Primary Gastrointestinal T-Cell Lymphoma and Indolent Lymphoproliferative Disorders: Practical Diagnostic and Treatment Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Enteropathy-Associated T-Cell Lymphoma
2.1. Definition and Epidemiology
2.2. Pathogenesis
2.3. Cell Origin
2.4. Histopathology
2.5. Immunophenotype and Genetic Alternations
2.6. Clinical Manifestations
2.7. Prognosis and Treatment Strategies
3. Monomorphic Epitheliotropic Intestinal T-Cell Lymphoma (MEITL)
3.1. Definition and Epidemiology
3.2. Histopathology
3.3. Immunophenotype and Genetic Alternations
3.4. Clinical Manifestations
3.5. Prognosis and Treatment Strategies
4. Intestinal T-Cell Lymphoma, Not Otherwise Specified (ITL, NOS)
4.1. Definition and Epidemiology
4.2. Immunophenotype and Genetic Alternations
4.3. Clinical Manifestations
5. Indolent T-Cell Lymphoproliferative Disorder of the Gastrointestinal Tract (ITLPD-GI)
5.1. Definition and Epidemiology
5.2. Histopathology
5.3. Immunophenotype and Genetic Alterations
5.4. Clinical Manifestations
5.5. Prognosis and Treatment Strategies
6. Differential Diagnoses and Diagnostic Pitfalls
6.1. Differential Diagnoses
6.1.1. Anaplastic Large Cell Lymphoma (ALCL)
6.1.2. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTCL)
6.1.3. Adult T-Cell Leukemia/Lymphoma (ATLL)
6.1.4. NK-Cell Enteropathy (NK-ENT)
6.1.5. Chronic Active Epstein-Barr Virus Infection (CAEBV)
6.2. Diagnostic Pitfalls
6.2.1. Discriminating Subtypes with Different Clinical Prognoses
- Epitheliotropism is more clearly observed with cytokeratin (CK20) staining. Epitheliotropism is most frequently observed in EATL and MEITL but is also observed in other lymphomas or NK-ENT and is not specific to EATL and MEITL.
- EATL with large, atypical cell proliferation with CD30 expression is challenging to differentiate from ALK-negative ALCL, and a reliable differential approach has not been established.
- In MEITL, abnormal expression of CD20 is infrequently observed and may lead to misdiagnosis of B-cell lymphoma. In such cases, other B-cell markers such as CD79a and PAX5 should be evaluated to prevent misdiagnosis.
- In MEITL, CD8 or CD56 can be negative. CD56 negativity may cause difficulties in differentiating MEITL from EATL. In the case of CD8-negative MEITL, the immunostaining pattern may resemble that of NK-ENT, but the PCR results of NK-ENT do not exhibit TCR rearrangement.
- CD103 expression may represent neoplastic changes in mucosal intraepithelial lymphocytes, but it should be noted that approximately half of ATLL cases are also positive for CD103.
- Despite NK-ENT being clinically indolent, Ki-67 labeling index has been reported to be high in some cases. In this situation, high rate of Ki-67 LI is not necessarily an indicator of aggressive clinical course.
- NK-ENT can be misdiagnosed as ENKTCL, but EBER in situ hybridization will always show negative results for the former and positive results for the latter.
6.2.2. Distinguishing Primary GI Neoplasms from Secondary Involvement
6.2.3. Differentiating Neoplasms from Non-Specific Inflammatory Changes at an Early Stage
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohno, S.; Ohshima, K.; Yoneda, S.; Kodama, T.; Shirakusa, T.; Kikuchi, M. Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification. Histopathology 2003, 43, 135–143. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, C.W.; Mun, Y.C.; Oh, S.Y.; Kang, H.J.; Lee, S.I.; Won, J.H.; Kim, M.K.; Kwon, J.H.; Kim, J.S.; et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer 2011, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Zhao, S.; Wang, J.; Yang, Q.; Sun, H.; Yan, J.; Gao, L.; Yao, W.; Zhang, W.; Liu, W. Gastrointestinal Lymphoma in Southwest China: Subtype Distribution of 1,010 Cases Using the WHO (2008) Classification in a Single Institution. Acta Haematol. 2016, 135, 21–28. [Google Scholar] [CrossRef]
- Chott, A.; Haedicke, W.; Mosberger, I.; Fodinger, M.; Winkler, K.; Mannhalter, C.; Muller-Hermelink, H.K. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am. J. Pathol. 1998, 153, 1483–1490. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Attygalle, A.D.; Cabecadas, J.; Gaulard, P.; Jaffe, E.S.; de Jong, D.; Ko, Y.H.; Said, J.; Klapper, W. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward—Report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology 2014, 64, 171–199. [Google Scholar] [CrossRef]
- Delabie, J.; Holte, H.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Savage, K.J.; Connors, J.M.; Rimsza, L.; Harris, N.L.; Muller-Hermelink, K.; et al. Enteropathy-associated T-cell lymphoma: Clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011, 118, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Gale, J.; Simmonds, P.D.; Mead, G.M.; Sweetenham, J.W.; Wright, D.H. Enteropathy-type intestinal T-cell lymphoma: Clinical features and treatment of 31 patients in a single center. J. Clin. Oncol. 2000, 18, 795–803. [Google Scholar] [CrossRef]
- Malamut, G.; Chandesris, O.; Verkarre, V.; Meresse, B.; Callens, C.; Macintyre, E.; Bouhnik, Y.; Gornet, J.M.; Allez, M.; Jian, R.; et al. Enteropathy associated T cell lymphoma in celiac disease: A large retrospective study. Dig. Liver Dis. 2013, 45, 377–384. [Google Scholar] [CrossRef]
- Sieniawski, M.; Angamuthu, N.; Boyd, K.; Chasty, R.; Davies, J.; Forsyth, P.; Jack, F.; Lyons, S.; Mounter, P.; Revell, P.; et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 2010, 115, 3664–3670. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, W.H.; Van De Water, J.M.; Al-Toma, A.; Oudejans, J.J.; Mulder, C.J.; Coupe, V.M. Incidence of enteropathy--associated T-cell lymphoma: A nation-wide study of a population-based registry in The Netherlands. Scand. J. Gastroenterol. 2008, 43, 1322–1328. [Google Scholar] [CrossRef]
- O’Farrelly, C.; Feighery, C.; O’Briain, D.S.; Stevens, F.; Connolly, C.E.; McCarthy, C.; Weir, D.G. Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br. Med. J. Clin. Res. Ed. 1986, 293, 908–910. [Google Scholar] [CrossRef] [Green Version]
- Swinson, C.M.; Slavin, G.; Coles, E.C.; Booth, C.C. Coeliac disease and malignancy. Lancet 1983, 1, 111–115. [Google Scholar] [CrossRef]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef]
- Howell, W.M.; Leung, S.T.; Jones, D.B.; Nakshabendi, I.; Hall, M.A.; Lanchbury, J.S.; Ciclitira, P.J.; Wright, D.H. HLA-DRB, -DQA, and -DQB polymorphism in celiac disease and enteropathy-associated T-cell lymphoma. Common features and additional risk factors for malignancy. Hum. Immunol. 1995, 43, 29–37. [Google Scholar] [CrossRef]
- Silano, M.; Volta, U.; Vincenzi, A.D.; Dessi, M.; Vincenzi, M.D. Effect of a gluten-free diet on the risk of enteropathy-associated T-cell lymphoma in celiac disease. Dig. Dis. Sci. 2008, 53, 972–976. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- de Mascarel, A.; Belleannee, G.; Stanislas, S.; Merlio, C.; Parrens, M.; Laharie, D.; Dubus, P.; Merlio, J.P. Mucosal intraepithelial T-lymphocytes in refractory celiac disease: A neoplastic population with a variable CD8 phenotype. Am. J. Surg. Pathol. 2008, 32, 744–751. [Google Scholar] [CrossRef]
- Daum, S.; Ipczynski, R.; Schumann, M.; Wahnschaffe, U.; Zeitz, M.; Ullrich, R. High rates of complications and substantial mortality in both types of refractory sprue. Eur. J. Gastroenterol. Hepatol. 2009, 21, 66–70. [Google Scholar] [CrossRef]
- Ilus, T.; Kaukinen, K.; Virta, L.J.; Huhtala, H.; Maki, M.; Kurppa, K.; Heikkinen, M.; Heikura, M.; Hirsi, E.; Jantunen, K.; et al. Refractory coeliac disease in a country with a high prevalence of clinically-diagnosed coeliac disease. Aliment. Pharmacol. Ther. 2014, 39, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Al-Toma, A.; Verbeek, W.H.; Hadithi, M.; von Blomberg, B.M.; Mulder, C.J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: Retrospective evaluation of single-centre experience. Gut 2007, 56, 1373–1378. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Tapia, A.; Kelly, D.G.; Lahr, B.D.; Dogan, A.; Wu, T.T.; Murray, J.A. Clinical staging and survival in refractory celiac disease: A single center experience. Gastroenterology 2009, 136, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishi, A.R.; Rubio-Tapia, A.; Murray, J.A. Refractory celiac disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Afchain, P.; Verkarre, V.; Lecomte, T.; Amiot, A.; Damotte, D.; Bouhnik, Y.; Colombel, J.F.; Delchier, J.C.; Allez, M.; et al. Presentation and long-term follow-up of refractory celiac disease: Comparison of type I with type II. Gastroenterology 2009, 136, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Granath, F.; Ekbom, A.; Smedby, K.E.; Murray, J.A.; Neugut, A.I.; Green, P.H.; Ludvigsson, J.F. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: A population-based cohort study. Ann. Intern. Med. 2013, 159, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheminant, M.; Bruneau, J.; Malamut, G.; Sibon, D.; Guegan, N.; van Gils, T.; Cording, S.; Trinquand, A.; Verkarre, V.; Lhermitte, L.; et al. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: A CELAC study. Gut 2019, 68, 1396–1405. [Google Scholar] [CrossRef]
- Cellier, C.; Patey, N.; Mauvieux, L.; Jabri, B.; Delabesse, E.; Cervoni, J.P.; Burtin, M.L.; Guy-Grand, D.; Bouhnik, Y.; Modigliani, R.; et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998, 114, 471–481. [Google Scholar] [CrossRef]
- Tack, G.J.; van Wanrooij, R.L.; Langerak, A.W.; Tjon, J.M.; von Blomberg, B.M.; Heideman, D.A.; van Bergen, J.; Koning, F.; Bouma, G.; Mulder, C.J.; et al. Origin and immunophenotype of aberrant IEL in RCDII patients. Mol. Immunol. 2012, 50, 262–270. [Google Scholar] [CrossRef]
- Schmitz, F.; Tjon, J.M.; Lai, Y.; Thompson, A.; Kooy-Winkelaar, Y.; Lemmers, R.J.; Verspaget, H.W.; Mearin, M.L.; Staal, F.J.; Schreurs, M.W.; et al. Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 2013, 62, 509–519. [Google Scholar] [CrossRef]
- Ettersperger, J.; Montcuquet, N.; Malamut, G.; Guegan, N.; Lopez-Lastra, S.; Gayraud, S.; Reimann, C.; Vidal, E.; Cagnard, N.; Villarese, P.; et al. Interleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Develop in the Intestine and Transform into Lymphomas in Celiac Disease. Immunity 2016, 45, 610–625. [Google Scholar] [CrossRef]
- Chander, U.; Leeman-Neill, R.J.; Bhagat, G. Pathogenesis of Enteropathy-Associated T Cell Lymphoma. Curr. Hematol. Malig. Rep. 2018, 13, 308–317. [Google Scholar] [CrossRef]
- Isaacson, P.; Wright, D.H. Malignant histiocytosis of the intestine. Its relationship to malabsorption and ulcerative jejunitis. Hum. Pathol. 1978, 9, 661–677. [Google Scholar] [CrossRef]
- Isaacson, P.G.; Bhagat, G. Enteropathy-associated T-cell lymphoma and other primary intestinal T-cell lymphomas. In Hematopathology, 2nd ed.; Elsevier: Saunders, MO, USA, 2017. [Google Scholar]
- Murray, A.; Cuevas, E.C.; Jones, D.B.; Wright, D.H. Study of the immunohistochemistry and T cell clonality of enteropathy-associated T cell lymphoma. Am. J. Pathol. 1995, 146, 509–519. [Google Scholar] [PubMed]
- Zettl, A.; Ott, G.; Makulik, A.; Katzenberger, T.; Starostik, P.; Eichler, T.; Puppe, B.; Bentz, M.; Muller-Hermelink, H.K.; Chott, A. Chromosomal gains at 9q characterize enteropathy-type T-cell lymphoma. Am. J. Pathol. 2002, 161, 1635–1645. [Google Scholar] [CrossRef] [Green Version]
- Moffitt, A.B.; Ondrejka, S.L.; McKinney, M.; Rempel, R.E.; Goodlad, J.R.; Teh, C.H.; Leppa, S.; Mannisto, S.; Kovanen, P.E.; Tse, E.; et al. Enteropathy-associated T cell lymphoma subtypes are characterized by loss of function of SETD2. J. Exp. Med. 2017, 214, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Deleeuw, R.J.; Zettl, A.; Klinker, E.; Haralambieva, E.; Trottier, M.; Chari, R.; Ge, Y.; Gascoyne, R.D.; Chott, A.; Muller-Hermelink, H.K.; et al. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology 2007, 132, 1902–1911. [Google Scholar] [CrossRef]
- Baumgartner, A.K.; Zettl, A.; Chott, A.; Ott, G.; Muller-Hermelink, H.K.; Starostik, P. High frequency of genetic aberrations in enteropathy-type T-cell lymphoma. Lab. Investig. 2003, 83, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Cejkova, P.; Zettl, A.; Baumgartner, A.K.; Chott, A.; Ott, G.; Muller-Hermelink, H.K.; Starostik, P. Amplification of NOTCH1 and ABL1 gene loci is a frequent aberration in enteropathy-type T-cell lymphoma. Virchows Arch. 2005, 446, 416–420. [Google Scholar] [CrossRef]
- Nicolae, A.; Xi, L.; Pham, T.H.; Pham, T.A.; Navarro, W.; Meeker, H.G.; Pittaluga, S.; Jaffe, E.S.; Raffeld, M. Mutations in the JAK/STAT and RAS signaling pathways are common in intestinal T-cell lymphomas. Leukemia 2016, 30, 2245–2247. [Google Scholar] [CrossRef] [Green Version]
- Cording, S.; Lhermitte, L.; Malamut, G.; Berrabah, S.; Trinquand, A.; Guegan, N.; Villarese, P.; Kaltenbach, S.; Meresse, B.; Khater, S.; et al. Oncogenetic landscape of lymphomagenesis in coeliac disease. Gut 2021. [Google Scholar] [CrossRef]
- Novakovic, B.J.; Novakovic, S.; Frkovic-Grazio, S. A single-center report on clinical features and treatment response in patients with intestinal T cell non-Hodgkin’s lymphomas. Oncol. Rep. 2006, 16, 191–195. [Google Scholar]
- Daum, S.; Ullrich, R.; Heise, W.; Dederke, B.; Foss, H.D.; Stein, H.; Thiel, E.; Zeitz, M.; Riecken, E.O. Intestinal non-Hodgkin’s lymphoma: A multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2003, 21, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Delabesse, E.; Helmer, C.; Patey, N.; Matuchansky, C.; Jabri, B.; Macintyre, E.; Cerf-Bensussan, N.; Brousse, N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000, 356, 203–208. [Google Scholar] [CrossRef]
- Egan, L.J.; Walsh, S.V.; Stevens, F.M.; Connolly, C.E.; Egan, E.L.; McCarthy, C.F. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J. Clin. Gastroenterol. 1995, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Allez, M.; Treton, X.; Fieschi, C.; Galicier, L.; Joly, F.; Gornet, J.M.; Oksenhendler, E.; Lemann, M.; Bouhnik, Y. High frequency of fatal haemophagocytic lymphohistiocytosis syndrome in enteropathy-associated T cell lymphoma. Dig. Liver Dis. 2012, 44, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.Y.; Tashi, T.; Lee, Y.Y.; Fu, T.Y.; Shih, C.A. Enteropathy-associated T-cell Lymphoma (EATL) with intracranial metastasis: A rare and dismal condition. Acta Gastroenterol. Belg. 2020, 83, 77–80. [Google Scholar] [PubMed]
- Horvath, L.; Oberhuber, G.; Chott, A.; Effenberger, M.; Tilg, H.; Gunsilius, E.; Wolf, D.; Iglseder, S. Multiple cerebral lesions in a patient with refractory celiac disease: A case report. World J. Gastroenterol. 2020, 26, 7584–7592. [Google Scholar] [CrossRef]
- Berman, E.L.; Zauber, N.P.; Rickert, R.R.; Diss, T.C.; Isaacson, P.G. Enteropathy-associated T cell lymphoma with brain involvement. J. Clin. Gastroenterol. 1998, 26, 337–341. [Google Scholar] [CrossRef]
- Nijeboer, P.; Malamut, G.; Mulder, C.J.; Cerf-Bensussan, N.; Sibon, D.; Bouma, G.; Cellier, C.; Hermine, O.; Visser, O. Enteropathy-associated T-cell lymphoma: Improving treatment strategies. Dig. Dis. 2015, 33, 231–235. [Google Scholar] [CrossRef]
- d’Amore, F.; Relander, T.; Lauritzsen, G.F.; Jantunen, E.; Hagberg, H.; Anderson, H.; Holte, H.; Osterborg, A.; Merup, M.; Brown, P.; et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J. Clin. Oncol. 2012, 30, 3093–3099. [Google Scholar] [CrossRef]
- Jantunen, E.; Boumendil, A.; Finel, H.; Luan, J.J.; Johnson, P.; Rambaldi, A.; Haynes, A.; Duchosal, M.A.; Bethge, W.; Biron, P.; et al. Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: A retrospective study by the EBMT. Blood 2013, 121, 2529–2532. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, W.F.; Caldwell, M.E.; Reddy, N. Brentuximab in the treatment of CD30-positive enteropathy-associated T-cell lymphoma. J. Natl. Compr. Cancer Netw. 2013, 11, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Voorhees, T.J.; Ghosh, N.; Grover, N.; Block, J.; Cheng, C.; Morrison, K.; Ivanova, A.; Dotti, G.; Serody, J.; Savoldo, B.; et al. Long-term remission in multiply relapsed enteropathy-associated T-cell lymphoma following CD30 CAR T-cell therapy. Blood Adv. 2020, 4, 5925–5928. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Mukewar, A.S.; Rubio-Tapia, A.; Wu, T.-T.; Jabri, B.; Murray, J.A. Open-Capsule Budesonide for Refractory Celiac Disease. Am. J. Gastroenterol. 2017, 112, 959–967. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Jaffe, E.S.; Brousset, P.; Chan, J.K.; de Leval, L.; Gaulard, P.; Harris, N.L.; Pileri, S.; Weiss, L.M.; International Lymphoma Study, G. Cytotoxic T-cell and NK-cell lymphomas: Current questions and controversies. Am. J. Surg. Pathol. 2014, 38, e60–e71. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.; Gill, H.; Loong, F.; Kim, S.J.; Ng, S.B.; Tang, T.; Ko, Y.H.; Chng, W.J.; Lim, S.T.; Kim, W.S.; et al. Type II enteropathy-associated T-cell lymphoma: A multicenter analysis from the Asia Lymphoma Study Group. Am. J. Hematol. 2012, 87, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Chuang, S.S.; Tang, T.; Tan, L.; Ko, Y.H.; Chuah, K.L.; Ng, S.B.; Chng, W.J.; Gatter, K.; Loong, F.; et al. Type II EATL (epitheliotropic intestinal T-cell lymphoma): A neoplasm of intra-epithelial T-cells with predominant CD8alphaalpha phenotype. Leukemia 2013, 27, 1688–1696. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.K.; Chan, A.C.; Cheuk, W.; Wan, S.K.; Lee, W.K.; Lui, Y.H.; Chan, W.K. Type II enteropathy-associated T-cell lymphoma: A distinct aggressive lymphoma with frequent gammadelta T-cell receptor expression. Am. J. Surg. Pathol. 2011, 35, 1557–1569. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Z.; Yang, D.; Chen, J. Primary intestinal T-cell and NK-cell lymphomas: A clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod. Pathol. 2011, 24, 983–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.Y.; Ooi, A.S.; Ang, M.K.; Koh, M.; Wong, J.C.; Dykema, K.; Ngeow, J.; Loong, S.; Gatter, K.; Tan, L.; et al. Nuclear expression of MATK is a novel marker of type II enteropathy-associated T-cell lymphoma. Leukemia 2011, 25, 555–557. [Google Scholar] [CrossRef]
- Tomita, S.; Kikuti, Y.Y.; Carreras, J.; Kojima, M.; Ando, K.; Takasaki, H.; Sakai, R.; Takata, K.; Yoshino, T.; Bea, S.; et al. Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod. Pathol. 2015, 28, 1286–1296. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.H.; Karnan, S.; Kim, K.M.; Park, C.K.; Kang, E.S.; Kim, Y.H.; Kang, W.K.; Kim, S.J.; Kim, W.S.; Lee, W.Y.; et al. Enteropathy-associated T-cell lymphoma--a clinicopathologic and array comparative genomic hybridization study. Hum. Pathol. 2010, 41, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nairismagi, M.L.; Tan, J.; Lim, J.Q.; Nagarajan, S.; Ng, C.C.; Rajasegaran, V.; Huang, D.; Lim, W.K.; Laurensia, Y.; Wijaya, G.C.; et al. JAK-STAT and G-protein-coupled receptor signaling pathways are frequently altered in epitheliotropic intestinal T-cell lymphoma. Leukemia 2016, 30, 1311–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberti, A.; Dobay, M.P.; Bisig, B.; Vallois, D.; Boechat, C.; Lanitis, E.; Bouchindhomme, B.; Parrens, M.C.; Bossard, C.; Quintanilla-Martinez, L.; et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat. Commun. 2016, 7, 12602. [Google Scholar] [CrossRef]
- Tomita, S.; Kikuti, Y.Y.; Carreras, J.; Sakai, R.; Takata, K.; Yoshino, T.; Bea, S.; Campo, E.; Missiaglia, E.; Bouilly, J.; et al. Monomorphic Epitheliotropic Intestinal T-Cell Lymphoma in Asia Frequently Shows SETD2 Alterations. Cancers 2020, 12, 3539. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Jang, M.; Yang, W.I.; Yoon, S.O. Primary Gastrointestinal T/NK Cell Lymphoma. Cancers 2021, 13, 2679. [Google Scholar] [CrossRef] [PubMed]
- Kikuma, K.; Yamada, K.; Nakamura, S.; Ogami, A.; Nimura, S.; Hirahashi, M.; Yonemasu, H.; Urabe, S.; Naito, S.; Matsuki, Y.; et al. Detailed clinicopathological characteristics and possible lymphomagenesis of type II intestinal enteropathy-associated T-cell lymphoma in Japan. Hum. Pathol. 2014, 45, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, A.L.; Lymphoma, G.; Eastern Cooperative Oncology, G.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Yi, J.H.; Lee, G.W.; Do, Y.R.; Jung, H.R.; Hong, J.Y.; Yoon, D.H.; Suh, C.; Choi, Y.S.; Yi, S.Y.; Sohn, B.S.; et al. Multicenter retrospective analysis of the clinicopathologic features of monomorphic epitheliotropic intestinal T-cell lymphoma. Ann. Hematol. 2019, 98, 2541–2550. [Google Scholar] [CrossRef]
- Liu, T.Z.; Zheng, Y.J.; Zhang, Z.W.; Li, S.S.; Chen, J.T.; Peng, A.H.; Huang, R.W. Chidamide based combination regimen for treatment of monomorphic epitheliotropic intestinal T cell lymphoma following radical operation: Two case reports. World J. Clin. Cases 2020, 8, 1278–1286. [Google Scholar] [CrossRef]
- Nato, Y.; Miyazaki, K.; Imai, H.; Nakano, E.; Kageyama, Y.; Ino, K.; Fujieda, A.; Matsumoto, T.; Tawara, I.; Tanaka, K.; et al. Early central nervous system relapse of monomorphic epitheliotropic intestinal T-cell lymphoma after cord blood transplantation. Int. J. Hematol. 2021, 114, 129–135. [Google Scholar] [CrossRef]
- Tang, X.F.; Yang, L.; Duan, S.; Guo, H.; Guo, Q.N. Intestinal T-cell and NK/T-cell lymphomas: A clinicopathological study of 27 Chinese patients. Ann. Diagn. Pathol. 2018, 37, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ko, Y.H. Peripheral T cell lymphoma in Asia. Int. J. Hematol. 2014, 99, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonnel, F.; Lavergne, A.; Messing, B.; Tsapis, A.; Berger, R.; Galian, A.; Nemeth, J.; Brouet, J.C.; Rambaud, J.C. Extensive small intestinal T-cell lymphoma of low-grade malignancy associated with a new chromosomal translocation. Cancer 1994, 73, 1286–1291. [Google Scholar] [CrossRef]
- Egawa, N.; Fukayama, M.; Kawaguchi, K.; Hishima, T.; Hayashi, Y.; Funata, N.; Ibuka, T.; Koike, M.; Miyashita, H.; Tajima, T. Relapsing oral and colonic ulcers with monoclonal T-cell infiltration. A low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer 1995, 75, 1728–1733. [Google Scholar] [CrossRef]
- Hirakawa, K.; Fuchigami, T.; Nakamura, S.; Daimaru, Y.; Ohshima, K.; Sakai, Y.; Ichimaru, T. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology 1996, 111, 778–782. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Inada, K.; Morita, K.; Suzuki, T. T-cell lymphomas diffusely involving the intestine: Report of two rare cases. Jpn. J. Clin. Oncol. 1996, 26, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Carbonnel, F.; d’Almagne, H.; Lavergne, A.; Matuchansky, C.; Brouet, J.C.; Sigaux, F.; Beaugerie, L.; Nemeth, J.; Coffin, B.; Cosnes, J.; et al. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut 1999, 45, 662–667. [Google Scholar] [CrossRef]
- Ranheim, E.A.; Jones, C.; Zehnder, J.L.; Warnke, R.; Yuen, A. Spontaneously relapsing clonal, mucosal cytotoxic T-cell lymphoproliferative disorder: Case report and review of the literature. Am. J. Surg. Pathol. 2000, 24, 296–301. [Google Scholar] [CrossRef]
- Isomoto, H.; Maeda, T.; Akashi, T.; Tsuchiya, T.; Kawaguchi, Y.; Sawayama, Y.; Koida, S.; Ohnita, K.; Kohno, S.; Tomonaga, M. Multiple lymphomatous polyposis of the colon originating from T-cells: A case report. Dig. Liver Dis. 2004, 36, 218–221. [Google Scholar] [CrossRef]
- Zivny, J.; Banner, B.F.; Agrawal, S.; Pihan, G.; Barnard, G.F. CD4+ T-cell lymphoproliferative disorder of the gut clinically mimicking celiac sprue. Dig. Dis. Sci. 2004, 49, 551–555. [Google Scholar] [CrossRef]
- Svrcek, M.; Garderet, L.; Sebbagh, V.; Rosenzwajg, M.; Parc, Y.; Lagrange, M.; Bennis, M.; Lavergne-Slove, A.; Flejou, J.F.; Fabiani, B. Small intestinal CD4+ T-cell lymphoma: A rare distinctive clinicopathological entity associated with prolonged survival. Virchows Arch. 2007, 451, 1091–1093. [Google Scholar] [CrossRef]
- Margolskee, E.; Jobanputra, V.; Lewis, S.K.; Alobeid, B.; Green, P.H.; Bhagat, G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS ONE 2013, 8, e68343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.M.; Warnke, R.A.; Hu, Q.; Gaulard, P.; Copie-Bergman, C.; Alkan, S.; Wang, H.Y.; Cheng, J.X.; Bacon, C.M.; Delabie, J.; et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 2013, 122, 3599–3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leventaki, V.; Manning, J.T., Jr.; Luthra, R.; Mehta, P.; Oki, Y.; Romaguera, J.E.; Medeiros, L.J.; Vega, F. Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. Hum. Pathol. 2014, 45, 421–426. [Google Scholar] [CrossRef]
- Malamut, G.; Meresse, B.; Kaltenbach, S.; Derrieux, C.; Verkarre, V.; Macintyre, E.; Ruskone-Fourmestraux, A.; Fabiani, B.; Radford-Weiss, I.; Brousse, N.; et al. Small intestinal CD4+ T-cell lymphoma is a heterogenous entity with common pathology features. Clin. Gastroenterol. Hepatol. 2014, 12, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Sena Teixeira Mendes, L.; Attygalle, A.D.; Cunningham, D.; Benson, M.; Andreyev, J.; Gonzales-de-Castro, D.; Wotherspoon, A. CD4-positive small T-cell lymphoma of the intestine presenting with severe bile-acid malabsorption: A supportive symptom control approach. Br. J. Haematol. 2014, 167, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Edison, N.; Belhanes-Peled, H.; Eitan, Y.; Guthmann, Y.; Yeremenko, Y.; Raffeld, M.; Elmalah, I.; Trougouboff, P. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn’s colitis. Hum. Pathol. 2016, 57, 45–50. [Google Scholar] [CrossRef]
- Sharma, A.; Oishi, N.; Boddicker, R.L.; Hu, G.; Benson, H.K.; Ketterling, R.P.; Greipp, P.T.; Knutson, D.L.; Kloft-Nelson, S.M.; He, R.; et al. Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood 2018, 131, 2262–2266. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ng, C.S.; Chen, C.; Yu, G.; Yin, W. An unusual case report of indolent T-cell lymphoproliferative disorder with aberrant CD20 expression involving the gastrointestinal tract and bone marrow. Diagn. Pathol. 2018, 13, 82. [Google Scholar] [CrossRef]
- Guo, L.; Wen, Z.; Su, X.; Xiao, S.; Wang, Y. Indolent T-cell lymphoproliferative disease with synchronous diffuse large B-cell lymphoma: A case report. Medicine 2019, 98, e15323. [Google Scholar] [CrossRef]
- Nagaishi, T.; Yamada, D.; Suzuki, K.; Fukuyo, R.; Saito, E.; Fukuda, M.; Watabe, T.; Tsugawa, N.; Takeuchi, K.; Yamamoto, K.; et al. Indolent T cell lymphoproliferative disorder with villous atrophy in small intestine diagnosed by single-balloon enteroscopy. Clin. J. Gastroenterol. 2019, 12, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Perry, A.M.; Bailey, N.G.; Bonnett, M.; Jaffe, E.S.; Chan, W.C. Disease Progression in a Patient With Indolent T-Cell Lymphoproliferative Disease of the Gastrointestinal Tract. Int. J. Surg. Pathol. 2019, 27, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.H.; Gu, H.Y.; Lin, D.L.; Shi, H.L.; Zhang, Y.J.; Li, Y.J. Clinicopathological features of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: A report of five cases. Zhonghua Bing Li Xue Za Zhi 2019, 48, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Baciorri, F.; Di Prete, M.; Zizzari, A.G.; Anemona, L. Oral manifestation of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: A potential diagnostic pitfall. J. Cutan. Pathol. 2020, 47, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Tsukasaki, K.; Kohri, M.; Akuzawa, Y.; Saeki, T.; Okamura, D.; Ishikawa, M.; Maeda, T.; Kawai, N.; Matsuda, A.; et al. Indolent T-cell lymphoproliferative disorder of the stomach successfully treated by radiotherapy. J. Clin. Exp. Hematopathol. 2020, 60, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, L.G.; Zhang, X.Y.; Wang, L.L.; Zhang, L.; Xiao, Y.J.; Xing, X.M.; Lin, D.L. Indolent T cell lymphoproliferative disorder of the gastrointestinal tract: An uncommon case with lymph node involvement and the classic Hodgkin’s lymphoma. J. Gastrointest. Oncol. 2020, 11, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Zizzo, M.; Sanguedolce, F.; Martino, G.; Soriano, A.; Ricci, S.; Castro Ruiz, C.; Annessi, V.; Ascani, S. Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: A tricky diagnosis of a gastric case. BMC Gastroenterol. 2020, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Luminari, S.; Martino, G.; Soriano, A.; Ricci, L.; Caprera, C.; Ascani, S. Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers 2021, 13, 2790. [Google Scholar] [CrossRef]
- Chan, J.K.C.; Fukuyama, M. Haematolymphoid tumours of the digestive system. In WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of the Digestive System, 5th ed.; IARC: Lyon, France, 2019; Volume 1. [Google Scholar]
- Soderquist, C.R.; Patel, N.; Murty, V.V.; Betman, S.; Aggarwal, N.; Young, K.H.; Xerri, L.; Leeman-Neill, R.; Lewis, S.K.; Green, P.H.; et al. Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica 2020, 105, 1895–1906. [Google Scholar] [CrossRef] [Green Version]
- Soderquist, C.R.; Bhagat, G. Gastrointestinal T- and NK-cell lymphomas and indolent lymphoproliferative disorders. Semin. Diagn. Pathol. 2020, 37, 11–23. [Google Scholar] [CrossRef]
- Jaffe, E.S. T-cell and NK-cell neoplasms of the gastrointestinal tract—Recurrent themes, but clinical and biological distinctions exist. Haematologica 2020, 105, 1760–1762. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Takata, K.; Wang, R.C.; Yang, S.F.; Chuang, S.S. Primary gastrointestinal anaplastic large cell lymphoma. Pathology 2017, 49, 479–485. [Google Scholar] [CrossRef] [PubMed]
- ten Berge, R.L.; Oudejans, J.J.; Ossenkoppele, G.J.; Pulford, K.; Willemze, R.; Falini, B.; Chott, A.; Meijer, C.J. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J. Clin. Pathol. 2000, 53, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Liu, F.; Li, S.; Liu, N.; Li, L.; Li, C.; Peng, T. Primary rare anaplastic large cell lymphoma, ALK positive in small intestine: Case report and review of the literature. Diagn. Pathol. 2016, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Carey, M.J.; Medeiros, L.J.; Roepke, J.E.; Kjeldsberg, C.R.; Elenitoba-Johnson, K.S. Primary anaplastic large cell lymphoma of the small intestine. Am. J. Clin. Pathol. 1999, 112, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.; Fields, P.; Simo, R. Anaplastic lymphoma of the cervical esophagus presenting as a tracheoesophageal fistula. Head Neck 2008, 30, 1264–1268. [Google Scholar] [CrossRef]
- Sadiya, N.; Ghosh, M. Primary ALK positive Anaplastic large cell lymphoma of T-cell type of jejunum: Report of a rare extranodal entity with review of literature. Arch. Int. Surg. 2014, 4, 50–53. [Google Scholar] [CrossRef]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Savage, K.J.; Harris, N.L.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Connors, J.M.; Rimsza, L.; Pileri, S.A.; Chhanabhai, M.; Gascoyne, R.D.; et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008, 111, 5496–5504. [Google Scholar] [CrossRef]
- Au, W.Y.; Weisenburger, D.D.; Intragumtornchai, T.; Nakamura, S.; Kim, W.S.; Sng, I.; Vose, J.; Armitage, J.O.; Liang, R.; International Peripheral, T.C.L.P. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: A study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009, 113, 3931–3937. [Google Scholar] [CrossRef] [Green Version]
- Pongpruttipan, T.; Sukpanichnant, S.; Assanasen, T.; Wannakrairot, P.; Boonsakan, P.; Kanoksil, W.; Kayasut, K.; Mitarnun, W.; Khuhapinant, A.; Bunworasate, U.; et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: A comprehensive clinicopathologic and phenotypic study. Am. J. Surg. Pathol. 2012, 36, 481–499. [Google Scholar] [CrossRef]
- Chuang, S.S.; Chang, S.T.; Chuang, W.Y.; Huang, W.T.; Hsieh, P.P.; Tsou, M.H.; Liao, Y.L.; Lin, S.H.; Hsieh, Y.C.; Lu, C.L.; et al. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am. J. Surg. Pathol. 2009, 33, 1230–1240. [Google Scholar] [CrossRef]
- Fang, J.C.; Xia, Z.X.; Wang, C.N.; Li, Z. Clinicopathologic and Immunophenotypic Features of Primary Intestinal Extranodal NK/T-Cell Lymphoma, Nasal Type. Int. J. Surg. Pathol. 2015, 23, 609–616. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.; Baek, H.L.; Jung, J.H.; Kang, I.G.; Sym, S.J.; Park, J.; Ahn, J.Y.; Cho, E.K.; Kim, S.T.; et al. Tumor cell nuclear diameter and CD30 expression as potential prognostic parameter in patients with extranodal NK/T-cell lymphoma, nasal type. Int. J. Clin. Exp. Pathol. 2012, 5, 939–947. [Google Scholar] [PubMed]
- Hu, L.M.; Takata, K.; Miyata-Takata, T.; Asano, N.; Takahashi, E.; Furukawa, K.; Miyoshi, H.; Satou, A.; Kohno, K.; Kosugi, H.; et al. Clinicopathological analysis of 12 patients with Epstein-Barr virus-positive primary intestinal T/natural killer-cell lymphoma (EBV(+) ITNKL). Histopathology 2017, 70, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yang, W.I.; Min, Y.H.; Ko, Y.H.; Yoon, S.O. The role of the polycomb repressive complex pathway in T and NK cell lymphoma: Biological and prognostic implications. Tumour. Biol. 2016, 37, 2037–2047. [Google Scholar] [CrossRef]
- Kim, W.Y.; Nam, S.J.; Kim, S.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk. Lymphoma 2015, 56, 1778–1786. [Google Scholar] [CrossRef]
- Kuo, T.T.; Shih, L.Y.; Tsang, N.M. Nasal NK/T cell lymphoma in Taiwan: A clinicopathologic study of 22 cases, with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene association, and treatment modalities. Int. J. Surg. Pathol. 2004, 12, 375–387. [Google Scholar] [CrossRef]
- Mraz-Gernhard, S.; Natkunam, Y.; Hoppe, R.T.; LeBoit, P.; Kohler, S.; Kim, Y.H. Natural killer/natural killer-like T-cell lymphoma, CD56+, presenting in the skin: An increasingly recognized entity with an aggressive course. J. Clin. Oncol. 2001, 19, 2179–2188. [Google Scholar] [CrossRef]
- Takahashi, E.; Asano, N.; Li, C.; Tanaka, T.; Shimada, K.; Shimada, S.; Yoshino, T.; Kojima, M.; Hara, K.; Eimoto, T.; et al. Nodal T/NK-cell lymphoma of nasal type: A clinicopathological study of six cases. Histopathology 2008, 52, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.H.; Shui, R.H.; Sheng, W.Q.; Wang, C.F.; Lu, H.F.; Zhou, X.Y.; Zhu, X.Z.; Li, X.Q. Primary Intestinal Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: A Comprehensive Clinicopathological Analysis of 55 Cases. PLoS ONE 2016, 11, e0161831. [Google Scholar] [CrossRef]
- Ishibashi, H.; Nimura, S.; Kayashima, Y.; Takamatsu, Y.; Iwasaki, H.; Harada, N.; Momosaki, S.; Takedatsu, H.; Sakisaka, S.; Takeshita, M. Endoscopic and clinicopathological characteristics of gastrointestinal adult T-cell leukemia/lymphoma. J. Gastrointest. Oncol. 2019, 10, 723–733. [Google Scholar] [CrossRef]
- Baba, U.; Toubai, T.; Ota, S.; Miura, Y.; Toyosima, N.; Tanaka, J.; Asaka, M.; Imamura, M. A case report of primary gastric adult T cell lymphoma. Nihon Ronen Igakkai Zasshi 2004, 41, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Ishitsuka, K.; Utsunomiya, A.; Aosaki, S.; Tashiro, Y.; Takeshita, T. Indolent primary gastric adult T-cell leukemia/lymphoma with recurrent lesions limited to the stomach and duodenum. Rinsho Ketsueki 2002, 43, 554–559. [Google Scholar]
- Nozoe, T.; Matsumata, T. Primary gastric lymphoma associated with human T-cell leukaemia virus I. Eur. J. Gastroenterol. Hepatol. 2000, 12, 357–360. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamadori, I.; Hasuo, T.; Oue, H.; Kunitomo, T.; Takase, S.; Hayashi, K.; Sadahira, Y.; Akagi, T. Primary Gastric T-cell Lymphoma Associated with Human T-cell Leukemia Virus Type I shows ʻLymphoepithelial Lesions’: Case report. J. Clin. Exp. Hematopathol. 2002, 42, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Yaita, H.; Nakamura, S.; Kurahara, K.; Nagasue, T.; Kochi, S.; Oshiro, Y.; Ohshima, K.; Ikeda, Y.; Fuchigami, T. Primary small-bowel adult T-cell leukemia/lymphoma with gastric AL amyloidosis. Endoscopy 2014, 46, E613–E614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Yokoyama, M.; Ishizawa, S.; Terui, Y.; Nomura, K.; Marutsuka, K.; Nunomura, M.; Fukushima, N.; Yagyuu, T.; Nakamine, H.; et al. Lymphomatoid gastropathy: A distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood 2010, 116, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Noujima-Harada, M.; Miyata-Takata, T.; Ichimura, K.; Sato, Y.; Miyata, T.; Naruse, K.; Iwamoto, T.; Tari, A.; Masunari, T.; et al. Clinicopathologic analysis of 6 lymphomatoid gastropathy cases: Expanding the disease spectrum to CD4-CD8+ cases. Am. J. Surg. Pathol. 2015, 39, 1259–1266. [Google Scholar] [CrossRef]
- Mansoor, A.; Pittaluga, S.; Beck, P.L.; Wilson, W.H.; Ferry, J.A.; Jaffe, E.S. NK-cell enteropathy: A benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: Clinicopathologic features and follow-up in a unique case series. Blood 2011, 117, 1447–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.; Morgan, E.A.; Berger, D.; Pinkus, G.S.; Ferry, J.A.; Zukerberg, L.R. NK-Cell Enteropathy and Similar Indolent Lymphoproliferative Disorders: A Case Series With Literature Review. Am. J. Clin. Pathol. 2019, 151, 75–85. [Google Scholar] [CrossRef]
- Xiao, W.; Gupta, G.K.; Yao, J.; Jang, Y.J.; Xi, L.; Baik, J.; Sigler, A.; Kumar, A.; Moskowitz, A.J.; Arcila, M.E.; et al. Recurrent somatic JAK3 mutations in NK-cell enteropathy. Blood 2019, 134, 986–991. [Google Scholar] [CrossRef]
- Cohen, J.I.; Kimura, H.; Nakamura, S.; Ko, Y.H.; Jaffe, E.S. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: A status report and summary of an international meeting, 8-9 September 2008. Ann. Oncol. 2009, 20, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Kimura, H.; Yoshino, T.; Kim, C.W.; Ko, Y.H.; Lee, S.S.; Peh, S.C.; Chan, J.K.; Group, C.S. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: Overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol. Int. 2008, 58, 209–217. [Google Scholar] [CrossRef]
- Tian, S.; Westbrook, L.M.; Xiao, S.Y.; Zhang, Y.; Huang, Y.; Wang, H.L. The Morphologic Features of Primary Epstein-Barr Virus Infection in the Gastrointestinal Tract: An Approach to Correct Diagnosis. Am. J. Surg. Pathol. 2019, 43, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiao, S.Y.; Chen, Q.; Liu, H.; Ping, J. Monomorphic epitheliotropic intestinal T-cell lymphoma may mimic intestinal inflammatory disorders. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419829387. [Google Scholar] [CrossRef]
- Pardi, D.S. Diagnosis and Management of Microscopic Colitis. Am. J. Gastroenterol. 2017, 112, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Matnani, R.; Ganapathi, K.A.; Lewis, S.K.; Green, P.H.; Alobeid, B.; Bhagat, G. Indolent T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: A review and update. Hematol. Oncol. 2017, 35, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
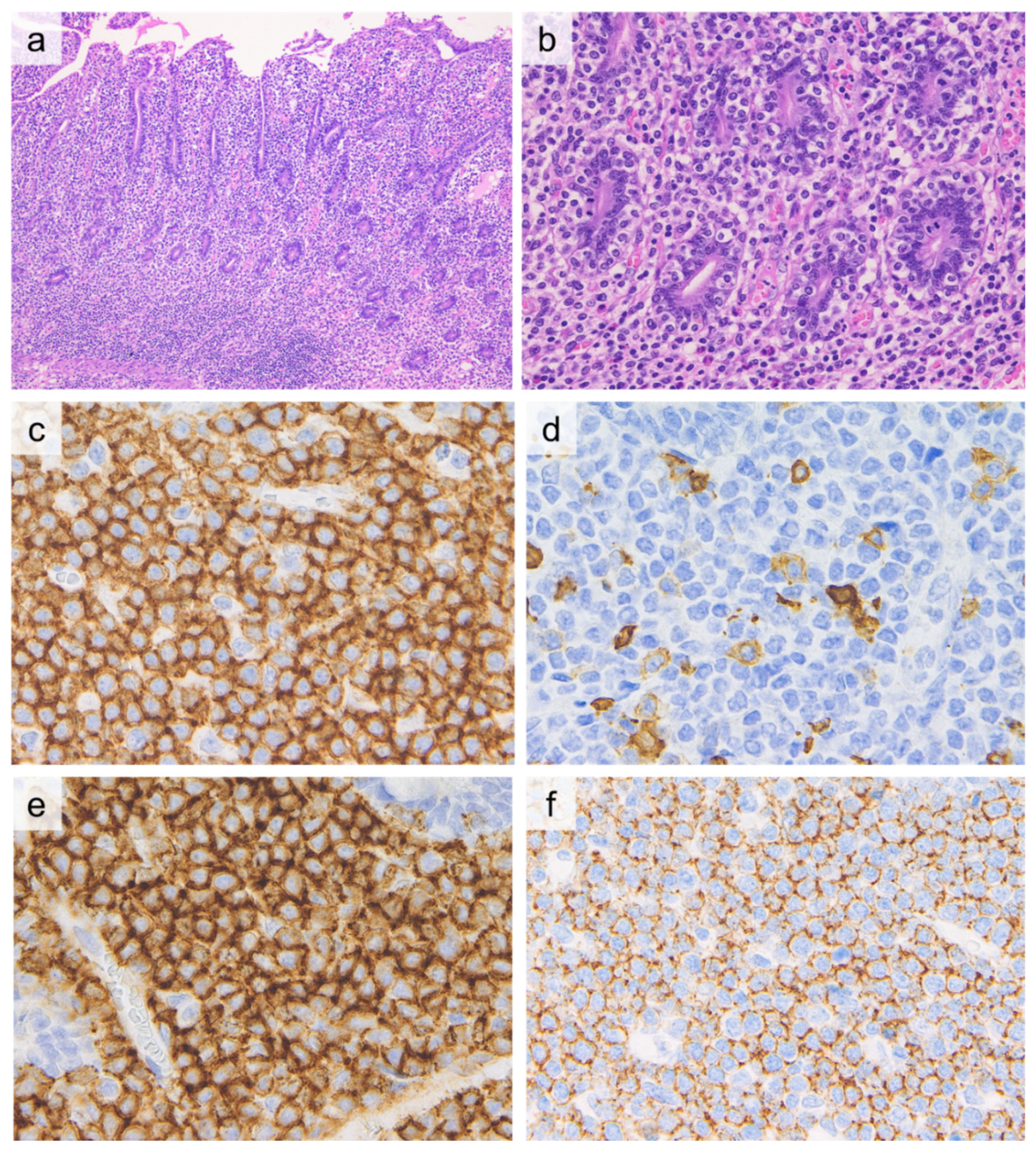

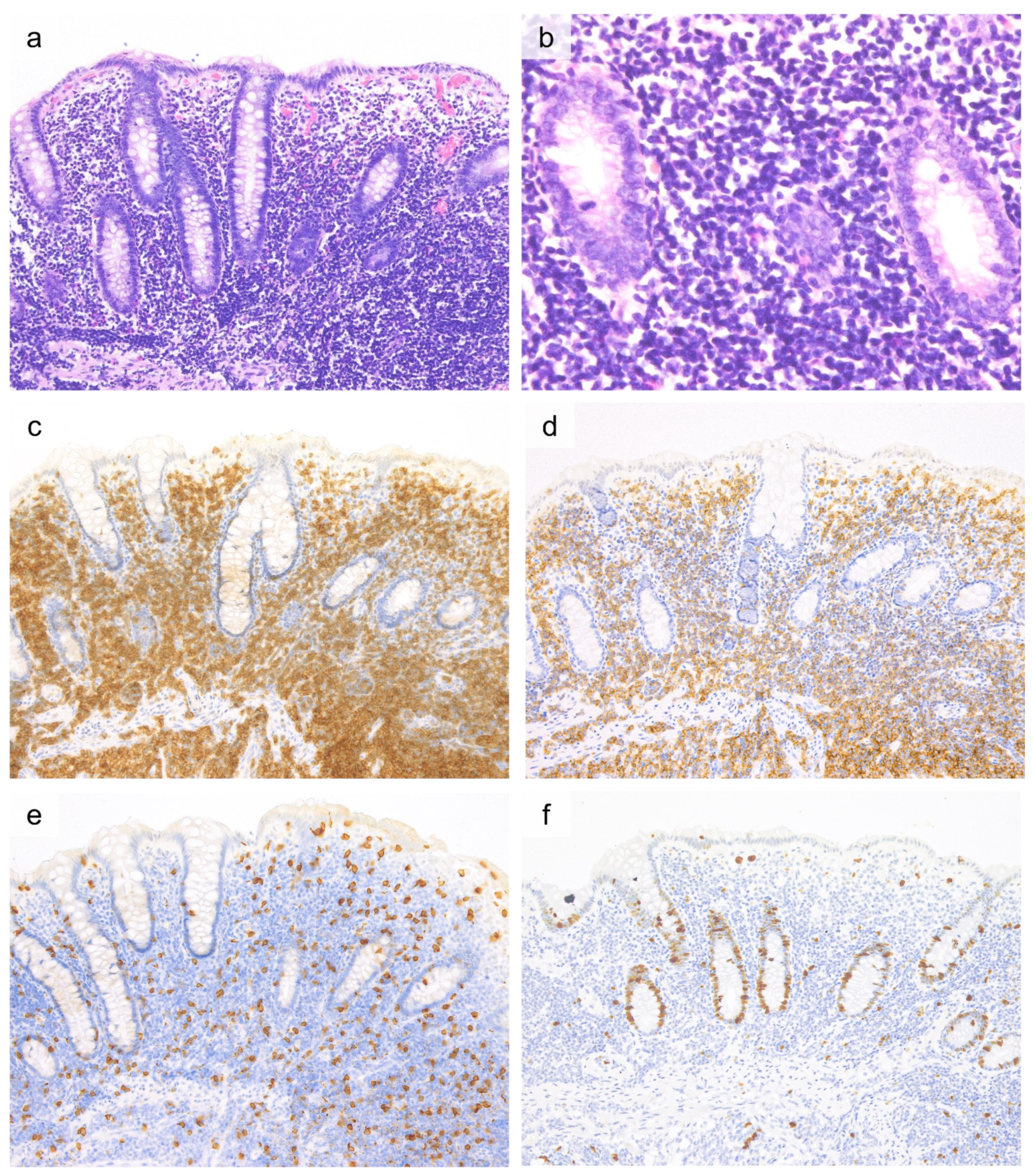
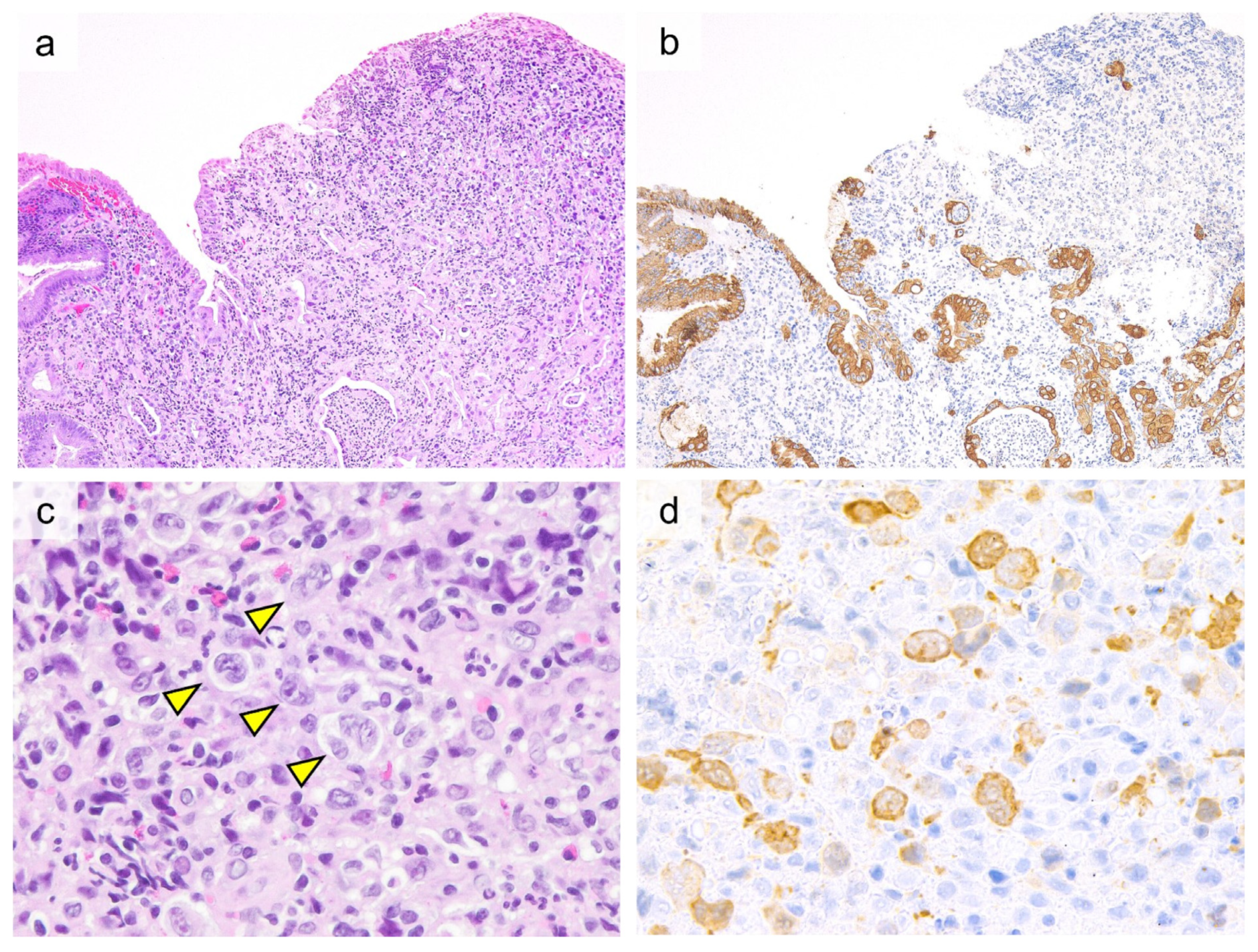



| Investigations | Celiac Disease | RCD I | RCD II | EATL |
|---|---|---|---|---|
| Disease type | Chronic enteropathy triggered by dietary gluten | Persistent autoinflammatory immune response, gluten independent | Low-grade lymphoproliferative disorder | High-grade lymphoma |
| Immunophenotype | sCD3+, cCD3+, CD5−/+, CD8+, CD103+ | sCD3+, cCD3+, CD5−/+, CD8+, CD103+ | sCD3−, cCD3+, CD5−, CD8−, CD103+, CD30− | CD3+, CD8−, CD30+, Ki67 LI: high (>50%) |
| T-cell receptor | polyclonal | polyclonal | monoclonal | monoclonal |
| 5-year survival | 80–96% [18,20,21] | 45–58% [18,20,21] | ~20% [5,9,20] | |
| Rate of progression to EATL in 5 years | 0.7% [24] | 3–14% [19,20,21,22,23] | 33–52% [19,20,21,22,23] | - |
| Investigation | EATL | MEITL | ALCL | ENKTCL | ATLL | ITLPD-GI | NK-ENT |
|---|---|---|---|---|---|---|---|
| Common sites | Jejunum Ileum | Jejunum Ileum | Small intestine Stomach Colon Esophagus | Small intestine Colon Ileocecal junction | Stomach Small intestine Colorectal | Small intestine Colon Esophagus Oral cavity | Stomach Duodenum Small intestine Colon |
| Morphology |
|
|
|
|
|
|
|
| Immuno- phenotype | |||||||
| CD2 | + | + | Most cases are positive for at least one of the T-cell markers | + | + | + | −/+ |
| CD3 | + | + | + | + | + | + (cytoplasmic) | |
| CD5 | − | − | −/+ | + | + | − | |
| CD4 | − | − | − | + | CD4+ or CD8+ | − | |
| CD8 | −/+ | + | − | −/+ | − | − | |
| CD30 | frequently + in large cells | − | + (in Golgi and cytomembrane) | sometimes + (26–47%) | sometimes + (32%) | − | − |
| CD56 | − | + | − sometimes+ (~20%) | + | − | − | + |
| TIA-1 | + | + | + (+for at least one of the cytotoxic markers) | + | − | + in CD8+ cells | + |
| EBER in situ | − | − | − | + | − | − | − |
| Other findings | CD103+, MATK+ < 40% | CD103+, MATK+ > 85%, Aberrant CD20+ (11–24%) | ALK+ for approximately 24% in GI-ALCL | Cases derived from NK cells are sCD3− and cCD3+ | CD25+ CD103+ (48%) | Ki-67 LI is very low (<10%) | CD103− Ki-67 LI varies in the range of 10–90% |
| Chromosomal features | Gains of 9q, 7q, 1q, 5q Losses of 6q | Gains of 9q, 8q | (ALK+ cases) Gains of 17p, 17q, 7p Losses of 4q, 11q (ALK− cases) Gains of 6p, 7p | Gains of 1q, 17q, 20q Losses of 6q, 8p, 11q | Gains of 14q Losses of 6q | − | − |
| Genetic features | JAK-STAT RAS | JAK-STAT RAS SETD2 | (ALK+ cases) NPM-ALK fusion JAK/STAT RAS-ERK PIK-AKT | JAK-STAT Epigenetic regulators | TCR-NF-κB JAK/STAT | (CD4+ cases) JAK/STAT RAS Epigenetic modifiers | JAK/STAT |
| Clinical course | Aggressive | Aggressive | Aggressive | Aggressive | Aggressive | Indolent, Slight risk of progression and transformation | Indolent, No transformation reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, M.F.; Nishimura, Y.; Nishikori, A.; Yoshino, T.; Sato, Y. Primary Gastrointestinal T-Cell Lymphoma and Indolent Lymphoproliferative Disorders: Practical Diagnostic and Treatment Approaches. Cancers 2021, 13, 5774. https://doi.org/10.3390/cancers13225774
Nishimura MF, Nishimura Y, Nishikori A, Yoshino T, Sato Y. Primary Gastrointestinal T-Cell Lymphoma and Indolent Lymphoproliferative Disorders: Practical Diagnostic and Treatment Approaches. Cancers. 2021; 13(22):5774. https://doi.org/10.3390/cancers13225774
Chicago/Turabian StyleNishimura, Midori Filiz, Yoshito Nishimura, Asami Nishikori, Tadashi Yoshino, and Yasuharu Sato. 2021. "Primary Gastrointestinal T-Cell Lymphoma and Indolent Lymphoproliferative Disorders: Practical Diagnostic and Treatment Approaches" Cancers 13, no. 22: 5774. https://doi.org/10.3390/cancers13225774
APA StyleNishimura, M. F., Nishimura, Y., Nishikori, A., Yoshino, T., & Sato, Y. (2021). Primary Gastrointestinal T-Cell Lymphoma and Indolent Lymphoproliferative Disorders: Practical Diagnostic and Treatment Approaches. Cancers, 13(22), 5774. https://doi.org/10.3390/cancers13225774








