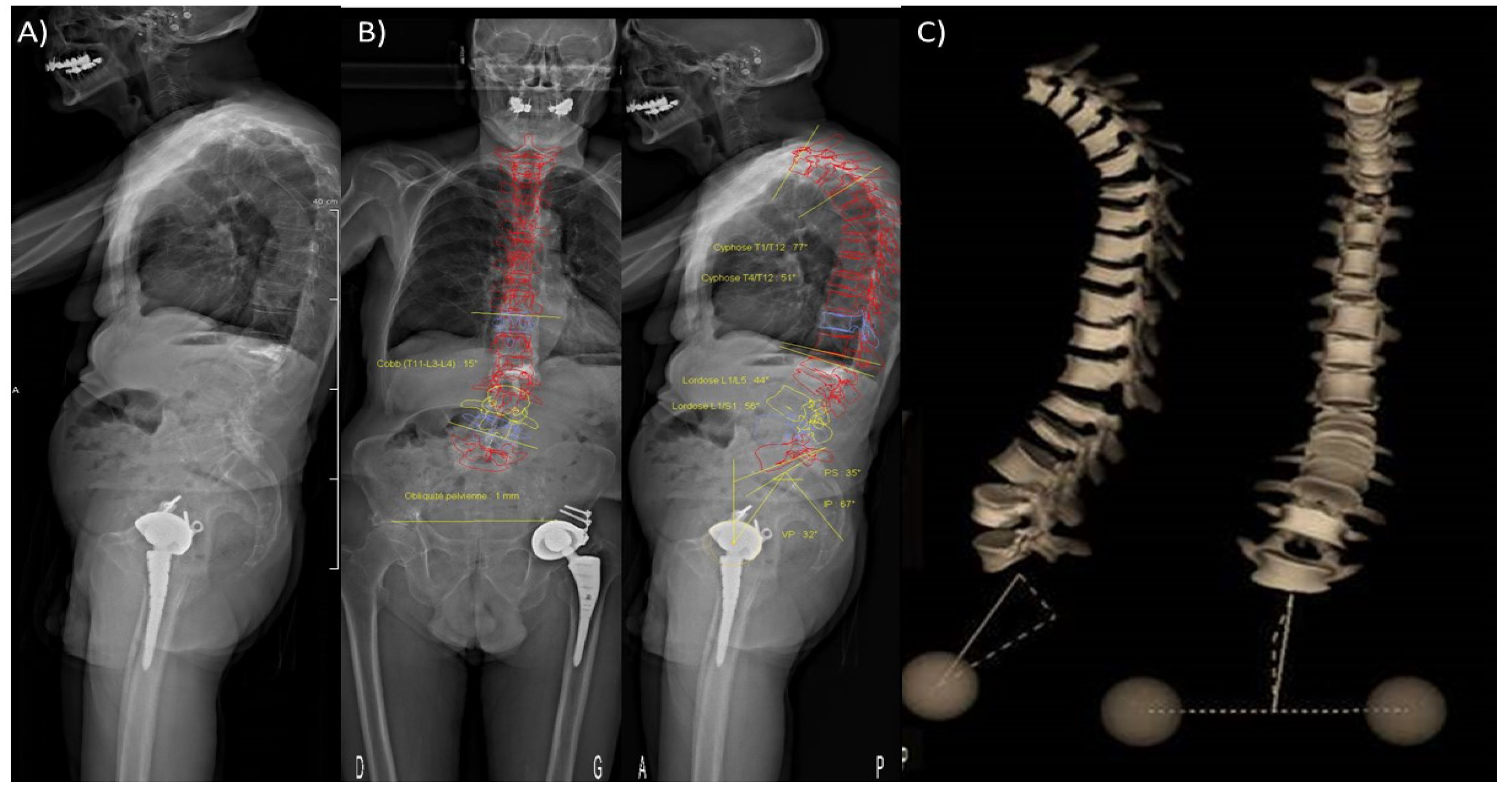
5.4. Tools to Assess Loadings Applied to Metastatic Bones
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiang, A.C.; Massague, J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J. Epithelio-mesenchymal transformation and cancer. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.R.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Karrison, T.G.; Ferguson, D.J.; Meier, P. Dormancy of Mammary Carcinoma After Mastectomy. J. Natl. Cancer Inst. 1999, 91, 80–85. [Google Scholar] [CrossRef]
- Haug, J.S.; He, X.C.; Grindley, J.C.; Wunderlich, J.P.; Gaudenz, K.; Ross, J.T.; Paulson, A.; Wagner, K.P.; Xie, Y.; Zhu, R.; et al. N-Cadherin Expression Level Distinguishes Reserved versus Primed States of Hematopoietic Stem Cells. Cell Stem Cell 2008, 2, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Côté, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nat. Cell Biol. 2005, 435, 969–973. [Google Scholar] [CrossRef]
- Banys, M.; Solomayer, E.-F.; Gebauer, G.; Janni, W.; Krawczyk, N.; Lueck, H.-J.; Becker, S.; Huober, J.; Kraemer, B.; Wackwitz, B.; et al. Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: Results of a prospective clinical trial. BMC Cancer 2013, 13, 480. [Google Scholar] [CrossRef] [Green Version]
- Puppo, M.; Taipaleenmäki, H.; Hesse, E.; Clézardin, P. Non-coding RNAs in bone remodelling and bone metastasis: Mechanisms of action and translational relevance. Br. J. Pharmacol. 2021, 178, 1936–1954. [Google Scholar] [CrossRef]
- Jensen, A.Ø.; Jacobsen, J.B.; Nørgaard, M.; Yong, M.; Fryzek, J.P.; Sørensen, H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 2018, CD003474. [Google Scholar] [CrossRef]
- Pavlakis, N.; Schmidt, R.; Stockler, M.R. Bisphosphonates for breast cancer. Cochrane Database Syst. Rev. 2005, 3, CD003474. [Google Scholar] [CrossRef] [Green Version]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; De Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Zoledronic Acid Versus Placebo in the Treatment of Skeletal Metastases in Patients with Lung Cancer and Other Solid Tumors: A Phase III, Double-Blind, Randomized Trial—The Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003, 21, 3150–3157. [Google Scholar] [CrossRef]
- Decroisette, C.; Monnet, I.; Berard, H.; Quere, G.; Le Caer, H.; Bota, S.; Audigier-Valette, C.; Geriniere, L.; Vernejoux, J.-M.; Chouaid, C. Epidemiology and Treatment Costs of Bone Metastases from Lung Cancer: A French Prospective, Observational, Multicenter Study (GFPC 0601). J. Thorac. Oncol. 2011, 6, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Confavreux, C.B.; Pialat, J.-B.; Bellière, A.; Brevet, M.; Decroisette, C.; Tescaru, A.; Wegrzyn, J.; Barrey, C.; Mornex, F.; Souquet, P.-J.; et al. Bone metastases from lung cancer: A paradigm for multidisciplinary onco-rheumatology management. Jt. Bone Spine 2019, 86, 185–194. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Malatray, M.; Al-Qahtani, T.; Pibarot, V.; Confavreux, C.; Freyer, G. Total Hip Arthroplasty for Periacetabular Metastatic Disease. An Original Technique of Reconstruction According to the Harrington Classification. J. Arthroplast. 2018, 33, 2546–2555. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Coleman, R.; Smith, P.; Rubens, R. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 1998, 77, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [Green Version]
- Lohinai, Z.; Klikovits, T.; Moldvay, J.; Ostoros, G.; Raso, E.; Timar, J.; Fabian, K.; Kovalszky, I.; Kenessey, I.; Aigner, C.; et al. KRAS-mutation incidence and prognostic value are metastatic site-specific in lung adenocarcinoma: Poor prognosis in patients with KRAS mutation and bone metastasis. Sci. Rep. 2017, 7, 39721. [Google Scholar] [CrossRef] [Green Version]
- Doebele, R.C.; Lu, X.; Sumey, C.; Bs, D.A.M.; Weickhardt, A.J.; Oton, A.B.; Bunn, P.A.; Barón, A.E.; Franklin, W.A.; Aisner, D.L.; et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012, 118, 4502–4511. [Google Scholar] [CrossRef]
- Hu, D.; Zhen, W.; Bi, J.; Han, G.; Wei, X.; Pi, G.; Zhang, Y.; Li, Y.; Wang, M. The features and prognostic impact of extracranial metastases in patients with epidermal growth factor receptor-mutant lung adenocarcinoma. J. Cancer Res. Ther. 2018, 14, 799–806. [Google Scholar] [CrossRef]
- Fujimoto, D.; Ueda, H.; Shimizu, R.; Kato, R.; Otoshi, T.; Kawamura, T.; Tamai, K.; Shibata, Y.; Matsumoto, T.; Nagata, K.; et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: Importance of bone metastasis. Clin. Exp. Metastasis 2014, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, C.; Hendriks, L.; Derks, J.; Dingemans, A.-M.; van Lindert, A.; Heuvel, M.V.D.; Damhuis, R.; Willems, S. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer 2018, 121, 76–81. [Google Scholar] [CrossRef]
- Confavreux, C.B.; Girard, N.; Pialat, J.-B.; Bringuier, P.-P.; Devouassoux-Shisheboran, M.; Rousseau, J.-C.; Isaac, S.; Thivolet-Bejui, F.; Clezardin, P.; Brevet, M. Mutational profiling of bone metastases from lung adenocarcinoma: Results of a prospective study (POUMOS-TEC). BoneKEy Rep. 2014, 3, 580. [Google Scholar] [CrossRef] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Chambard, L.; Girard, N.; Ollier, E.; Rousseau, J.-C.; Duboeuf, F.; Carlier, M.-C.; Brevet, M.; Szulc, P.; Pialat, J.-B.; Wegrzyn, J.; et al. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone 2018, 108, 202–209. [Google Scholar] [CrossRef]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A.; et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Himelstein, A.L.; Foster, J.C.; Khatcheressian, J.L.; Roberts, J.D.; Seisler, D.K.; Novotny, P.J.; Qin, R.; Go, R.S.; Grubbs, S.S.; O’Connor, T.; et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases. JAMA 2017, 317, 48–58. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Van Poznak, C.; Harker, W.G.; Gradishar, W.J.; Chew, H.; Dakhil, S.R.; Haley, B.B.; Sauter, N.; Mohanlal, R.; Zheng, M.; et al. Continued Treatment Effect of Zoledronic Acid Dosing Every 12 vs 4 Weeks in Women with Breast Cancer Metastatic to Bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA Oncol. 2017, 3, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Amadori, D.; Aglietta, M.; Alessi, B.; Gianni, L.; Ibrahim, T.; Farina, G.; Gaion, F.; Bertoldo, F.; Santini, D.; Rondena, R.; et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): A phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013, 14, 663–670. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical Features of 24 Patients with Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Miner. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rodriguez, E.; Aubry-Rozier, B.; Stoll, D.; Zaman, K.; Lamy, O. Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res. Treat. 2019, 179, 153–159. [Google Scholar] [CrossRef]
- Tyan, A.; Patel, S.P.; Block, S.; Hughes, T.; McCowen, K.C. Rebound Vertebral Fractures in a Patient with Lung Cancer After Oncology-Dose Denosumab Discontinuation: A Cautionary Tale. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Garfield, D. Increasing osteoblastic lesions as a manifestation of a major response to gefitinib. J. Thorac. Oncol. 2006, 1, 859–860. [Google Scholar] [CrossRef]
- Canon, J.; Bryant, R.; Roudier, M.; Osgood, T.; Jones, J.; Miller, R.; Coxon, A.; Radinsky, R.; Dougall, W.C. Inhibition of RANKL increases the anti-tumor effect of the EGFR inhibitor panitumumab in a murine model of bone metastasis. Bone 2010, 46, 1613–1619. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Krüger, S.; Buck, A.K.; Mottaghy, F.M.; Hasenkamp, E.; Pauls, S.; Schumann, C.; Wibmer, T.; Merk, T.; Hombach, V.; Reske, S.N. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Bunyaviroch, T.; Coleman, R.E. PET evaluation of lung cancer. J. Nucl. Med. 2006, 47, 451–469. [Google Scholar] [PubMed]
- Choi, J.; Raghavan, M. Diagnostic Imaging and Image-Guided Therapy of Skeletal Metastases. Cancer Control. 2012, 19, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Cuccurullo, V.; Cascini, G.L.; Tamburrini, O.; Rotondo, A.; Mansi, L. Bone metastases radiopharmaceuticals: An overview. Curr. Radiopharm. 2013, 6, 41–47. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Zhang, L.; Tong, G.; Ou, Z.; Wang, Z.; Zhang, H.; Qiao, G. Pathologic complete response to preoperative immunotherapy in a lung adenocarcinoma patient with bone metastasis: A case report. Thorac. Cancer 2020, 11, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Shionoya, Y.; Hirohashi, Y.; Takahashi, H.; Hashimoto, M.; Nishiyama, K.; Takakuwa, Y.; Nakatsugawa, M.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; et al. Possible Pseudo-progression of Non-small Cell Lung Carcinoma in a Patient with Clinical Hyper-progression Associated with Trousseau Syndrome Who Was Treated with Pembrolizumab: A Case Report. Anticancer. Res. 2021, 41, 3699–3706. [Google Scholar] [CrossRef]
- Ishiwata, Y.; Hieda, Y.; Kaki, S.; Aso, S.; Horie, K.; Kobayashi, Y.; Nakamura, M.; Yamada, K.; Yamashiro, T.; Utsunomiya, D. Improved Diagnostic Accuracy of Bone Metastasis Detection by Water-HAP Associated to Non-contrast CT. Diagnostics 2020, 10, 853. [Google Scholar] [CrossRef]
- Rajapakse, C.S.; Gupta, N.; Evans, M.; Alizai, H.; Shukurova, M.; Hong, A.L.; Cruickshank, N.J.; Tejwani, N.; Egol, K.; Honig, S.; et al. Influence of bone lesion location on femoral bone strength assessed by MRI-based finite-element modeling. Bone 2019, 122, 209–217. [Google Scholar] [CrossRef]
- Florkow, M.C.; Willemsen, K.; Zijlstra, F.; Foppen, W.; van der Wal, B.C.; van der Voort van Zyp, J.R.N.; Viergever, M.A.; Castelein, R.M.; Weinans, H.; van Stralen, M.; et al. MRI-based synthetic CT shows equivalence to conventional CT for the morphological assessment of the hip joint. J. Orthop. Res. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Arita, Y.; Takahara, T.; Yoshida, S.; Kwee, T.C.; Yajima, S.; Ishii, C.; Ishii, R.; Okuda, S.; Jinzaki, M.; Fujii, Y. Quantitative Assessment of Bone Metastasis in Prostate Cancer Using Synthetic Magnetic Resonance Imaging. Investig. Radiol. 2019, 54, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Mirels, H. The Classic: Metastatic Disease in Long Bones A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 2003, 415, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.A.; Nazarian, A.; Entezari, V.; Brown, C.; Grant, W.; Calderon, N.; Zurakowski, D.; Terek, R.; Anderson, M.E.; Cheng, E.; et al. CT-based Structural Rigidity Analysis Is More Accurate Than Mirels Scoring for Fracture Prediction in Metastatic Femoral Lesions. Clin. Orthop. Relat. Res. 2016, 474, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, E.L.; Shepherd, K.L.; Cribb, G.; Cool, P. The validity of the Mirels score for predicting impending pathological fractures of the lower limb. Bone Jt. J. 2018, 100, 1100–1105. [Google Scholar] [CrossRef]
- Sternheim, A.; Traub, F.; Trabelsi, N.; Dadia, S.; Gortzak, Y.; Snir, N.; Gorfine, M.; Yosibash, Z. When and where do patients with bone metastases actually break their femurs? Bone Jt. J. 2020, 10, 638–645. [Google Scholar] [CrossRef]
- Benca, E.; Patsch, J.M.; Mayr, W.; Pahr, D.H.; Windhager, R. The insufficiencies of risk analysis of impending pathological fractures in patients with femoral metastases: A literature review. Bone Rep. 2016, 5, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Crenn, V.; Carlier, C.; Gouin, F.; Sailhan, F.; Bonnevialle, P. High rate of fracture in long-bone metastasis: Proposal for an improved Mirels predictive score. Orthop. Traumatol. Surg. Res. 2020, 106, 1005–1011. [Google Scholar] [CrossRef]
- Algra, P.R.; Heimans, J.J.; Valk, J.; Nauta, J.J.; Lachniet, M.; Van Kooten, B. Do metastases in vertebrae begin in the body or the pedicles? Imaging study in 45 patients. Am. J. Roentgenol. 1992, 158, 1275–1279. [Google Scholar] [CrossRef]
- Selvaggi, G.; Scagliotti, G.V. Management of bone metastases in cancer: A review. Crit. Rev. Oncol. 2005, 56, 365–378. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.; Boriani, S.; Chou, D.; et al. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence-Based Approach and Expert Consensus From the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourney, D.R.; Frangou, E.M.; Ryken, T.C.; DiPaola, C.P.; Shaffrey, C.I.; Berven, S.H.; Bilsky, M.; Harrop, J.S.; Fehlings, M.; Boriani, S.; et al. Spinal Instability Neoplastic Score: An Analysis of Reliability and Validity from the Spine Oncology Study Group. J. Clin. Oncol. 2011, 29, 3072–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, C.G.; Schouten, R.; Versteeg, A.L.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Kawahara, N.; Fourney, D.; Weir, L.; Reynolds, J.J.; et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: An assessment of instability secondary to spinal metastases. Radiat. Oncol. 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.D.; Hertan, L.M.; Lam, T.C.; Skamene, S.; Chi, J.H.; Groff, M.; Cho, C.H.; Ferrone, M.L.; Harris, M.; Chen, Y.-H.; et al. Assessing the utility of the spinal instability neoplastic score (SINS) to predict fracture after conventional radiation therapy (RT) for spinal metastases. Pr. Radiat. Oncol. 2018, 8, e285–e294. [Google Scholar] [CrossRef]
- Kim, Y.R.; Lee, C.-H.; Yang, S.H.; Hyun, S.-J.; Kim, C.H.; Park, S.B.; Kim, K.-J.; Chung, C.K. Accuracy and precision of the spinal instability neoplastic score (SINS) for predicting vertebral compression fractures after radiotherapy in spinal metastases: A meta-analysis. Sci. Rep. 2021, 11, 5553. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A Revised Scoring System for Preoperative Evaluation of Metastatic Spine Tumor Prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Tanck, E.; van Aken, J.B.; van der Linden, Y.M.; Schreuder, H.B.; Binkowski, M.; Huizenga, H.; Verdonschot, N. Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone 2009, 45, 777–783. [Google Scholar] [CrossRef]
- Derikx, L.C.; van Aken, J.B.; Janssen, D.; Snyers, A.; van der Linden, Y.M.; Verdonschot, N.; Tanck, E. The assessment of the risk of fracture in femora with metastatic lesions: Comparing case-specific finite element analyses with predictions by clinical experts. J. Bone Jt. Surg. 2012, 94, 1135–1142. [Google Scholar] [CrossRef]
- Derikx, L.C.; Verdonschot, N.; Tanck, E. Towards clinical application of biomechanical tools for the prediction of fracture risk in metastatic bone disease. J. Biomech. 2015, 48, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, F.; Derikx, L.C.; Verdonschot, N.; Van Der Geest, I.C.M.; De Jong, M.A.A.; Snyers, A.; Van Der Linden, Y.M.; Tanck, E. Can patient-specific finite element models better predict fractures in metastatic bone disease than experienced clinicians? owards computational modelling in daily clinical practice. Bone Jt. Res. 2018, 7, 430–439. [Google Scholar] [CrossRef]
- Eggermont, F.; van der Wal, G.; Westhoff, P.; Laar, A.; de Jong, M.; Rozema, T.; Kroon, H.M.; Ayu, O.; Derikx, L.; Dijkstra, S.; et al. Patient-specific finite element computer models improve fracture risk assessments in cancer patients with femoral bone metastases compared to clinical guidelines. Bone 2020, 130, 115101. [Google Scholar] [CrossRef]
- Knowles, N.K.; Reeves, J.M.; Ferreira, L.M. Quantitative Computed Tomography (QCT) derived Bone Mineral Density (BMD) in finite element studies: A review of the literature. J. Exp. Orthop. 2016, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleps, I.; Bahaloo, H.; Zysset, P.K.; Ferguson, S.J.; Pálsson, H.; Helgason, B. Empirical relationships between bone density and ultimate strength: A literature review. J. Mech. Behav. Biomed. Mater. 2020, 110, 103866. [Google Scholar] [CrossRef] [PubMed]
- Sandino, C.; McErlain, D.D.; Schipilow, J.; Boyd, S.K. Mechanical stimuli of trabecular bone in osteoporosis: A numerical simulation by finite element analysis of microarchitecture. J. Mech. Behav. Biomed. Mater. 2017, 66, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, M.; Bagherian, A.; Sheidaei, A.; Baniassadi, M.; Baghani, M.; Rémond, Y.; George, D. Numerical Simulation of Osteoporosis Degradation at Local Scale: A Preliminary Study on the Kinematic Loss of Mechanical Bone Stiffness and Microstructure. Stem Cells Regen. Med. 2019, 79, 86–93. [Google Scholar]
- Keyak, J.; Skinner, H. Three-dimensional finite element modelling of bone: Effects of element size. J. Biomed. Eng. 1992, 14, 483–489. [Google Scholar] [CrossRef]
- Burkhart, T.A.; Andrews, D.M.; Dunning, C.E. Finite element modeling mesh quality, energy balance and validation methods: A review with recommendations associated with the modeling of bone tissue. J. Biomech. 2013, 46, 1477–1488. [Google Scholar] [CrossRef]
- Knowles, N.K.; Ip, K.; Ferreira, L.M. The Effect of Material Heterogeneity, Element Type, and Down-Sampling on Trabecular Stiffness in Micro Finite Element Models. Ann. Biomed. Eng. 2018, 47, 615–623. [Google Scholar] [CrossRef]
- Imai, K.; Ohnishi, I.; Bessho, M.; Nakamura, K. Nonlinear Finite Element Model Predicts Vertebral Bone Strength and Fracture Site. Spine 2006, 31, 1789–1794. [Google Scholar] [CrossRef]
- Zysset, P.K.; Dall’Ara, E.; Varga, P.; Pahr, D.H. Finite element analysis for prediction of bone strength. BoneKEy Rep. 2013, 2, 386. [Google Scholar] [CrossRef] [Green Version]
- Benca, E.; Reisinger, A.; Patsch, J.; Hirtler, L.; Synek, A.; Stenicka, S.; Windhager, R.; Mayr, W.; Pahr, D.H. Effect of simulated metastatic lesions on the biomechanical behavior of the proximal femur. J. Orthop. Res. 2017, 35, 2407–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilayphiou, N.; Boutroy, S.; Szulc, P.; van Rietbergen, B.; Munoz, F.; Delmas, P.D.; Chapurlat, R. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J. Bone Miner. Res. 2011, 26, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Panyasantisuk, J.; Dall’Ara, E.; Pretterklieber, M.; Pahr, D.; Zysset, P. Mapping anisotropy improves QCT-based finite element estimation of hip strength in pooled stance and side-fall load configurations. Med Eng. Phys. 2018, 59, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Demirtas, A.; Rajapakse, C.S.; Ural, A. Assessment of the multifactorial causes of atypical femoral fractures using a novel multiscale finite element approach. Bone 2020, 135, 115318. [Google Scholar] [CrossRef]
- Spruijt, S.; Van Der Linden, J.C.; Dijkstra, P.D.S.; Wiggers, T.; Oudkerk, M.; Snijders, C.J.; Van Keulen, F.; Verhaar, J.A.N.; Weinans, H.; Swierstra, B.A. Prediction of torsional failure in 22 cadaver femora with and without simulated subtrochanteric metastatic defects: A CT scan-based finite element analysis. Acta Orthop. 2006, 77, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.E.; Gutierrez, S.; Nayak, A.; Palumbo, B.T.; Cheong, D.; Letson, G.D.; Santoni, B.G. Biomechanical model of a high risk impending pathologic fracture of the femur: Lesion creation based on clinically implemented scoring systems. Clin. Biomech. 2013, 28, 408–414. [Google Scholar] [CrossRef]
- Benca, E.; Synek, A.; Amini, M.; Kainberger, F.; Hirtler, L.; Windhager, R.; Mayr, W.; Pahr, D.H. QCT-based finite element prediction of pathologic fractures in proximal femora with metastatic lesions. Sci. Rep. 2019, 9, 10305. [Google Scholar] [CrossRef] [Green Version]
- Sas, A.; Tanck, E.; Sermon, A.; van Lenthe, G.H. Finite element models for fracture prevention in patients with metastatic bone disease. A literature review. Bone Rep. 2020, 12, 100286. [Google Scholar] [CrossRef]
- Johnson, J.E.; Brouillette, M.J.; Permeswaran, P.T.; Miller, B.J.; Goetz, J.E. Simulated lesions representative of metastatic disease predict proximal femur failure strength more accurately than idealized lesions. J. Biomech. 2020, 106, 109825. [Google Scholar] [CrossRef]
- Yosibash, Z.; Mayo, R.P.; Dahan, G.; Trabelsi, N.; Amir, G.; Milgrom, C. Predicting the stiffness and strength of human femurs with real metastatic tumors. Bone 2014, 69, 180–190. [Google Scholar] [CrossRef]
- Keyak, J.H.; Kaneko, T.S.; Tehranzadeh, J.; Skinner, H.B. Predicting Proximal Femoral Strength Using Structural Engineering Models. Clin. Orthop. Relat. Res. 2005, 437, 219–228. [Google Scholar] [CrossRef]
- Keyak, J.H.; Kaneko, T.S.; Skinner, H.B.; Hoang, B.H. The Effect of Simulated Metastatic Lytic Lesions on Proximal Femoral Strength. Clin. Orthop. Relat. Res. 2007, 459, 139–145. [Google Scholar] [CrossRef]
- Van der Wal, C.; Eggermont, F.; Fiocco, M.; Kroon, H.; Ayu, O.; Slot, A.; Snyers, A.; Rozema, T.; Verdonschot, N.; Dijkstra, P.; et al. Axial cortical involvement of metastatic lesions to identify impending femoral fractures; a clinical validation study. Radiother. Oncol. 2020, 144, 59–64. [Google Scholar] [CrossRef]
- Falcinelli, C.; Di Martino, A.; Gizzi, A.; Vairo, G.; Denaro, V. Fracture risk assessment in metastatic femurs: A patient-specific CT-based finite-element approach. Meccanica 2020, 55, 861–881. [Google Scholar] [CrossRef]
- Falcinelli, C.; Di Martino, A.; Gizzi, A.; Vairo, G.; Denaro, V. Mechanical behavior of metastatic femurs through patient-specific computational models accounting for bone-metastasis interaction. J. Mech. Behav. Biomed. Mater. 2019, 93, 9–22. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Derikx, L.C.; Verdonschot, N.; Calderon, N.; Zurakowski, D.; Snyder, B.D.; Nazarian, A.; Tanck, E. Finite element analysis and CT-based structural rigidity analysis to assess failure load in bones with simulated lytic defects. Bone 2014, 58, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Sternheim, A.; Giladi, O.; Gortzak, Y.; Drexler, M.; Salai, M.; Trabelsi, N.; Milgrom, C.; Yosibash, Z. Pathological fracture risk assessment in patients with femoral metastases using CT-based finite element methods. A retrospective clinical study. Bone 2018, 110, 215–220. [Google Scholar] [CrossRef]
- Shinoda, Y.; Sawada, R.; Ishibashi, Y.; Akiyama, T.; Zhang, L.; Hirai, T.; Oka, H.; Ohki, T.; Ikegami, M.; Okajima, K.; et al. Prediction of pathological fracture in patients with lower limb bone metastasis using computed tomography imaging. Clin. Exp. Metastasis 2020, 37, 607–616. [Google Scholar] [CrossRef]
- Delpuech, B.; Confavreux, C.; Bouazza, L.; Geraci, S.; Clezardin, P.; Mitton, D.; Follet, H. Effect of intra-tibial injection on mechanical properties of mouse bone. Comput. Methods Biomech. Biomed. Eng. 2017, 20, S57–S58. [Google Scholar] [CrossRef] [Green Version]
- Delpuech, B.; Nicolle, S.; Confavreux, C.; Bouazza, B.; Geraci, S.; Clézardin, P.; Mitton, D.; Folle, F. Failure Prediction of Metastatic Bone with Osteolytic Lesion in Mice. In Proceedings of the 25th Congress of the European Society of Biomechanics, Vienna, Austria, 7–10 July 2019; Available online: https://hal.archives-ouvertes.fr/hal-02441862 (accessed on 12 December 2020).
- Delpuech, B.; Nicolle, S.; Confavreux, C.; Bouazza, B.; Geraci, S.; Clézardin, P.; Mitton, D.; Folle, F. Determination of Tumor Tissue Mechanical Properties, toward Quantification of Implication of Tumor in Whole Bone Resistance: A Preliminary Study. In Proceedings of the 8th World Congress of Biomechanics, Dublin, Ireland, 8–12 July 2018; Available online: https://hal.archives-ouvertes.fr/hal-02086220 (accessed on 12 December 2020).
- Delpuech, B.; Nicolle, S.; Confavreux, C.B.; Bouazza, L.; Clezardin, P.; Mitton, D.; Follet, H. Failure Prediction of Tumoral Bone with Osteolytic Lesion in Mice. In Developments and Novel Approaches in Biomechanics and Metamaterials; Abali, B.E., Giorgio, I., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 17–34. [Google Scholar]
- Voutouri, C.; Stylianopoulos, T. Accumulation of mechanical forces in tumors is related to hyaluronan content and tissue stiffness. PLoS ONE 2018, 13, e0193801. [Google Scholar] [CrossRef] [Green Version]
- Stadelmann, M.A.; Schenk, D.E.; Maquer, G.; Lenherr, C.; Buck, F.M.; Bosshardt, D.D.; Hoppe, S.; Theumann, N.; Alkalay, R.N.; Zysset, P.K. Conventional finite element models estimate the strength of metastatic human vertebrae despite alterations of the bone’s tissue and structure. Bone 2020, 141, 115598. [Google Scholar] [CrossRef]
- Choisne, J.; Valiadis, J.-M.; Travert, C.; Kolta, S.; Roux, C.; Skalli, W. Vertebral strength prediction from Bi-Planar dual energy x-ray absorptiometry under anterior compressive force using a finite element model: An in vitro study. J. Mech. Behav. Biomed. Mater. 2018, 87, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Campello, L.B.; Ryan, M.; Rochester, J.; Viceconti, M.; Dall’Ara, E. Effect of size and location of simulated lytic lesions on the structural properties of human vertebral bodies, a micro-finite element study. Bone Rep. 2020, 12, 100257. [Google Scholar] [CrossRef]
- Chevalier, Y.; Charlebois, M.; Pahr, D.; Varga, P.; Heini, P.; Schneider, E.; Zysset, P. A patient-specific finite element methodology to predict damage accumulation in vertebral bodies under axial compression, sagittal flexion and combined loads. Comput. Methods Biomech. Biomed. Eng. 2008, 11, 477–487. [Google Scholar] [CrossRef]
- Anitha, D.; Baum, T.; Kirschke, J.S.; Subburaj, K. Risk of vertebral compression fractures in multiple myeloma patients. Medicine 2017, 96, e5825. [Google Scholar] [CrossRef]
- Palanca, M.; Barbanti-Bròdano, G.; Cristofolini, L.; Palanco, M.; Barbanti, G.B. The Size of Simulated Lytic Metastases Affects the Strain Distribution on the Anterior Surface of the Vertebra. J. Biomech. Eng. 2018, 140, 111005. [Google Scholar] [CrossRef]
- Whyne, C.M.; Hu, S.S.; Lotz, J.C. Burst Fracture in the Metastatically Involved Spine: Development, validation, and parametric analysis of a three-dimensional poroelastic finite-element model. Spine 2003, 28, 652–660. [Google Scholar] [CrossRef]
- McGowan, D.P.; Hipp, J.A.; Takeuchi, T.; White, A.A., 3rd; Hayes, W.C. Strength reductions from trabecular destruction within thoracic vertebrae. J. Spinal Disord. 1993, 6, 130–136. [Google Scholar] [CrossRef]
- Alkalay, R.; Adamson, R.; Miropolsky, A.; Hackney, D. Female Human Spines with Simulated Osteolytic Defects: CT-based Structural Analysis of Vertebral Body Strength. Radiology 2018, 288, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Whealan, K.M.; Kwak, S.D.; Tedrow, J.R.; Inoue, K.; Snyder, B.D. Noninvasive Imaging Predicts Failure Load of the Spine with Simulated Osteolytic Defects. J. Bone Jt. Surg. Am. Vol. 2000, 82, 1240–1251. [Google Scholar] [CrossRef]
- Windhagen, H.J.; Hipp, J.; Silva, M.; Lipson, S.J.; Hayes, W.C. Predicting Failure of Thoracic Vertebrae with Simulated and Actual Metastatic Defects. Clin. Orthop. Relat. Res. 1997, 344, 313–319. [Google Scholar] [CrossRef]
- Taneichi, H.; Kaneda, K.; Takeda, N.; Abumi, K.; Satoh, S. Risk Factors and Probability of Vertebral Body Collapse in Metastases of the Thoracic and Lumbar Spine. Spine 1997, 22, 239–245. [Google Scholar] [CrossRef]
- Silva, M.J.; Hipp, J.A.; McGowan, D.P.; Takeuchi, T.; Hayes, W.C. Strength reductions of thoracic vertebrae in the presence of transcortical osseous defects: Effects of defect location, pedicle disruption, and defect size. Eur. Spine J. 1993, 2, 118–125. [Google Scholar] [CrossRef]
- Alkalay, R.N. Effect of the metastatic defect on the structural response and failure process of human vertebrae: An experimental study. Clin. Biomech. 2015, 30, 121–128. [Google Scholar] [CrossRef]
- Giambini, H.; Fang, Z.; Zeng, H.; Camp, J.J.; Yaszemski, M.J.; Lu, L. Noninvasive Failure Load Prediction of Vertebrae with Simulated Lytic Defects and Biomaterial Augmentation. Tissue Eng. Part C Methods 2016, 22, 717–724. [Google Scholar] [CrossRef]
- Groenen, K.H.J.; Bitter, T.; Bsc, T.C.V.V.; Van Der Linden, Y.M.; Verdonschot, N.; Tanck, E.; Janssen, D. Case-specific non-linear finite element models to predict failure behavior in two functional spinal units. J. Orthop. Res. 2018, 36, 3208–3218. [Google Scholar] [CrossRef] [Green Version]
- Dall’Ara, E.; Schmidt, R.; Pahr, D.; Varga, P.; Chevalier, Y.; Patsch, J.; Kainberger, F.; Zysset, P. A nonlinear finite element model validation study based on a novel experimental technique for inducing anterior wedge-shape fractures in human vertebral bodies in vitro. J. Biomech. 2010, 43, 2374–2380. [Google Scholar] [CrossRef]
- Palanca, M.; Cristofolini, L.; Gasbarrini, A.; Tedesco, G.; Barbanti-Bròdano, G. Assessing the Mechanical Weakness of Vertebrae Affected by Primary Tumors: A Feasibility Study. Materials 2020, 13, 3256. [Google Scholar] [CrossRef]
- Palanca, M.; Barbanti-Bròdano, G.; Marras, D.; Marciante, M.; Serra, M.; Gasbarrini, A.; Dall’Ara, E.; Cristofolini, L. Type, size, and position of metastatic lesions explain the deformation of the vertebrae under complex loading conditions. Bone 2021, 151, 116028. [Google Scholar] [CrossRef]
- Dubousset, J.; Charpak, G.; Dorion, I.; Skalli, W.; Lavaste, F.; DeGuise, J.; Kalifa, G.; Ferey, S. A new 2D and 3D imaging approach to musculoskeletal physiology and pathology with low-dose radiation and the standing position: The EOS system. Bull. Académie Natl. Méd. 2005, 189, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Skalli, W.; Mitton, D.; Rouch, P.; Dubousset, J. Biomechanics and Spinal Modelling. In Spinal Anatomy: Modern Concepts; Vital, J.M., Cawley, D.T., Eds.; Springer International Publishing: Cham, Germany, 2019; pp. 491–503. [Google Scholar]
- Deschênes, S.; Charron, G.; Beaudoin, G.; Labelle, H.; Dubois, J.; Miron, M.-C.; Parent, S. Diagnostic Imaging of Spinal Deformities: Reducing Patients Radiation Dose with a New Slot-Scanning X-ray Imager. Spine 2010, 35, 989–994. [Google Scholar] [CrossRef]
- Brosses, E.S.-D.; Jolivet, E.; Travert, C.; Mitton, D.; Skalli, W. Prediction of the Vertebral Strength Using a Finite Element Model Derived from Low-Dose Biplanar Imaging: Benefits of Subject-Specific Material Properties. Spine 2012, 37, E156–E162. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Dymke, J.; Duda, G.; Damm, P. Standardized Loads Acting in Hip Implants. PLoS ONE 2016, 11, e0155612. [Google Scholar] [CrossRef]
- Duda, G.N.; Schneider, E.; Chao, E.Y. Internal forces and moments in the femur during walking. J. Biomech. 1997, 30, 933–941. [Google Scholar] [CrossRef]
- Moissenet, F.; Cheze, L.; Dumas, R. Influence of the Level of Muscular Redundancy on the Validity of a Musculoskeletal Model. J. Biomech. Eng. 2016, 138, 021019. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confavreux, C.B.; Follet, H.; Mitton, D.; Pialat, J.B.; Clézardin, P. Fracture Risk Evaluation of Bone Metastases: A Burning Issue. Cancers 2021, 13, 5711. https://doi.org/10.3390/cancers13225711
Confavreux CB, Follet H, Mitton D, Pialat JB, Clézardin P. Fracture Risk Evaluation of Bone Metastases: A Burning Issue. Cancers. 2021; 13(22):5711. https://doi.org/10.3390/cancers13225711
Chicago/Turabian StyleConfavreux, Cyrille B., Helene Follet, David Mitton, Jean Baptiste Pialat, and Philippe Clézardin. 2021. "Fracture Risk Evaluation of Bone Metastases: A Burning Issue" Cancers 13, no. 22: 5711. https://doi.org/10.3390/cancers13225711
APA StyleConfavreux, C. B., Follet, H., Mitton, D., Pialat, J. B., & Clézardin, P. (2021). Fracture Risk Evaluation of Bone Metastases: A Burning Issue. Cancers, 13(22), 5711. https://doi.org/10.3390/cancers13225711






